Abstract
Flavopiridol (NSC 649890; Behringwerke L86-8275, Marburg, Germany), is a potent inhibitor of cyclin dependent kinases (CDKs) 1, 2, and 4. It has potent antiproliferative effects in vitro and is active in tumor models in vivo. While surveying the effect of flavopiridol on cell cycle progression in different cell types, we discovered that hematopoietic cell lines, including SUDHL4, SUDHL6 (B-cell lines), Jurkat, and MOLT4 (T-cell lines), and HL60 (myeloid), displayed notable sensitivity to flavopiridol-induced apoptosis. For example, after 100 nmol/L for 12 hours, SUDHL4 cells displayed a similar degree of DNA fragmentation to that shown by the apoptosis-resistant PC3 prostate carcinoma cells only after 3,000 nmol/L for 48 hours. After exposure to 1,000 nmol/L flavopiridol for 12 hours, typical apoptotic morphology was observed in SUDHL4 cells, but not in PC3 prostate carcinoma cells despite comparable potency (SUDHL4:120 nmol/L; PC3: 203 nmol/L) in causing growth inhibition by 50% (IC50). Flavopiridol did not induce topoisomerase I or II cleavable complex activity. A relation of p53, bcl2, or bax protein levels to apoptosis in SUDHL4 was not appreciated. While flavopiridol caused cell cycle arrest with decline in CDK1 activity in PC3 cells, apoptosis of SUDHL4 cells occurred without evidence of cell cycle arrest. These results suggest that antiproliferative activity of flavopiridol (manifest by cell cycle arrest) may be separated in different cell types from a capacity to induce apoptosis. Cells from hematopoietic neoplasms appear in this limited sample to be very susceptible to flavopiridol-induced apoptosis and therefore clinical trials in hematopoietic neoplasms should be of high priority.
FLAVOPIRIDOL IS A NOVEL flavonoid with potent antiproliferative effects. Its capacity to inhibit cell growth by 50% (IC50) is 60 and 400 times more potent than the structurally related flavone, quercetin and the isoflavone, genistein, respectively.1 Flavopiridol also has antitumor effects in vivo.2 Previous studies have shown that flavopiridol is a potent inhibitor of cyclin-dependent kinase (CDK)1,3 as well as CDKs, 2 and 4.4 In addition, the drug can indirectly affect CDK activity by inhibiting the normal regulatory phosphorylation of CDKs.5 These activities can be explained by the recent demonstration that an analog of flavopiridol can bind directly to the adenosine triphosphate (ATP) binding site of CDK2.6
Apoptosis is the process by which physiologic regulation of cell number in developing organs and organisms is achieved. Apoptotic cell death is characterized by the activation of proteases and nucleases leading to chromatin condensation.7,8 Recent experiments have underscored that apoptosis can be activated by many types of cancer chemotherapeutic agents.9-11 Of great ongoing interest is how agents of such diverse structural types can activate the apoptotic program.
Because flavopiridol has recently entered clinical trials,12 we have sought to acquire a basis for prioritizing entry into Phase II trials. We report here that several hematopoietic cell types are notably sensitive to induction of apoptosis by flavopiridol. This sensitivity cannot be ascribed to induction of cleavable complex activity by topoisomerases, or is it relatable to changes in p53, bcl2, and bax levels in the apoptosis-prone B-cell line, SUDHL4.
MATERIALS AND METHODS
Drugs and cell culture.
Flavopiridol (NSC 649890; Behringwerke, L86-8275 [(-) cis-6,7-dihydroxy-2-(2-chlorophenyl)-8[4-(3-hydroxy-1-methyl)-piperidinyl]-4h-benzopyran-4-one]) was provided by Behringwerke AG, Marburg, Germany, to the Developmental Therapeutics Program, National Cancer Institute. Flavopiridol was dissolved in dimethyl sulfoxide as 50 mmol/L stock solutions. SUDHL4 and SUDHL6 cell lines (Southwestern University Diffuse Histiocytic Lymphoma), histologically transformed follicular lymphoma cell lines, were provided by Dr M. Stetler-Stevenson, Laboratory of Pathology, NCI. MOLT4 Jurkat (T-cell, acute lymphoblastic leukemia [ALL]); HL60, K562 (myeloid); and PC3 prostate carcinoma cell lines were obtained from (ATCC, Rockville, MD). The cells (doubling time, ≈24 hr) were maintained in RPMI 1640 containing 10% (vol/vol) heat-inactivated fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 2 mmol/L glutamine (complete medium) in an atmosphere containing 5% (vol/vol) CO2. All chemical reagents were from Sigma, St Louis, MO, unless noted otherwise. Morphologic assessment of the effect of flavopiridol was achieved by cytospin of 100 μL of cell suspension, stained with Leukostat Kit (Fisher Scientific, Pittsburgh, PA) and viewed under oil immersion microscope.
Drug effect on cell growth.
Exponentially growing hematopoietic and prostate cells were treated as described in figure legends. In the case of PC3 cells, 2 × 103 cells per well were incubated with either drug or vehicle. After drug exposure, cells were incubated with 10% trichloroacetic acid and then stained with sulforhodamine B (SRB) solution, as described in detail elsewhere.13 In the case of hematopoietic cells, exponentially growing cells in suspension were harvested after drug treatment, and cell numbers were counted electronically (Coulter Electronics, Hialeah, FL) or by hemocytometer and viability assessed by Trypan Blue exclusion.
DNA gel fractionation.
Cells grown at a density of 1 × 106 cells/mL were exposed to flavopiridol for different concentrations and time periods as described in the figure legends. DNA was extracted, as described by Wang et al.14 Briefly, cells were washed once with cold phosphate-buffered saline (PBS) and lysed with 3 mL lysis buffer (5 mmol/L Tris-HCL [pH 7.5]; 20 mmol/L EDTA; 0.5% Triton X-100) for 15 minutes at 4°C. The chromatin of the cell lysates was isolated by centrifugation (20 minutes at 26,000g, 4°C). The supernatants containing small DNA fragments were extracted sequentially with phenol, phenol:chloroform (1:1), and chloroform. Nucleic acids were precipitated in 0.5 mol/L NaCl, 90% ethanol at -20°C overnight. RNA was then digested by bovine RNAase A (60 μg/mL). After sequential reextraction and reprecipitation, DNA was dissolved in 10 mmol/L Tris-HCL (pH 7.5), 1 mmol/L EDTA, 0.5% sodium dodecyl sulfate (SDS) before electrophoresis on 1.6% agarose gel.
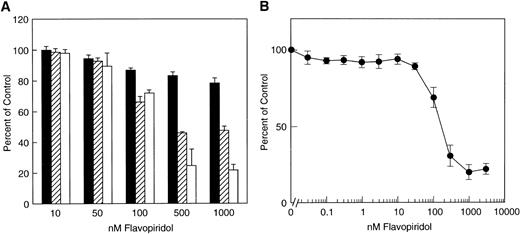
Effect of flavopiridol on cell growth. (A) SUDHL4 cell line. Cultures were exposed to various concentrations of flavopiridol and at the indicated points counted by electronic counter at 24 hours (▪), 48 hours (□), and 72 hours (□). The data are shown as percentage of untreated control. Total untreated control cell number/culture at 24 hours was 104,040 ± 3,750; 48 hours, 183,880 ± 6,687; and 72 hours, 371,670 ± 11,956. (B) PC3 cell line. Exponentially growing cells were incubated with flavopiridol at the indicated concentrations for 48 hours, and cell growth was assessed by the colorimetric SRB assay, as described in Materials and Methods. The data are the mean of four determinations ± standard deviation (SD) and are representative of three experiments for each cell line.
Effect of flavopiridol on cell growth. (A) SUDHL4 cell line. Cultures were exposed to various concentrations of flavopiridol and at the indicated points counted by electronic counter at 24 hours (▪), 48 hours (□), and 72 hours (□). The data are shown as percentage of untreated control. Total untreated control cell number/culture at 24 hours was 104,040 ± 3,750; 48 hours, 183,880 ± 6,687; and 72 hours, 371,670 ± 11,956. (B) PC3 cell line. Exponentially growing cells were incubated with flavopiridol at the indicated concentrations for 48 hours, and cell growth was assessed by the colorimetric SRB assay, as described in Materials and Methods. The data are the mean of four determinations ± standard deviation (SD) and are representative of three experiments for each cell line.
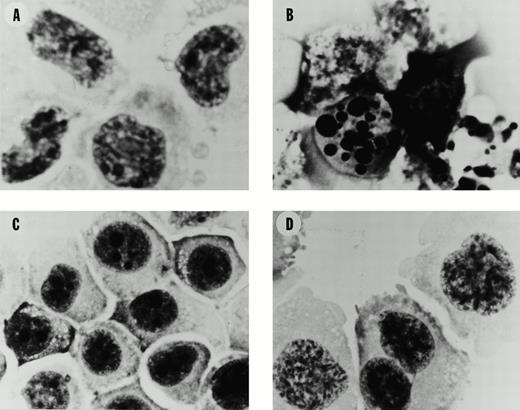
Morphology of SUDHL4 and PC-3 cells after exposure to flavopiridol. (A) Untreated control SUDHL4s. (B) SUDHL4s after 12 hours exposure to flavopiridol at 1,000 nmol/L. (C) Untreated control PC-3s. (D) PC-3s after 12 hours exposure to flavopiridol at 1,000 nmol/L. Photography was at 1,000 ×, oil immersion microscope, after cytospin preparation as described in Materials and Methods.
Morphology of SUDHL4 and PC-3 cells after exposure to flavopiridol. (A) Untreated control SUDHL4s. (B) SUDHL4s after 12 hours exposure to flavopiridol at 1,000 nmol/L. (C) Untreated control PC-3s. (D) PC-3s after 12 hours exposure to flavopiridol at 1,000 nmol/L. Photography was at 1,000 ×, oil immersion microscope, after cytospin preparation as described in Materials and Methods.
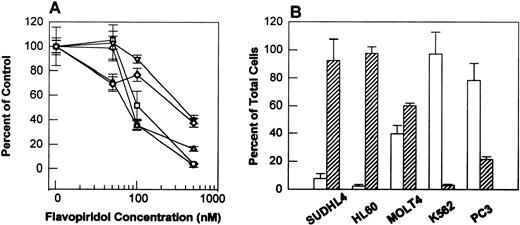
Effect of flavopiridol on growth of SUDHL4, HL60, MOLT4, K562, and PC3 cells. Cells (2 × 104 cells/mL) were plated into each well of a six-well plate. After 24 hours, cells were treated in triplicate either with vehicle or with 50, 100, and 500 nmol/L of flavopiridol for 48 hours. Cell growth was determined by counting live (Trypan Blue excluded) and dead (Trypan Blue stained) cells on a hemacytometer. (A) Represents growth inhibition as percent of control for SUDHL4 (○), HL60 (□), MOLT4 (▵), K562 (▿), and PC3 (◊) cells after 48 hours drug exposure. (B) Presents the results as percent of total live cells (open bar) and dead cells (hatched bar) after 500 nmol/L flavopiridol exposure for 48 hours. The experiments represent the mean of three determinations ± SD.
Effect of flavopiridol on growth of SUDHL4, HL60, MOLT4, K562, and PC3 cells. Cells (2 × 104 cells/mL) were plated into each well of a six-well plate. After 24 hours, cells were treated in triplicate either with vehicle or with 50, 100, and 500 nmol/L of flavopiridol for 48 hours. Cell growth was determined by counting live (Trypan Blue excluded) and dead (Trypan Blue stained) cells on a hemacytometer. (A) Represents growth inhibition as percent of control for SUDHL4 (○), HL60 (□), MOLT4 (▵), K562 (▿), and PC3 (◊) cells after 48 hours drug exposure. (B) Presents the results as percent of total live cells (open bar) and dead cells (hatched bar) after 500 nmol/L flavopiridol exposure for 48 hours. The experiments represent the mean of three determinations ± SD.
DNA alkaline filter elution.
Quantitation of DNA fragmentation was performed by a modification of a previously published procedure.15 Briefly, cells were labeled with [14C]thymidine (0.02 μCi/mL; specific activity, 59 mCi/mmol, from Amersham Corp, Arlington Heights, IL) for one doubling time, followed by incubation in isotope-free medium to allow chase of radioactivity into high molecular weight DNA. After exposure to flavopiridol, cells were loaded onto a filter (Poretics Corporation, Livermore, CA). The culture medium and wash fractions were collected as “the extracellular fraction”. Lysis solution (0.2% Na Sarkosyl, 2 mol/L NaCl, 0.04 mol/L EDTA, pH 10) was added, followed by another wash with 0.02 mol/L EDTA. This was collected as the “lysis fraction”, containing the protein-free DNA double-strand breaks occurring as a result of induction of apoptosis. The filter was placed into a scintillation bottle containing 0.4 mL 1 N HCL . The filter was placed into a 65°C oven for 60 minutes, followed by addition of 0.4 N NaOH for 60 minutes to solubilize the filter-bound label. Radioactivity was then measured in each fraction by liquid scintillation spectrometry, and the data plotted as the fraction of DNA eluting from the filter.
Flavopiridol effects on topoisomerase activity.
To assess the capacity of flavopiridol to activate topoisomerase, a DNA fragment corresponding to the 5′-end–labeled sense strand of the c-myc proto-oncogene was used.16 The DNA was reacted with purified topoisomerase I and II in the presence of the indicated concentrations of flavopiridol. Reactions were incubated at 30°C for 30 minutes and stopped by adding 0.5% SDS followed by proteinase K digestion. DNA fragments were separated on a 7% denaturing polyacrylamide gel and visualized by Phosphorimager.
DNA content and flow cytometry analysis.
Exponentially growing cells (4 × 106/20 mL) were treated with flavopiridol as indicated in the figure legends. At each time point, cells were washed twice with PBS, fixed in suspension in 70% ethanol and stored at -20°C. For DNA content, cells were washed twice with PBS and resuspended in 2 mL of PBS. A total of 2 mL of phosphate-citric acid buffer (192 mL of 0.2 mol/L Na2HPO8 and 8 mL of 0.1 mol/L citric acid, pH 7.8) was added to each sample, and cells were incubated for 15 minutes at room temperature. Cells were washed with PBS, incubated with 50 μg/mL of propidium Iidide (Calbiochem, San Diego, CA) and 250 μg of DNase-free Rnase A (Sigma) in the dark for 30 minutes. DNA content was measured using a FACScan (Beckton Dickinson, San Jose, CA) flow cytometer. Data acquisition and analysis was performed using Modfit software (Becton Dickinson).
Western blot analysis.
Exponentially growing cells (5 × 106/25 mL) were treated with flavopiridol as described in the figure legends. At indicated times, cells were washed with PBS and lysed in 500 μL of lysis buffer (50 mmol/L hepes, 150 mmol/L NaCl, 1% Triton X-100, 10% glycerol, 5 mmol/L EGTA, 15 mmol/L MgCl2, 20 mmol/L NaF, 50 mmol/L β-glycerophosphate, 2 mmol/L phenylmethylsulfonyl fluoride [PMSF], 1 mmol/L Na3VO4, 10 μg/mL leupeptin, and 10 μg/mL aprotinin). Cell lysates were centrifuged at 14,000 rpm for 15 minutes at 4°C. Protein content of clarified supernatants was determined by Bradford protein assay. Cell lysates containing equal amounts of protein (50 μg) were resolved on 12% mini gels (Novex, San Diego, CA).
After SDS-polyacrylamide gel electrophoresis (PAGE), proteins were transferred onto immobilon polyvinyldiene difluoride (PVDF) membranes (Millipore, Bedford, MA) at 500 mA for 2.5 hours at 4°C using CAPS (3-[cyclohexylamino]-1-propanesulfonic acid) buffer (10 mmol/L CAPS, pH 11, 10% MeOH). Residual binding sites on the membrane were blocked by incubation in TTS (20 mmol/L Tris, pH 7.4, 0.9% NaCl, and 0.05% Tween 20) containing 3% bovine serum albumin (BSA) overnight at 4°C or for 1 hour at room temperature. Blots were probed with either anti-bcl2 monoclonal antibody (MoAb) (Dako, Inc, Carpinteria, CA) anti-p53 MoAb (Calbiochem) or with rabbit polyclonal bax (Santa Cruz Biotech, Santa Cruz, CA). Immune complexes were detected using goat antirabbit or antimouse horseradish proxidase conjugated secondary antibodies (Amersham Corp) and were visualized using enhanced chemilumminescence reagents (Amersham Corp).
CDK1 activity.
The activity of CDK1 was assessed, as described previously3using CDKs1 substrate peptide.
RESULTS
Antiproliferative and morphologic effects of flavopiridol.
Flavopiridol inhibits the growth of SUDHL4 lymphoma cells with an IC50 of approximately 120 nmol/L at 48 or 72 hours (Fig 1A). For comparison, the PC3 prostate carcinoma epithelial cell line displays comparable inhibition of growth, with IC50 of 203 nmol/L over 48 hours (Fig 1B). A striking finding, however, is the effect of the drug on the morphology of the different cell types. Untreated SUDHL4 cells display homogeneous chromatin with prominent nucleoli, similar to that expected for lymphoid blasts (Fig 2A). After a 12-hour exposure to flavopiridol at 1,000 nmol/L, there are typical “apoptotic” changes, including prominent chromatin condensation, loss of normal nuclear architecture (Fig 2B), and accumulation of nuclear debris. In contrast, exposure of PC3 prostate carcinoma cells to flavopiridol at the same concentrations and durations only causes loss of nucleoli (compare Fig 2C with 2D), with no evidence of nuclear debris or apoptotic body formation. No morphologic changes compatible with apoptosis were observed with several other epithelial lines examined, such as MDA 468 breast carcinoma cells and DU-145 prostate cells, despite similar IC50s for growth inhibition over 48 hours in these cell lines (data not shown).
To expand the variety of cell types examined, we directly contrasted the behavior of SUDHL4 with a variety of other cell types. Figure 3A shows that after 48 hours, PC3, HL60, SUDHL4, K562, and MOLT4 cells have comparable IC50s of 80 to 300 nmol/L. However, SUDHL4 cultures have ≈ 95% dead cells by Trypan Blue exclusion at 500 nmol/L (Fig 3B), where growth is inhibited by 90%. Similar behavior to this is shown by HL60 and MOLT4 cells, with 90% and 60% Trypan Blue positive, respectively (Fig 3C and D). In contrast, PC3 cells, while growth inhibited by 80% at 500 nmol/L, show little Trypan Blue staining (Fig 3F). Interestingly, K562 chronic myelogenous leukemia cells are more similar to PC3 cells in that they are efficiently inhibited by flavopiridol, but show little tendency toward cell death. Qualitatively, similar conclusions were apparent after 24 hours, where at 500 nmol/L, SUDHL4, HL60, and MOLT4 showed 80%, 90%, and 40% dead cells, respectively (data not shown).
DNA fragmentation after flavopiridol treatment.
The appearance of morphologic changes consistent with induction of apoptosis in SUDHL4 cells suggested that DNA fragmentation might be readily evident in these cells. Figure 4A shows that exposure to as little as 100 nmol/L flavopiridol (approximately the IC50; compare with Fig 1) for 14 hours, induced DNA fragmentation with a typical “DNA ladder” in lymphoid neoplastic cells derived from T- (MOLT4 and Jurkat) or B-cell lineage (SUDHL4 and 6). A similar effect was observed in another B-cell line, Wilson (data not shown).
DNA fragmentation after exposure to flavopiridol. (A) Exponentially growing SUDHL4, SUDHL6, MOLT4, and Jurkat cells were exposed to the indicated concentrations of flavopiridol for 14 hours and genomic DNA extracted as described in Materials and Methods before electrophoresis in a 1.6% agarose gel. (B) SUDHL4 cells were exposed to the following concentrations of flavopiridol for the indicated periods after prelabelling DNA with [14C]-thymidine. The fraction of DNA eluting from filters is indicated. Untreated control (▵); 100 nmol/L (▪); 300 nmol/L (▴); 500 nmol/L (□); 1,000 nmol/L (•). (C) PC-3 cells were exposed to either untreated control (○); 1,000 nmol/L (•), or 3,000 nmol/L (▿) flavopiridol-containing medium for 24 or 48 hours. Each symbol represents the mean of three independent experiments.
DNA fragmentation after exposure to flavopiridol. (A) Exponentially growing SUDHL4, SUDHL6, MOLT4, and Jurkat cells were exposed to the indicated concentrations of flavopiridol for 14 hours and genomic DNA extracted as described in Materials and Methods before electrophoresis in a 1.6% agarose gel. (B) SUDHL4 cells were exposed to the following concentrations of flavopiridol for the indicated periods after prelabelling DNA with [14C]-thymidine. The fraction of DNA eluting from filters is indicated. Untreated control (▵); 100 nmol/L (▪); 300 nmol/L (▴); 500 nmol/L (□); 1,000 nmol/L (•). (C) PC-3 cells were exposed to either untreated control (○); 1,000 nmol/L (•), or 3,000 nmol/L (▿) flavopiridol-containing medium for 24 or 48 hours. Each symbol represents the mean of three independent experiments.
To quantitate the degree of DNA damage more rigorously in SUDHL4 and PC3 cells, we used the filter elution assay described in Materials and Methods. After exposure to 100 nmol/L flavopiridol for 6 hours, there is clearly evidence of DNA fragmentation. By 12 hours of exposure to 300 nmol/L, there is virtually complete fragmentation of DNA (Fig 4B). In contrast to the behavior of SUDHL4 cells, PC3 prostate carcinoma cells are considerably more resistant to induction of DNA fragmentation: exposure to 3,000 nmol/L of drug for 24 or 48 hours causes only 10% or 35% of the DNA fragmentation, respectively (Fig4C). These results, therefore, are concordant with the idea that the SUDHL4 lymphoma cell line, similar to most of the other hematopoietic cell lines studied here, is very sensitive to induction of apoptosis with DNA fragmentation after exposure to flavopiridol. These data further indicate that PC3 cells are relatively resistant to this effect despite a similar IC50 for inhibition of cell growth in short-term assays with continuous exposure to drug.
Activity of topoisomerase I and II after flavopiridol.
Previous studies17 18 have suggested that potential protein kinase antagonists at relatively high concentration can modulate topoisomerase activity. The readily apparent induction of apoptosis by flavopiridol raises the concern that flavopiridol might perturb the activity of topoisomerases. Flavopiridol at concentrations 100-fold greater than those associated with apoptosis in cells does not stimulate topoisomerase I or II-induced cleavage of DNA, conditions where, for example, VP-16 clearly causes topoisomerase II-induced DNA cleavage of a defined target DNA sequence (data not shown).
Modulation of bcl2, p53, and cell cycle.
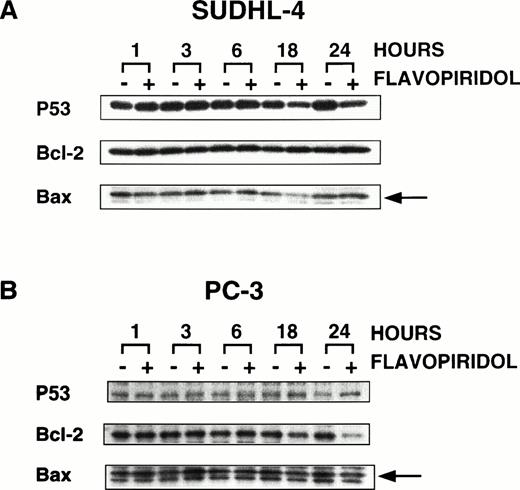
To begin to address the mechanisms by which flavopiridol causes apoptosis selectively, we examined the effect of the drug on bcl2, bax, and p53 protein levels. Figure 5A shows essentially no change in bcl2 or bax proteins in SUDHL4 cells after exposure to flavopiridol, and by 18 to 24 hours, there is a slight decrease in p53. In PC3 cells, 24 hours after exposure to flavopiridol, there is a decrease in bcl2 levels (Fig 5B), without change of p53. These changes accompanied or preceded arrest of PC3 cells with a decrease in S and increase in G2 phase fractions (Fig 6A), with decrease in CKD1 activity to 58% of untreated controls (data not shown), as has been described in other cell types previously.1,4,5 In contrast, in SUDHL4 cells, CDK1 activity did not decrease, but at 3 hours after addition of 500 nmol/L flavopiridol, there was increased CDK1 activity (Fig 6B), comparable to the threefold increases in CDK1, CDK2, and CDK4 immunoprecipitated activity induced by flavopiridol at short times after drug addition in breast cancer cells and attributable to decreased CDK tyrosine phosphorylation.4 5 Also in contrast to PC3 cells, at 6, 18, and 24 hours in SUDHL4 cells, CDK1 activity does not decrease, despite florid induction of apoptosis of SUDHL4 after 8 hours expsoure to 300 nmol/L (Fig 6C), manifest here as a hypodiploid DNA content.
Effect of flavopiridol on p53, bcl2, and bax proteins. Exponentially growing SUDHL4 cells (A) and PC3 cells (B) were treated with 500 nmol/L of flavopiridol for 1, 3, 6, 18, and 24 hours. Cells were washed with PBS, lysed, and Western blot analysis performed as described in Materials and Methods. Proteins were visualized by autoradiography using ECL. The arrows indicate the position of the bax protein.
Effect of flavopiridol on p53, bcl2, and bax proteins. Exponentially growing SUDHL4 cells (A) and PC3 cells (B) were treated with 500 nmol/L of flavopiridol for 1, 3, 6, 18, and 24 hours. Cells were washed with PBS, lysed, and Western blot analysis performed as described in Materials and Methods. Proteins were visualized by autoradiography using ECL. The arrows indicate the position of the bax protein.
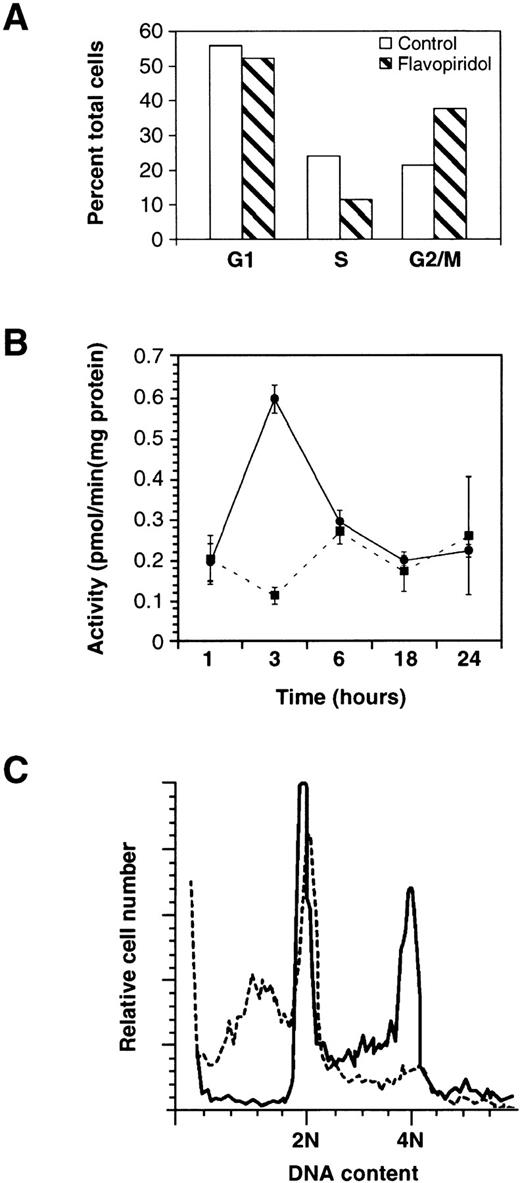
Cell cycle distribution and effect on CDK1 activity after exposure to flavopiridol. In (A), the fraction of PC3 cells in G1, S, and G2/M is indicated after 12 hours exposure to 300 nmol/L flavopiridol. The experiment shown is the mean of duplicate samples with a range of < 5% and is representative of two experiments. In (B), SUDHL4 cells were exposed to 500 nmol/L flavopiridol (•) or vehicle (▪) for the indicated time periods and CDK1 activity assayed. The experiment shown is representative of two experiments, with each kinase determination the average ± SD of three determinations. In (C), SUDHL4 cells (A) were exposed to vehicle (solid line) or 300 nmol/L flavopiridol (dashed line) for 8 hours and cell cycle distribution assayed by flow cytometry.
Cell cycle distribution and effect on CDK1 activity after exposure to flavopiridol. In (A), the fraction of PC3 cells in G1, S, and G2/M is indicated after 12 hours exposure to 300 nmol/L flavopiridol. The experiment shown is the mean of duplicate samples with a range of < 5% and is representative of two experiments. In (B), SUDHL4 cells were exposed to 500 nmol/L flavopiridol (•) or vehicle (▪) for the indicated time periods and CDK1 activity assayed. The experiment shown is representative of two experiments, with each kinase determination the average ± SD of three determinations. In (C), SUDHL4 cells (A) were exposed to vehicle (solid line) or 300 nmol/L flavopiridol (dashed line) for 8 hours and cell cycle distribution assayed by flow cytometry.
DISCUSSION
In this report, we have shown that the antiproliferative effect of flavopiridol, a known CDK inhibitor, can be temporally linked to induction of apoptosis in several hematopoietic cell lines. These apoptotic events were documented by three different independent methods and were more easily appreciated in the lymphoid cell lines, in contrast to PC3 cells and to all epithelial cell lines studied to date (data not shown), as well as, interestingly, the K562 myeloid cell line. The induction of apoptosis is observed as early as 3 to 6 hours after exposure to 100 nmol/L of flavopiridol in the SUDHL4 cell line. This may be compared with a delayed (48 hours) and less potent effect of the drug in the PC3 cell line in causing DNA fragmentation, despite similar IC50 for cell growth inhibition. PC3 and K562 cells never displayed typical apoptotic morphology after drug addition. In contrast to VP16-213, flavopiridol did not activate cleavable complex formation by topoisomerase II nor did it activate cleavable complex activity by topoisomerase I at concentrations almost 1,000 times higher than the concentration inducing apoptosis in living cells. Clear evidence of alteration of the bcl2/bax protein ratio in SUDHL4 cells was not obtained, or did p53 increase in SUDHL4 and PC3 cells.
Apoptosis (or programmed cell death) is a physiologic event in response to multiple stimuli including growth factor withdrawal, radiation therapy, and chemotherapeutic agents.19-22 Apoptotic cells undergo shrinkage, chromatin condensation, and plasma membrane blebbing with the activation of proteases and endonucleases. Their final phenotype is characterized by plasma membrane-bound “apoptotic bodies”.23 Several mechanisms apparently regulate this process, such as induction of a p53-dependent–pathway after DNA-damaging agents, modulation by the bcl2 family of proteins, and activation of effectors including the interleukin-converting enzyme (ICE) family of proteases and endonucleases.24-27
Interestingly, flavopiridol action appeared not to correlate with p53 status: while PC3 and K562, both p53 null, respectively,28,29 are relatively resistant to flavopiridol-induced apoptosis, both HL60 and Jurkat cells, also p53 null,30 31 are very sensitive to flavopiridol-induced apoptosis. Also, in neither SUDHL4 nor PC3 cells is p53 induced after exposure to flavopiridol.
CDKs have been implicated as modulators of apoptosis in at least two ways. Inappropriate activation of CDKs has been correlated with induction of apoptosis by cytotoxic lymphocytes,32 after exposure to staurosporine33,34 or the staurosporine congener, UCN-01.14 We have also observed transient cyclin B/cdc2 kinase activation in human leukemia HL60 treated with topoisomerase inhibitors and DNA alkylating agents.35Elevated expression of CDK dominant negative mutants can prevent cell death in Hela cells.36 In other cell types, such as proliferating PC12 cells, flavopiridol and olomucine (another CDK inhibitor) induce apoptosis, while in differentiated PC12 cells, flavopiridol protects from apoptosis after growth factor withdrawal.37 These results have led Meijer38to conclude that the influence of CDK activity on the apoptotic program may be cell-context or cell type-dependent. However, the phase of the cell cycle may influence the susceptibility to induction of apoptosis.
It is intriguing that despite similar IC50s for growth inhibition of the lymphoma and prostate cell lines studied here, there is a clear difference between the epithelial and hematopoietic cells (except K562) in the onset, concentration and apparent magnitude of the apoptotic phenomenon. Further experiments must define whether CDK inhibition can be related to induction of apoptosis. Consistent evidence of CDK inhibition was obtained only in the PC3 cells. SUDHL4 cells went into apoptosis so completely (Fig 6C) that evidence of cell cycle arrest was not observed in these cells. Of great interest, CDK1 activity was maintained even after florid induction of apoptosis. The meaning of maintained CDK activity is uncertain. One interpretation is that SUDHL4 and other “apoptosis-prone” cell types do not become growth arrested in the presence of stimuli that should cause cell cycle arrest, and the ocurrence of apoptosis reflects the continued influence of their growth-stimulating influences in the presence of the drug. Further experiments must focus on the nature and response to drug addition of putative CDK substrates in apoptosis-prone and apoptosis-resistance cell types. An important caution in this regard is that flavopiridol at concentrations > 5 μmol/L can also inhibit several other kinases including protein kinase C, protein kinase A, and epidermal growth factor receptor tyrosine kinase.39 Thus, it is possible that the action of flavopiridol on other targets or in addition to the effects of flavopiridol on CDKs contributes to flavopiridol-induced apoptosis. Other important influences may have an impact on susceptibility to apoptosis including altered generation of reactive oxygen or nitric oxide intermediates or propensity for mitrochondrial damage or protease activation. Further experiments must clarify which pathways are preferentially activated in hematopoietic cells34,40,41 and account for the observation that the combination of flavopiridol with “conventional” hemotherapeutic agents has been reported to enhance apoptosis.42 Finally, it is also possible that the uptake or metabolism of flavopiridol is very different in lymphoma cells in comparison to prostate carcinoma cells, and such differential flavopiridol metabolism may also be an explanation for the propensity to undergo apoptosis in hematopoietic cells.
While this report was in preparation, Bible and Kaufmann43presented evidence that high concentrations of flavopiridol (>500 nmol/L) for ≥ 24 hours was associated with cytotoxicity in several cell types. These investigators did note, however, that HL60 promyelocytic leukemia cells were exquisitely sensitive to flavopiridol-induced apoptosis, concordant with results presented here.
In summary, flavopiridol readily induces programmed cell death in most hematopoietic cell lines thus far examined. Irrespective of the mechanism by which this effect occurs, these results call for early consideration of hematopoietic neoplasms as targets for Phase II trials with flavopiridol.
ACKNOWLEDGMENT
We would like to acknowledge Carla Hemp for her valuable secretarial assistance.
Address reprint requests to Adrian M. Senderowicz, MD, DTP Clinical Trials Unit, Medicine Branch, National Cancer Institute, Bldg 10, Rm 6N113, NIH, Bethesda, MD 20892.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. DNA fragmentation after exposure to flavopiridol. (A) Exponentially growing SUDHL4, SUDHL6, MOLT4, and Jurkat cells were exposed to the indicated concentrations of flavopiridol for 14 hours and genomic DNA extracted as described in Materials and Methods before electrophoresis in a 1.6% agarose gel. (B) SUDHL4 cells were exposed to the following concentrations of flavopiridol for the indicated periods after prelabelling DNA with [14C]-thymidine. The fraction of DNA eluting from filters is indicated. Untreated control (▵); 100 nmol/L (▪); 300 nmol/L (▴); 500 nmol/L (□); 1,000 nmol/L (•). (C) PC-3 cells were exposed to either untreated control (○); 1,000 nmol/L (•), or 3,000 nmol/L (▿) flavopiridol-containing medium for 24 or 48 hours. Each symbol represents the mean of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.458/3/m_blod4020804.jpeg?Expires=1769526151&Signature=EMhxQCBL~UBhxrL0MJbNGLV2-fI~KVd7c3frA0qdBjMRVcgYb9pv8APc8Ufp0bWAYa9eBk7nhv6A7EP0M7FXddD7hQ~NUETbipZlsUGWmvAyji4gNPu~9EHZQa6zgtzstli88R6TdPiDXWVveLmYvoYAV3fVyXwLZLEU3kDf-EN-U-0XS8yqP5ZF8ktwrJkTQbCDSfMnzr5bq5akh8EYhErPc8GRsJTttmPjoiV2MV7WCTkLb~yeucPH4LBdxHVMtK0MIxP-VrYnhU5Ai-45kVMbEluxdb4xWa5~vbcKObdUJKqTphd~Q0j0AXJgAZ3b03wqztracHOd71UgFfMPLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal