Abstract
Parathyroid hormone-related protein (PTHrP) is considered to be one of the main causes of hypercalcemia associated with adult T-cell leukemia (ATL). To clarify the role of PTHrP and bone remodeling in the development of hypercalcemia in ATL, we examined the SCID mouse model of ATL that has previously been shown to mimic the disease in humans. Using this model, we found clear elevations in serum levels of calcium and C-terminal PTHrP (C-PTHrP). PTHrP mRNA was highly expressed in ATL cells proliferating in vivo. After the development of hypercalcemia, ATL mice were killed and bone histomorphometric analysis was performed. Bone volume was clearly decreased in the ATL mice. In comparison to control SCID mice, bone formation indices were very low in the ATL mice. Surprisingly, no significant difference was detected between the ATL mice and the control SCID mice in eroded surface/bone surface (ES/BS), a parameter of bone resorption. To our knowledge, the model presented here is the first animal model of ATL with humoral hypercalcemia. This is in contrast to previously reported, well-characterized animal models of human solid tumors associated with humoral hypercalcemia of malignancy (HHM). Furthermore, this model not only provides us with the opportunity to study the mechanisms underlying development of elevated calcium levels in ATL, but also allows us to test new therapeutic agents designed to treat hypercalcemia.
ADULT T-CELL LEUKEMIA (ATL) is caused by infection with human T-cell leukemia virus type-I (HTLV-I) and is associated with characteristic clinical features.1-4Hypercalcemia is frequently associated with ATL and contributes significantly to mortality in this disease.5 Parathyroid hormone-related protein (PTHrP) is considered to be one of the primary causes of hypercalcemia associated with ATL.6,7 PTHrP was originally identified as a factor produced by tumors with humoral hypercalcemia of malignancy (HHM)8-10 and is overexpressed by a variety of tumors.11 PTHrP is expressed in normal tissues12 and plays a wide range of physiological roles. ATL cells have been shown to produce PTHrP. HTLV-I Tax transactivates the PTHrP gene promoter,7 and interleukin-2 (IL-2) induces the production of PTHrP in ATL cells.13,14 However, we have found previously that IL-2 mRNA is not detected in peripheral blood leukemic cells from ATL patients.15 In addition, HTLV-I viral expression is usually undetectable or at very low levels in fresh ATL cells.16 17 Therefore, the mechanism by which the PTHrP gene is overexpressed in ATL cells remains unclear. Furthermore, due to the absence of an appropriate animal model, the role of PTHrP in the development of hypercalcemia in ATL in vivo has not been well characterized.
We have previously developed an in vivo proliferative model of ATL using severe combined immunodeficient (SCID) mice to study the mechanism of neoplastic cell growth of ATL in vivo.18-20 We found that the microenvironment provided by SCID mice was more suitable for leukemic cell growth than the conditions provided by in vitro cell culture. We have also recently developed a serial transplantation model of ATL cells in SCID mice, which closely resembles the disease in humans.21 Use of this model to study the function and regulation of PTHrP should provide additional information concerning the development of hypercalcemia in ATL.
In this study, we show that the serial transplantation model of ATL presents with hypercalcemia associated with marked elevations in serum C-PTHrP levels. We further characterize changes in bone metabolism seen in this model.
MATERIALS AND METHODS
Mice.
Immune-deficient SCID (CB17scid/scid) mice were obtained from Nihon Clea Inc (Tokyo, Japan). The mice were bred and maintained under specific pathogen-free conditions in the animal facility of the Institute for Virus Research, Kyoto University (Kyoto, Japan). Age-matched SCID mice were used as controls in all experiments.
Injection of ATL cells into SCID mice.
ATL cells were serially transplanted into SCID mice as previously described.21 In brief, lymph node cells (LNC) from a lymphoma-type ATL patient were injected intraperitoneally into SCID mice. Tumor cells were recovered from the mice engrafted with ATL cells and were serially transplanted into SCID mice.
Measurement of serum levels of calcium and C-terminal PTHrP (C-PTHrP).
Calcium and C-PTHrP levels were measured using blood obtained from the hearts of mice that had been killed. Serum calcium levels were determined by an o-cresol-phthalein complexone method using a Calcium-C-Test Wako kit (Wako, Osaka, Japan) according to the manufacturer's instruction. Serum C-PTHrP levels were determined by radioimmunoassay specific for the C-terminal region (109-141) of human PTHrP using a C-PTHrP RIA kit (Daiichi Isotope Co Ltd, Tokyo, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR).
Single-strand cDNA was synthesized in a volume of 20 μL, as previously described.20 The cDNA preparation was then diluted to 100 μL. cDNA (2.5 μL) was amplified in a volume of 25 μL in the presence of 800 nmol/L 5′ and 3′ primers, 200 μmol/L dNTPs, 1 U Taq polymerase (TAKARA, Otsu, Japan), and 2.5 mmol/L MgCl2. The PCR primers specific for PTHrP were used as previously described.22 PTHrP amplification was performed in a thermal cycler (Perkin Elmer Cetus, Norwalk, CT) for 38 cycles. The cycling conditions were 1 minute at 94°C for denaturation, 1 minute at 67°C for annealing, and 2 minutes at 72°C for elongation. β-Actin amplification was performed using the human-specific primers as previously described.20 The amplified products were then visualized after electrophoresis through 1.5% agarose gels by staining with ethidium bromide.
Bone histomorphometric analysis.
Mice were subcutaneously injected with calcein (10 mg/kg) and tetracyclin (20 mg/kg) at an interval of 7 days. Lumbar vertebrae obtained from mice that had been killed were fixed in 70% ethanol. Fixed bone specimens were stained with the Villanueva bone stain and then embedded in methyl methacrylate. Bone histomorphometric analysis using dry thin sections was performed with Osteoplan (Karl-Zeiss, Oberkochen, Germany). Statistical analysis was performed using the Student's t-test. The criterion of significance was P < .01. All values were reported as the mean ± standard deviation (SD). Wet thin sections were further stained with the Villanueva Goldner stain.
RESULTS
Mice engrafted with ATL cells.
As we have previously reported,21 mice engrafted with ATL cells developed disease closely resembling that of the original patient. Proliferating tumor cells obtained from the engrafted mice that were shown to be from the same clone as the original leukemic cells did not express either HTLV-I or IL-2 mRNA. No changes were noted in these characteristics during serial transplantations. In the present study, mice were analyzed at 12 to 14 passages. All the mice exhibited lethargy, ruffled fur, and a hunched posture and were found to have tumors within 3 weeks after inoculation of tumor cells.
Serum levels of calcium and C-PTHrP.
As shown in Table 1, both serum calcium levels and serum C-PTHrP levels were markedly elevated in SCID mice engrafted with ATL cells, as compared with the age-matched control SCID mice. Unfortunately, we were unable to measure serum vitamin D levels due to insufficient serum volume.
Serum Levels of Calcium and C-PTHrP in SCID Mice Engrafted With ATL Cells
| SCID-ATL (no.)-150 . | Calcium (mg/dL) . | C-PTHrP (pmol/μL) . |
|---|---|---|
| 1 | 13.3 | NE |
| 2 | 13.8 | 1,910 |
| 3 | 13.9 | 1,530 |
| 4 | 11.5 | 1,640 |
| 5 | 12 | 2,000 |
| 6 | 10.4 | 1,100 |
| 7 | 14.2 | 2,370 |
| Control (n = 8)-150 | 9.9 ± 0.29-151 | <80-152 |
| SCID-ATL (no.)-150 . | Calcium (mg/dL) . | C-PTHrP (pmol/μL) . |
|---|---|---|
| 1 | 13.3 | NE |
| 2 | 13.8 | 1,910 |
| 3 | 13.9 | 1,530 |
| 4 | 11.5 | 1,640 |
| 5 | 12 | 2,000 |
| 6 | 10.4 | 1,100 |
| 7 | 14.2 | 2,370 |
| Control (n = 8)-150 | 9.9 ± 0.29-151 | <80-152 |
Serum levels of calcium in SCID mice engrafted with ATL cells are markedly elevated according to serum C-PTHrP levels.
Abbreviation: NE, not examined.
No. indicates an individual mouse number, whereas n indicates a total number of mice examined.
This value was reported as the mean ± standard deviation.
This value represents an average of 8 age-matched control mice. Standard deviation cannot be calculated, because all the values were under the range of measurement.
Expression of PTHrP mRNA.
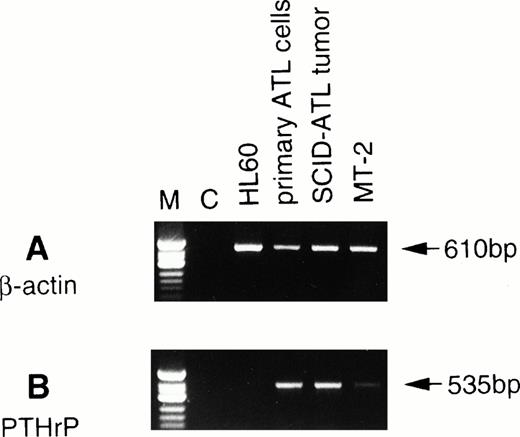
We next performed RT-PCR analysis to determine whether the tumor cells proliferating in vivo expressed PTHrP mRNA. RT-PCR analysis showed that the tumor cells expressed PTHrP mRNA at greater levels than MT-2, an HTLV-I–infected cell line,23 that has previously been shown to secrete PTHrP24 (Fig1). A strong signal for PTHrP mRNA was detected in both the original ATL cells as well as tumor cells obtained from the ATL mice.
Expression of PTHrP mRNA by RT-PCR analysis in the original leukemic cells and tumor cells from ATL mice. To detect β-actin and PTHrP mRNAs, PCR was performed for 25 and 38 cycles, respectively. C, negative control (RT reaction without RNA). ◊X174 DNA digested with HinfI was used as a molecular weight marker (M).
Expression of PTHrP mRNA by RT-PCR analysis in the original leukemic cells and tumor cells from ATL mice. To detect β-actin and PTHrP mRNAs, PCR was performed for 25 and 38 cycles, respectively. C, negative control (RT reaction without RNA). ◊X174 DNA digested with HinfI was used as a molecular weight marker (M).
Bone histomorphometric analysis.
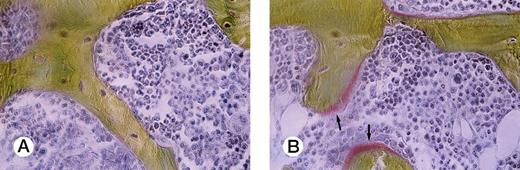
To study bone remodeling in this model, bone histomorphometric analysis was performed (Table 2). Bone volume/tissue volume (BV/TV) was clearly decreased in the mice engrafted with ATL cells (P = .0077). Indicators of bone formation, such as osteoid volume/bone volume (OV/BV), osteoid surface/bone surface (OS/BS), and bone formation rate/bone volume (BFR/BV), were markedly decreased in the ATL mice compared with those in the age-matched control SCID mice (P = .0011, .0023, and .0087, respectively). A marked decrease in osteoid surface in an ATL mouse compared with a control SCID mouse is shown in Fig 2. In contrast to these findings, no significant difference was detected between the ATL mice and the control mice in eroded surface/bone surface (ES/BS), a parameter of bone resorption.
Bone Histomorphometric Analysis of SCID Mice Engrafted With ATL Cells
| Parameter . | Control (n = 6)* . | SCID-ATL (n = 5)* . | Unit . |
|---|---|---|---|
| BV/TV | 14.994 ± 2.191† | 11.387 ± 0.921† | % |
| OV/BV | 4.382 ± 0.892† | 1.719 ± 0.975† | % |
| OS/BS | 15.376 ± 3.286† | 6.864 ± 3.423† | % |
| ES/BS | 8.539 ± 1.161 | 9.211 ± 1.216 | % |
| BFR/BV | 111.912 ± 20.923† | 68.58 ± 22.044† | mm3/cm3/yr |
| Parameter . | Control (n = 6)* . | SCID-ATL (n = 5)* . | Unit . |
|---|---|---|---|
| BV/TV | 14.994 ± 2.191† | 11.387 ± 0.921† | % |
| OV/BV | 4.382 ± 0.892† | 1.719 ± 0.975† | % |
| OS/BS | 15.376 ± 3.286† | 6.864 ± 3.423† | % |
| ES/BS | 8.539 ± 1.161 | 9.211 ± 1.216 | % |
| BFR/BV | 111.912 ± 20.923† | 68.58 ± 22.044† | mm3/cm3/yr |
Bone volume was decreased in SCID mice engrafted with ATL cells. OV/BV, OS/BS, and BFR/BV, indices of bone formation, were decreased in ATL mice. In contrast, ES/BS, an indicator of bone resorption, was not. All values were reported as the mean ± standard deviation.
Number of mice.
P < .01.
Villanueva Goldner staining of representative sections of lumbar vertebra from a control mouse and an ATL mouse. Arrows indicate osteoid surface, which is markedly decreased in an ATL mouse (A) compared with a control SCID mouse (B).
Villanueva Goldner staining of representative sections of lumbar vertebra from a control mouse and an ATL mouse. Arrows indicate osteoid surface, which is markedly decreased in an ATL mouse (A) compared with a control SCID mouse (B).
DISCUSSION
In the present study, we characterize a unique SCID mouse model of ATL that develops hypercalcemia, most likely through excess production of PTHrP by ATL cells proliferating in vivo. To our knowledge, this is the first animal model of ATL with hypercalcemia. Hypercalcemia is a frequent and fatal complication of ATL.5 In solid tumors, PTHrP has been shown to have a causative role in the development of hypercalcemia in studies using patient samples and animal models of HHM.25-27 PTHrP is also considered to be a causative factor of ATL-associated hypercalcemia,6,7 although other humoral factors such as IL-1 and transforming growth factor-β have been reported to be involved.28,29 Most of the studies on the function and regulation of PTHrP in ATL have been in vitro experiments, because no appropriate animal model has been available. Furthermore, most of the HTLV-I–infected cell lines used in these studies are derived from nonleukemic cell clones, which have properties quite different from those of leukemic cells.20 30Therefore, the precise mechanisms underlying the development of hypercalcemia in ATL as well as the function of PTHrP and the regulation of its expression in ATL remain to be determined. To help answer these questions, an appropriate animal model is needed.
The SCID mouse model of ATL-associated hypercalcemia presented in this study provides us with insights into the development of hypercalcemia in vivo. Mice engrafted with leukemic cells from an ATL patient who developed hypercalcemia in the terminal stage of the disease showed moderately high levels of serum C-PTHrP and calcium. In addition, PTHrP mRNA was detected both in the original leukemic cells and tumor cells proliferating in the ATL mice. Collectively, these data strongly suggest that PTHrP is one of the main causes of hypercalcemia in this model as well as in the original patient.
Bone histomorphometric analysis of this model showed that indices of bone formation were clearly lower in the ATL mice than in the age-matched control SCID mice. Surprisingly, ES/BS, an indicator of bone resorption, was normal in the ATL mice as compared with the control SCID mice. In addition, no significant difference was found in the number of osteoclasts in these two groups of mice (data not shown). A characteristic feature associated with PTHrP secretion found in patients with HHM is uncoupling, the process of excessive bone resorption with suppressed bone formation.11 31 To date, there has been no report on in vivo bone remodeling in ATL patients. In light of these data, another factor(s) produced by ATL cells may modulate the bone changes induced by PTHrP in this model.
Tax of HTLV-I and IL-2 have been reported to upregulate PTHrP gene expression.7,13,14 Tax transactivates the PTHrP gene through interaction with cellular transcription factors such as AP1, AP-2, Ets1, and Sp1.32-34 However, neither HTLV-I nor IL-2 mRNA was detectable in the tumor cells proliferating in vivo.20,21 The interaction between leukemic cells and the microenvironment provided by SCID mice may play an important role in the overexpression of the PTHrP gene in ATL cells proliferating in vivo. Wake et al35 have reported that PTHrP gene expression is induced via the leukocyte function-associated antigen-1 (LFA-1)/intracellular adhesion molecule-1 (ICAM-1) pathway in vitro. We have found that LFA-1 molecules are expressed on the tumor cells proliferating in the ATL mice (data not shown). However, as we reported previously, the LFA-1/ICAM-1 pathway is not functional in vivo as adhesion molecules, although LFA-1 molecules are expressed on fresh leukemic cells from ATL patients.36 It would be of interest to identify the factors or mechanisms that induce PTHrP gene expression in vivo using this model. Recently, it has been shown that PTHrP gene expression is affected by p53.37,38 Point mutation of the p53 gene has been found in a considerable proportion of ATL cases.39 40 Such a genetic change might be responsible for enhanced expression of the PTHrP gene, leading to the development of hypercalcemia in ATL.
It is still controversial whether PTHrP has any effect on ATL cell growth. McCauley et al41 reported that MT-2 cells have receptors for PTHrP and that PTHrP inhibits MT-2 cell growth. Inoue et al42 reported that 22-oxacalcitriol suppresses both cell proliferation and PTHrP gene expression in MT-2 cells. However, these data must be carefully interpreted, because MT-2 is a cell line transformed by the infection of HTLV-I in vitro. Our model will enable us to examine the effect of PTHrP on ATL cell growth in vivo.
Finally, hypercalcemia is one of the main causes of death in ATL. This model will be very useful not only for clarifying the in vivo role of PTHrP and bone remodeling in ATL, but also for developing novel therapeutic strategies to treat hypercalcemia and improve the prognosis of patients with this disease.
Supported in part by grants from the Ministry of Education, Science, Sport, and Culture, Japan, and Sankyo Foundation of Life Science.
Address reprint requests to Takashi Uchiyama, MD, Institute for Virus Research, Kyoto University, 53 Shogoin-Kawaracho, Sakyo-ku, Kyoto 606, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal