Abstract
Agonist antihuman gp130 transducer monoclonal antibodies (MoAbs) were used in SCID mice to grow myeloma cells whose survival and proliferation is dependent on gp130 transducer activation. The agonist anti-gp130 MoAbs neither bound to murine gp130 nor activated murine cells and, as a consequence, did not induce interleukin-6 (IL-6)–related toxicities in mice. They have a 2-week half-life in vivo when injected in the peritoneum. The agonist antibodies made possible the in vivo growth of exogenous IL-6–dependent human myeloma cells as well as that of freshly explanted myeloma cells from 1 patient with secondary plasma cell leukemia. Tumors occurred 4 to 10 weeks after myeloma cell graft and weighed 3 to 5 g. They grew as solid tumors in the peritoneal cavity and metastasized to the different peritoneal organs: liver, pancreas, spleen, and intestine. Tumoral cells were detected in blood and bone marrow of mice grafted with the XG-2 myeloma cells. Tumoral cells grown in SCID mice had kept the phenotypic characteristics of the original tumoral cells and their in vitro growth required the presence of IL-6 or agonist anti-gp130 MoAbs. Myeloma cells from 4 patients with medullary involvement persisted for more than 1 year as judged by detectable circulating human Ig. However, no tumors were detected, suggesting a long-term survival of human myeloma cells without major proliferation. These observations paralleled those made in in vitro cultures as well as the tumor growth pattern in these patients. This gp130 transducer-dependent SCID model of multiple myeloma should be useful to study various therapeutical approaches in multiple myeloma in vivo.
ANIMAL MODELS OF human tumors are essential to validate the development of novel therapies and especially that of immunotherapies. Tumoral samples taken from some patients with multiple myeloma (MM) may persist for several months in SCID mice, as judged by the presence of circulating human Ig without any significant tumor growth.1,2 A myeloma SCID model has been described using the ARH77 cell line,3 but this cell line is an Epstein-Barr virus (EBV)-infected B-cell line and not a myeloma cell line.4 Recently, several autonomously growing myeloma cells have been shown to grow close to femoral bones5 or in the bone marrow6 in SCID mice. One difficulty of getting a SCID model of MM might be due to the myeloma cell growth dependence on gp130 interleukin-6 (IL-6) transducer-activating cytokines.7,8Because murine gp130 cytokines do not activate human gp130 transducer,9 there is a need either to implant human stromal cells producing these cytokines or to inject human gp130 cytokines, in particular IL-6. However, these cytokines have a short half-life in vivo (20 to 60 minutes for IL-6)10,11 that necessitates a daily infusion. Another difficulty is that human IL-6 binds to murine IL-6R and activates murine gp130 transducer. As a consequence, human IL-6 may induce the toxicities reported for IL-6 in vivo, mainly an inflammatory and cachectic syndrome.12-14
In the present study, we have developed a SCID model of human MM, taking into account the myeloma cell growth dependence on gp130 cytokines and avoiding IL-6 toxicities. We used agonist monoclonal antibodies (MoAbs) to human gp130 transducer.15 16 These antibodies support the long-term growth of IL-6–dependent myeloma cell lines and the short-term proliferation of primary myeloma cells in vitro (unpublished results).
We show that these antibodies neither recognized nor activated murine gp130 IL-6 transducer and that they have a 2-week half-life in vivo. These antibodies made it possible to grow IL-6–dependent human cell lines as solid tumors in SCID mice. In addition, we have been able to grow primary myeloma cells from 1 patient with terminal MM and to further obtain a gp130-cytokine–dependent myeloma cell line.
PATIENTS, MATERIALS, AND METHODS
Patients.
Tumor samples were obtained from 5 patients with MM (median age, 57 years) after written informed consent was received. According to the Durie-Salmon classification, 4 patients were of stage IIIB and 1 of stage IIIA. Two patients had IgGλ MM, and 3 had IgGκ MM. One patient had a secondary plasma cell leukemia.
Reagents.
Recombinant IL-6 was provided by Dr A. Ytier (Ares Serono, Geneva, Swiss). The MI15 anti–syndecan-1 and the M91 anti–IL-6R MoAbs were obtained by our group.17 18 MoAbs against CD3, CD11a, CD18, CD19, CD28, CD80, CD38, CD40, CD45, CD54, CD56, CD58, MUC-1, HLA-DR, HLA-ABC antigens, fluorescein isothiocyanate (FITC)-conjugated (Fab′)2 fragments of goat antibodies to mouse IgG and phycoerythrin (PE)-conjugated (Fab′)2 fragments of goat antibodies to mouse IgG were purchased from Immunotech (Marseille, France). IgG1 and IgG2 control murine Igs (recognizing no human antigens) were purchased from Sigma (St Louis, MO), biotin-conjugated goat antimouse Ig and streptavidin-horseradish peroxidase from Amersham (Ulis, France), and Annexin-V-Fluos from Boehringer Mannheim (Meylan, France).
Agonist anti-gp130 antibodies.
Two different pairs of agonist anti-gp130 MoAb were used. B-S12 and B-P8 murine IgG1 MoAbs were cooperatively obtained by our group and the Diaclone Co (Besançon, France).15,19 B1 and I2 murine IgG1 MoAbs were produced by our group and recognized epitopes different from those recognized by B-S12 and B-P8 MoAbs.20 Both mixtures of MoAbs (used at 1/1 ratio) were proven to support long-term growth of IL-6–dependent myeloma cell lines.15 16 Most of the experiments were performed using the mixture of B-S12 and B-P8 MoAbs.
Isolation of primary myeloma cells.
Patients' myeloma cells were purified using the antimyeloma cell MI15 MoAb and Dynal magnetic beads (Dynal M450; Dynal, Oslo, Norway) coated with sheep antimouse IgG as previously described in detail.21 The MI15 MoAb recognizes syndecan-1, which is present only on myeloma cells in bone marrow samples.17 22Purified myeloma cells were resuspended in RPMI 1640 medium supplemented with 10% of fetal calf serum (FCS).
Cell lines.
Two human myeloma cell lines (HMCL) were used: XG-1 and XG-2. They had cytoplasmic Ig, expressed plasma cell antigens (CD38 and syndecan-1), and lacked the usual B-cell antigens (CD19 and CD20). Their growth was dependent on addition of exogenous IL-6. Detailed characteristics of these lines have been reported elsewhere.23 The IL-6–dependent B9 murine hybridoma cells were a generous gift of Dr L. Aarden (CLB, Amsterdam, NL).
Cell culture.
XG cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mmol/L L-glutamine, 5 × 10−5 mol/L 2-mercaptoethanol and with 3 ng/mL of IL-6 or 10 μg/mL of a pair of agonist anti-gp130 antibodies. The B9 cells were cultured in the same medium containing 100 pg of IL-6.
Proliferation assay of myeloma cell lines.
To investigate the effects of agonist anti-gp130 MoAbs or IL-6 on the proliferation of XG or B9 cells, cells were washed to remove bound IL-6. They were cultured for 5 hours in culture medium alone and washed again. They were then incubated in 96-well flat-bottomed microplates for 5 days with either culture medium alone or with a mixture of a pair of the anti-gp130 MoAbs (B-S12 + B-P8 or B1 + I2) or IL-6 at a concentration of 10,000 XG or 5,000 B9 cells per well. Tritiated thymidine (0.5 μCi, 25 Ci/mmol/L; CEA, Saclay, France) was added for the last 8 hours of culture to measure tritiated thymidine incorporation.
Measurement of blood murine and human Ig concentration.
Veinous blood was collected by retro-orbital puncture and Ig concentrations were determined by a standard double-antibody enzyme-linked immunoabsorbent assay (ELISA). To determine the concentration of murine Ig, microtiter plates (Maxisorb; Nunc, Rosklide, Denmark) were coated with rabbit antibodies to mouse Ig cross-absorbed to human and bovine Ig (DAKO Z0259; Dakopatts A/S, Glostrup, Denmark; 200 ng/100 μL in phosphate-buffered saline [PBS]) at 4°C overnight. The protein specific binding sites were saturated by 2 hours of incubation in PBS and 5% of bovine serum albumin (BSA; A-6793; Sigma). Serial dilutions of mouse serum samples were incubated for 2 hours at room temperature, and, after three washes, peroxidase-conjugated rabbit antibodies to mouse Ig (DAKO P0260) were used as a second antibody. Purified mouse IgG (I5381; Sigma) were used to get a standard curve. After additional washes, ortho-phenylene diamine (OPD P-6912; Sigma) in sodium acetate buffer (pH 5) was added as substrate solution and the optical density was determined using a Titertek Multiskan PLUS (ICN, Meckenheim, Germany).
Human Ig concentration was measured by the same method as the one used for mouse Ig determination, except that plates were coated with an affinity-purified goat antihuman IgG antiserum with minimal cross-reaction to mouse serum proteins (Jackson Immunoresearch Lab, West Grove, PA) and human Ig were detected by the same antiserum conjugated with peroxidase (Jackson Immunoresearch Laboratories). Home-purified human IgG were used to get the standard curve.
Measurement of circulating human soluble gp130 and IL-6R.
Circulating human soluble IL-6R (sIL-6R) and gp130 (sgp130) were measured using asymmetric ELISA, as already published.18,20The M182 and biotinylated M91 anti–IL-6R MoAbs and the A1 and biotinylated D2 anti-gp130 MoAbs were used to coat the immunoplates and show the bound material, respectively. The epitopes recognized by A1 and D2 anti-gp130 MoAbs are different from those recognized by the B-S12, B-P8, B1, and I2 MoAbs.20
Flow cytometry.
Cells (5 × 105) were incubated with 1 μg of murine MoAb in 100 μL of PBS, 30% human AB serum, and 0.01% sodium azide for 45 minutes at 4°C. Isotype-matched Ig was used as a control (human or murine IgG). After two washes, cells were stained with either FITC-conjugated or PE-conjugated (Fab′)2 fragments of goat antibodies to mouse IgG. Flow cytometry was performed with a FACScan apparatus (Becton Dickinson, Palo Alto, CA).
SCID mice.
SCID/SCID/Bg/Bg (CB-17/IcrHsd-scid-bg) were purchased from Harlan (Gannat, France) and bred in our sterile animal facility. Mice with a blood Ig concentration greater than 10 μg/mL were eliminated (leaky mice). Human myeloma cells (20 to 50 × 106) were washed and resuspended in 150 μL of RPMI 1640 medium without additives to be grafted in the mice. In the majority of the experiments, they were mixed with basement membrane matrix (Matrigel; 40234A; Becton Dickinson, Bedford, MA) using precooled pipettes to avoid gelling. The matrigel stock solution was frozen in 150 μL aliquots and stored at −20°C. An aliquot of 150 μL was thawed immediately before being mixed with 150 μL of cell suspension (50 × 106 cells) and the mixture was surgically implanted into the peritoneum. An autopsy examination was performed on each animal and aliquots of excised tissues were placed in 4% buffered formalin for immunohistological studies.
Immunohistological studies.
Tissues were embedded in paraffin and sections were stained with haematoxylin and eosin for histological examination. Additional sections were used for immunochemical staining for cytoplasmic human κ or λ Ig light chains and syndecan-1. Sections were incubated with murine antihuman κ or λ Ig light chains MoAbs or the MI15 anti–syndecan-1 MoAb. They were then incubated with biotin-conjugated goat antimouse antibodies followed by streptavidin-horseradish peroxidase. The staining reaction was performed for 10 minutes with 3,3-diamino-benzidine-tetrahydrochloride in PBS. Tissues of control SCID mice were not stained with MoAbs to human κ or λ Ig light chains or to human syndecan-1.
RESULTS
The agonist antihuman gp130 MoAbs do not recognize or activate murine gp130.
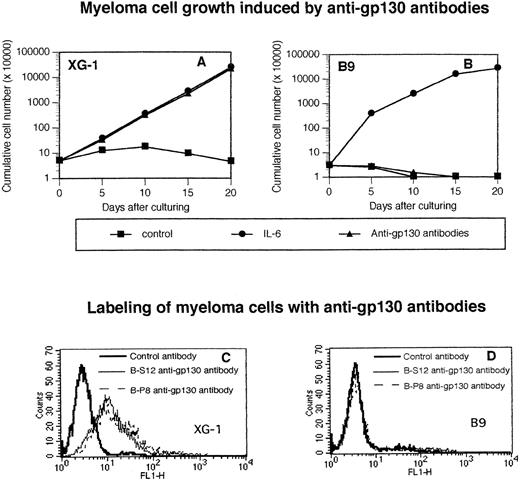
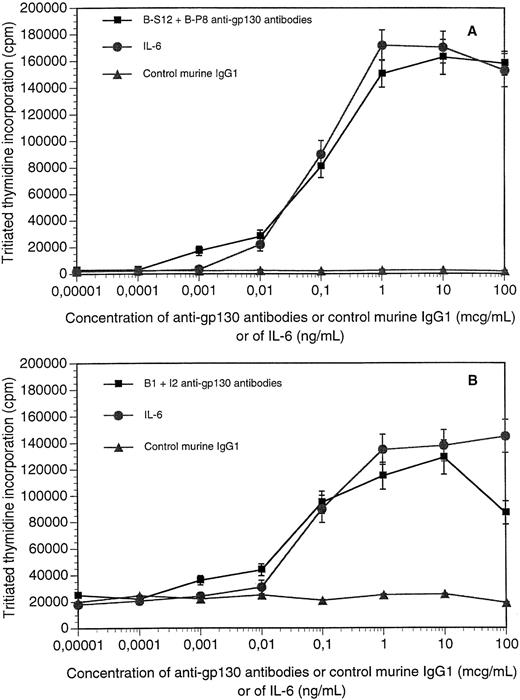
The survival and growth of XG myeloma cell lines was dependent on addition of IL-6; after starvation of IL-6, XG cells ceased to proliferate and progressively died through apoptosis.23,24In agreement with our previous results,15 the B-S12 and B-P8 MoAbs to human gp130 supported the long-term growth of the IL-6–dependent human myeloma XG-1 cell line and labeled membrane gp130 (Fig 1A through C). We have previously shown that an optimal proliferation was obtained when the antibodies were added together at a 1:1 ratio.15 The two antibodies failed to label the murine hybridoma B9 cells or to support their long-term growth, unlike human IL-6 (Fig 1B through D). As shown in Fig 2A, the two antibodies induced an optimal survival and proliferation of XG-1 cells at concentrations ranging from 1 μg (0.5 μg each) to 100 μg (50 μg each). Control murine IgG1 had no effect (Fig 2A). As shown previously,23an optimal survival and proliferation was obtained with 1 ng/mL of IL-6. Similar results were obtained with the other IL-6–dependent human myeloma cell lines (results not shown). The B1 and I2 anti-gp130 MoAbs that recognized different epitopes than the B-S12 and B-P8 MoAbs20 also supported the growth of IL-6–dependent myeloma cell lines but not that of B9 cells.16 Optimal proliferation of myeloma cell lines was obtained when these MoAbs were used at concentrations similar to those of B-S12 and B-P8 MoAbs, except that concentrations greater than 40 μg/mL became slightly inhibitory (Fig 2B).
The agonist antihuman gp130 MoAb does not recognize or activate murine gp130. Human XG-1 (A) and murine B9 hybridoma (B) cells were extensively washed and cultured at 5 × 104cells/mL (XG-1) or 3 × 104 cells/mL (B9) with 10 μg/mL of control murine IgG1 (control) or with 3 ng/mL (XG-1) or 20 pg/mL (B9) of IL-6 or with 10 μg/mL of a mixture of B-S12 and B-P8 (5 μg each) antihuman gp130 IL-6 transducer MoAbs. Every 5 days, the cells were counted and cultures were diluted at the initial cell concentration with culture medium containing fresh cytokine or the initial concentration of anti-gp130 MoAb. Results are the cumulative numbers of cells generated in the cultures. XG-1 (C) or B9 (D) cells were labeled with biotinylated B-S12 or B-P8 MoAb or control biotinylated murine IgG1 and then with FITC-conjugated avidin. Results are the fluorescence profiles obtained with the different antibodies analyzed with a FACSCAN apparatus.
The agonist antihuman gp130 MoAb does not recognize or activate murine gp130. Human XG-1 (A) and murine B9 hybridoma (B) cells were extensively washed and cultured at 5 × 104cells/mL (XG-1) or 3 × 104 cells/mL (B9) with 10 μg/mL of control murine IgG1 (control) or with 3 ng/mL (XG-1) or 20 pg/mL (B9) of IL-6 or with 10 μg/mL of a mixture of B-S12 and B-P8 (5 μg each) antihuman gp130 IL-6 transducer MoAbs. Every 5 days, the cells were counted and cultures were diluted at the initial cell concentration with culture medium containing fresh cytokine or the initial concentration of anti-gp130 MoAb. Results are the cumulative numbers of cells generated in the cultures. XG-1 (C) or B9 (D) cells were labeled with biotinylated B-S12 or B-P8 MoAb or control biotinylated murine IgG1 and then with FITC-conjugated avidin. Results are the fluorescence profiles obtained with the different antibodies analyzed with a FACSCAN apparatus.
Proliferation response of XG cells in the presence of agonist anti-gp130 MoAbs. XG-1 or XG-2 myeloma cells were extensively washed and cultured for 5 days with various concentrations of a mixture (1:1) of agonist anti-gp130 MoAbs or of control murine IgG1 or of IL-6. At the end of the culture, the proliferation was assayed by tritiated thymidine incorporation. XG-1 cells were cultured with B-S12 + B-P8 antibodies and XG-2 cells with B1 + I2 antibodies. Results are the mean ± SD tritiated thymidine incorporation determined on sextuplate culture wells. For some points, the SD was too small to be visible on the graph.
Proliferation response of XG cells in the presence of agonist anti-gp130 MoAbs. XG-1 or XG-2 myeloma cells were extensively washed and cultured for 5 days with various concentrations of a mixture (1:1) of agonist anti-gp130 MoAbs or of control murine IgG1 or of IL-6. At the end of the culture, the proliferation was assayed by tritiated thymidine incorporation. XG-1 cells were cultured with B-S12 + B-P8 antibodies and XG-2 cells with B1 + I2 antibodies. Results are the mean ± SD tritiated thymidine incorporation determined on sextuplate culture wells. For some points, the SD was too small to be visible on the graph.
Protocol of injection of the anti-gp130 MoAb.
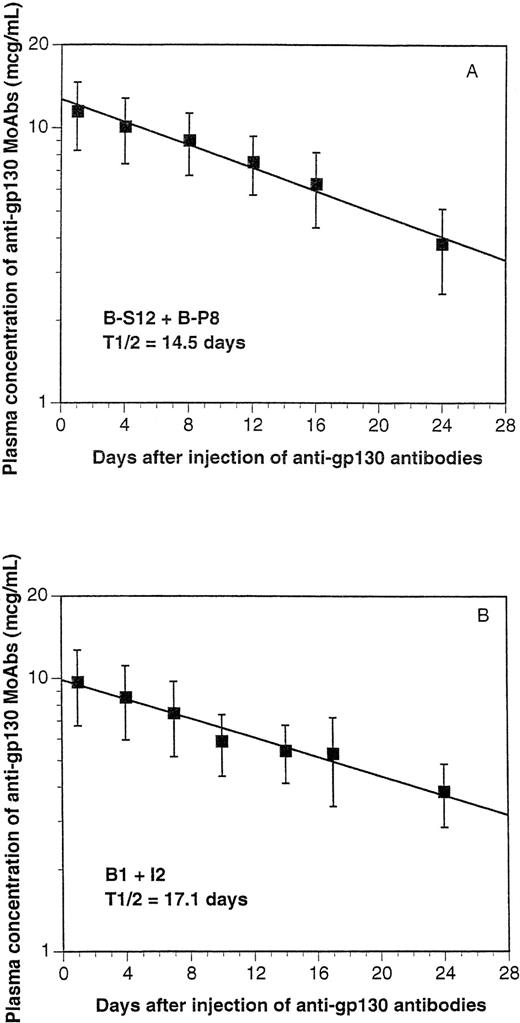
When 100 μg of the B-S12 + B-P8 mixture were injected intraperitoneally (IP), the concentration of the circulating MoAbs in the blood picked up to 12 μg/mL and progressively decreased, with a mean half-life of 14.5 days (Fig 3A). These data suggest a retention of the MoAbs in the peritoneum and a progressive release into the circulation (Fig 3A). The B1 and I2 MoAbs behave similarly, with a half-life of 17.1 days (Fig 3B). According to these data, the following protocol was used to achieve a mean blood concentration of 5 to 10 μg/mL of the pair of anti gp130 MoAbs according to the calculations we had previously developed for the diffusion of MoAb in humans25: IP injection of an initial dose of 100 μg (50 μg of each MoAb) and IP injection of 50 μg (25 μg of each MoAb) every fortnight. This is an easy way of delivering a human gp130-activating signal without any toxicity in the SCID mice. For more than 1 year, 20 mice were injected IP every fortnight with these antibodies without any obvious toxicity or mortality (results not shown).
Half-life of the anti-gp130 MoAbs in SCID mice. One hundred micrograms of a mixture of B-S12 and B-P8 (A) or B1 and I2 (B) anti-gp130 MoAbs (50 μg each) was injected IP in 4 SCID mice. Another 4 mice were injected with physiological saline. At days 1, 4, 8, 12, 16, 20, or 24 after injection, blood was collected and the concentration of circulating murine Ig was determined by ELISA. Results are the mean values ± SD obtained in the 4 mice. The concentration of murine Ig in mice injected with saline was less than 10 μg/mL.
Half-life of the anti-gp130 MoAbs in SCID mice. One hundred micrograms of a mixture of B-S12 and B-P8 (A) or B1 and I2 (B) anti-gp130 MoAbs (50 μg each) was injected IP in 4 SCID mice. Another 4 mice were injected with physiological saline. At days 1, 4, 8, 12, 16, 20, or 24 after injection, blood was collected and the concentration of circulating murine Ig was determined by ELISA. Results are the mean values ± SD obtained in the 4 mice. The concentration of murine Ig in mice injected with saline was less than 10 μg/mL.
Growth of IL-6–dependent human myeloma cell lines in SCID mice.
Having determined a protocol of an anti-gp130 MoAb injection to provide optimal concentration of circulating MoAb in SCID mice, we looked for a site where tumor cells could be grafted. XG-1 myeloma cells were injected in SCID mice either intravenously (IV), in the spleen (IS), or IP. In some experiments, myeloma cells were imbedded in matrigel before being implanted IP, as this gel has been shown to favor angiogenesis.26 We have previously shown that matrigel supported human myeloma cell growth in the presence of IL-6 in vitro (results not shown). Tumors and/or syndecan 1+ and HLA-class I+ human cells were looked for in bone marrow, spleen, blood, or liver 200 days after tumoral cell implantation. As outlined in Table 1, no tumoral infiltration or tumors were detected in the IV and IS groups. Tumor nodules were detected only when myeloma cells were imbedded in matrigel and implanted IP surgically. Tumors were detected 4 to 5 weeks after an XG-1 cell graft at the site of matrigel implantation. These tumors further developed along the peritoneal epithelium as a solid tumor. Mice died with a massive tumoral invasion of the peritoneum (tumor weight of about 5 g) 4 to 6 weeks after tumor detection. No myeloma cells were detected in the blood and bone marrow. Immunohistological examination showed infiltration of spleen, liver, and pancreas by tumoral plasmablasts that produced cytoplasmic human κ Ig light chains.
Tumoral Uptake in SCID Mice Injected With XG-1 Myeloma Cells
| Site of Tumor Injection . | No. of Mice Developing Tumors or With Detectable Tumoral Infiltration After 200 d of Myeloma Cell Implantation . |
|---|---|
| Blood | 0/5 |
| Spleen | 0/5 |
| Peritoneum | 0/5 |
| Peritoneum + matrigel (delay of tumor detection) | 15/15 (4-5 wks) |
| Peritoneum + matrigel with no anti-gp130 MoAbs (delay of tumor detection) | 5/5 (20-24 wks) |
| Site of Tumor Injection . | No. of Mice Developing Tumors or With Detectable Tumoral Infiltration After 200 d of Myeloma Cell Implantation . |
|---|---|
| Blood | 0/5 |
| Spleen | 0/5 |
| Peritoneum | 0/5 |
| Peritoneum + matrigel (delay of tumor detection) | 15/15 (4-5 wks) |
| Peritoneum + matrigel with no anti-gp130 MoAbs (delay of tumor detection) | 5/5 (20-24 wks) |
XG-1 myeloma cells (50 × 106) were injected either IV, IS, or IP in SCID mice. These mice were injected IP 2 days before with 100 μg of B-S12 + B-P8 anti-gp130 MoAbs. Then, 50 μg of B-S12 + B-P8 anti-gp130 MoAbs were injected IP every fortnight. In one group, 50 × 106 myeloma cells were imbedded in 150 μL of soft matrigel together with 3 μg of the anti-gp130 MoAb mixture (1.5 μg each), and the gel was implanted surgically IP. Mice either developed tumors (IP + matrigel group) or were killed at 200 days to look for human myeloma cells in the bone marrow, spleen, blood, or liver.
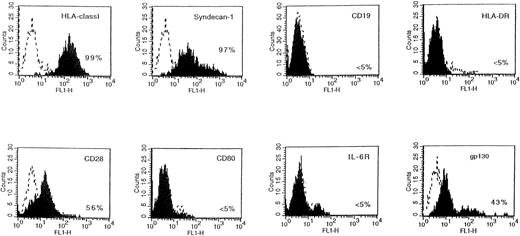
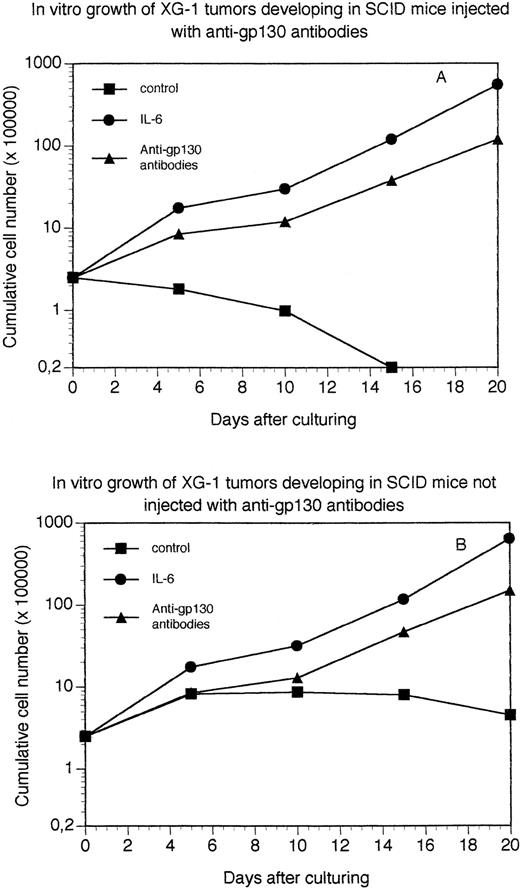
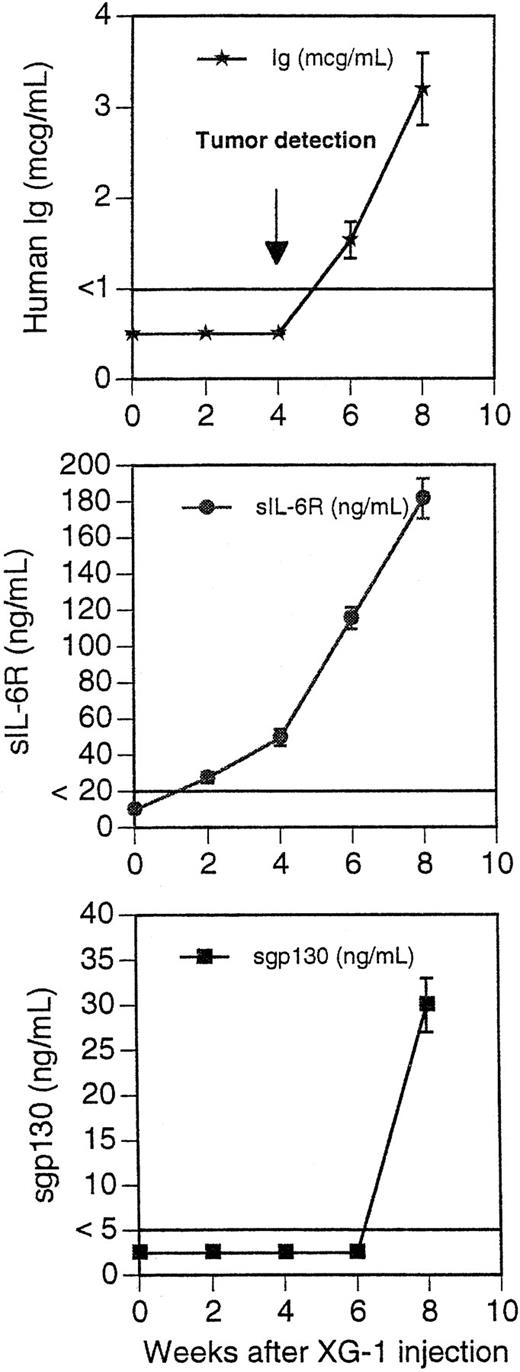
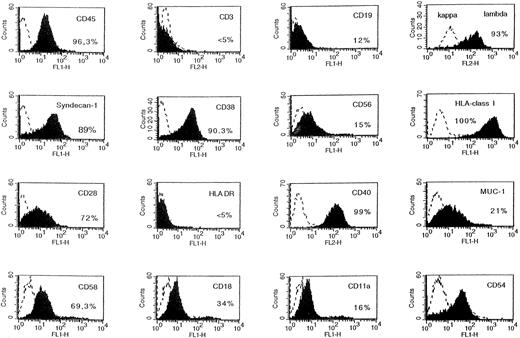
The tumors were put into suspension, and fluorescence-activated cell sorting (FACS) analysis indicated that they were human myeloma cells expressing human HLA class I antigen, human syndecan-1, but no B-cell antigens (Fig 4). In addition, tumoral cells had the phenotype of the original XG-1 cells: HLA-DR−, CD28+, and CD80−.23 They weakly expressed human gp130 (Fig 4). They had the cytology of plasmablasts and expressed cytoplasmic human κ Ig light chains. When cultured in vitro, tumoral cells failed to proliferate without addition of agonist anti-gp130 MoAbs or of IL-6 (Fig 5A). In a group of 10 mice grafted with XG-1 cells in matrigel, we measured sequentially the blood concentration of human Ig, sIL-6R, and sgp130 as markers of tumor cell mass. As shown in Fig 6, circulating sIL-6R was the earliest tumoral marker to be detected before the tumors were palpable. The blood concentration of human Ig or sgp130 increased later, 2 and 4 weeks after tumor detection, when the tumor size was already very large (Fig 6). As shown in Table 1, XG-1 cells ended up forming tumors in mice that did not receive anti-gp130 antibodies. However, these tumors were detected 20 weeks after the XG-1-cell graft, 16 weeks later than tumors arising in mice injected with anti-gp130 MoAbs. These tumors were composed of human myeloma cells (HLA-class I+, syndecan-1+, CD28+, CD19−; results not shown) that were able to survive without IL-6 for several weeks in vitro (Fig 5B). These findings were not surprising, because we had previously found that such autonomously growing clones could be obtained in vitro from this IL-6–dependent XG-1 cell line by long-term culture in vitro at a high density.27
Phenotype of XG-1 tumors in SCID mice. The tumors growing in SCID mice grafted with XG-1 cells and injected with agonist anti-gp130 MoAbs were harvested and put in suspension. Viable cells were recovered by centrifugation on a Ficoll-hypaque density medium and cells were labeled with MoAbs to various antigens or control murine antibodies. Fluorescence was analyzed with a FACSCAN apparatus. The dashed line represents the fluorescence profile with a control antibody and the solid line is with MoAbs to specific antigens. The percentages of cells labeled with the different MoAbs are indicated in the panels.
Phenotype of XG-1 tumors in SCID mice. The tumors growing in SCID mice grafted with XG-1 cells and injected with agonist anti-gp130 MoAbs were harvested and put in suspension. Viable cells were recovered by centrifugation on a Ficoll-hypaque density medium and cells were labeled with MoAbs to various antigens or control murine antibodies. Fluorescence was analyzed with a FACSCAN apparatus. The dashed line represents the fluorescence profile with a control antibody and the solid line is with MoAbs to specific antigens. The percentages of cells labeled with the different MoAbs are indicated in the panels.
Growth of XG-1 tumor cells in vitro. Tumors growing in SCID mice grafted with XG-1 cells and injected (A) or not (B) with agonist anti-gp130 MoAbs were put in suspension and viable cells were cultured at a concentration of 2.5 × 105 cells/mL in culture medium supplemented with 10 μg/mL of control murine IgG1 (control), 3 ng/mL of IL-6 (IL-6), or 10 μg/mL of a mixture of the B-S12 and B-P8 anti-gp130 MoAbs (5 μg each; anti-gp130 antibodies). Every 5 days, cells were counted and diluted at the initial cell concentration with fresh culture medium and the initial concentration of cytokine or antibodies. Results are the mean cumulative numbers of cells determined on six different culture wells.
Growth of XG-1 tumor cells in vitro. Tumors growing in SCID mice grafted with XG-1 cells and injected (A) or not (B) with agonist anti-gp130 MoAbs were put in suspension and viable cells were cultured at a concentration of 2.5 × 105 cells/mL in culture medium supplemented with 10 μg/mL of control murine IgG1 (control), 3 ng/mL of IL-6 (IL-6), or 10 μg/mL of a mixture of the B-S12 and B-P8 anti-gp130 MoAbs (5 μg each; anti-gp130 antibodies). Every 5 days, cells were counted and diluted at the initial cell concentration with fresh culture medium and the initial concentration of cytokine or antibodies. Results are the mean cumulative numbers of cells determined on six different culture wells.
Blood levels of human Ig, soluble IL-6R, and soluble gp130 in mice grafted with XG-1 cells. Ten mice received IP 100 μg of B-S12 + B-P8 MoAbs (50 μg each) 2 days before graft of 50 × 106 XG-1 cells in matrigel. They then received IP 50 μg of B-S12 + B-P8 MoAbs (25 μg each) every fortnight. Tumors were detected by palpation at the site of inoculation in all mice 4 to 5 weeks after the graft. Blood was collected every 2 weeks and assayed for human Ig, human sIL-6R, and human sgp130. The limit of sensitivity of the ELISA was 1 μg/mL for human Ig, 20 ng/mL for human sIL-6R, and 5 ng/mL for human sgp130. Results are the mean ± SD of the determinations. For some points, the error bars were too small to be visible on the graphs.
Blood levels of human Ig, soluble IL-6R, and soluble gp130 in mice grafted with XG-1 cells. Ten mice received IP 100 μg of B-S12 + B-P8 MoAbs (50 μg each) 2 days before graft of 50 × 106 XG-1 cells in matrigel. They then received IP 50 μg of B-S12 + B-P8 MoAbs (25 μg each) every fortnight. Tumors were detected by palpation at the site of inoculation in all mice 4 to 5 weeks after the graft. Blood was collected every 2 weeks and assayed for human Ig, human sIL-6R, and human sgp130. The limit of sensitivity of the ELISA was 1 μg/mL for human Ig, 20 ng/mL for human sIL-6R, and 5 ng/mL for human sgp130. Results are the mean ± SD of the determinations. For some points, the error bars were too small to be visible on the graphs.
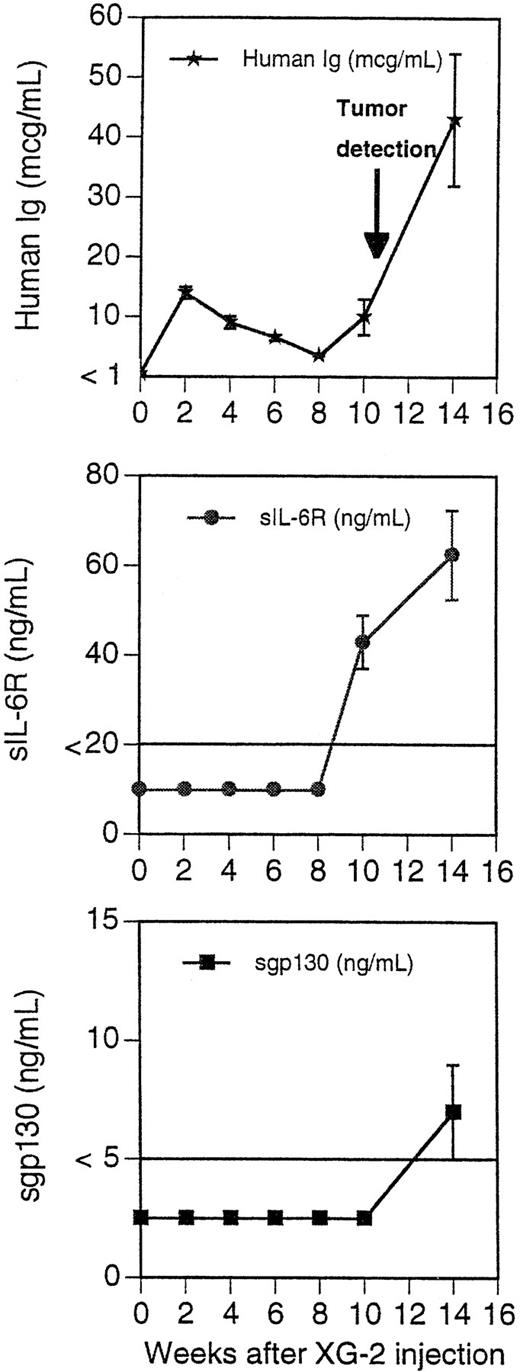
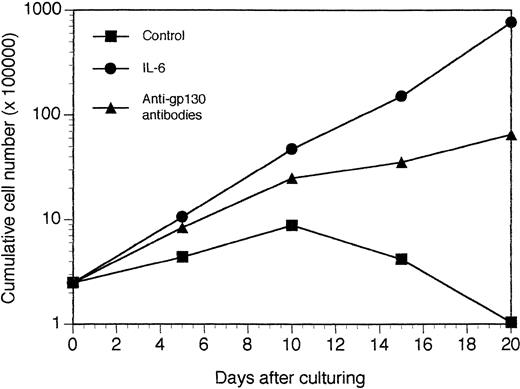
We next investigated whether tumoral cell growth could be obtained with other IL-6–dependent human cell lines in the presence or absence of agonist anti-gp130 MoAbs. XG-2 cells imbedded or not with matrigel formed tumors in vivo with a mean appearance time of 11 weeks (range, 9 to 14 weeks). No tumors were detected without anti-gp130 MoAb injections. These tumors had the characteristic phenotype of XG-2 myeloma cells (syndecan 1+, HLA-class I+, CD28+, CD40+++),23 expressed cytoplasmic κ chains, and had the morphology of malignant plasmablasts. Similar to XG-1 tumors, XG-2 tumors grew as solid tumors along the peritoneal epithelium. Unlike XG-1 tumors, a massive tumoral infiltration into the bone marrow was found at autopsy (60% of syndecan-1+ tumor cells in the bone marrow). Tumoral infiltration of spleen, liver, and pancreas were also found. Circulating human Ig were detected before clinical tumor detection (Fig 7). They were 12-fold higher than those observed in mice bearing XG-1 tumors in agreement with a larger in vitro Ig production by XG-2 cells compared with XG-1 cells. The tumor uptake was also associated with the presence of circulating sIL-6 and sgp130 (Fig 7). When cultured in vitro, the tumors grew in an agonist-gp130–dependent fashion similar to parental cells (Fig 8).
Concentration of blood murine and human Ig in SCID mice receiving XG-2 cells and anti-gp130 MoAbs. Three mice were injected IP with 50 × 106 XG-2 cells and B1 +I2 anti-gp130 MoAbs. Peripheral blood was harvested 2, 4, 6, 8, 10, and 14 weeks after XG-2-cell inoculation and the concentrations of human Ig, human sIL-6R, and human sgp130 were determined by ELISA. The limit of sensitivity of the ELISA was 1 μg/mL for human Ig, 20 ng/mL for human sIL-6R, and 5 ng/mL for human sgp130. Results are the mean ± SD of the determinations. For some points, the error bars were too small to be visible on the graphs.
Concentration of blood murine and human Ig in SCID mice receiving XG-2 cells and anti-gp130 MoAbs. Three mice were injected IP with 50 × 106 XG-2 cells and B1 +I2 anti-gp130 MoAbs. Peripheral blood was harvested 2, 4, 6, 8, 10, and 14 weeks after XG-2-cell inoculation and the concentrations of human Ig, human sIL-6R, and human sgp130 were determined by ELISA. The limit of sensitivity of the ELISA was 1 μg/mL for human Ig, 20 ng/mL for human sIL-6R, and 5 ng/mL for human sgp130. Results are the mean ± SD of the determinations. For some points, the error bars were too small to be visible on the graphs.
Growth of XG-2 tumors in vitro. Tumors growing in SCID mice injected with XG-2 cells and agonist anti-gp130 MoAbs were put in suspension and the viable cells recovered by centrifugation on Ficoll hypaque. These cells were cultured at a concentration of 2.5 × 105 cells/mL in culture medium supplemented with 10 μg/mL of control murine IgG1 (control), 3 ng/mL of IL-6 (IL-6), or 10 μg/mL of a mixture of the B1 and I2 anti-gp130 MoAbs (5 μg each; anti-gp130 antibodies). Every 5 days, cells were counted and diluted at the initial cell concentration with fresh culture medium and the initial concentration of cytokine or antibodies. Results are the mean cumulative numbers of cells determined on six different culture wells.
Growth of XG-2 tumors in vitro. Tumors growing in SCID mice injected with XG-2 cells and agonist anti-gp130 MoAbs were put in suspension and the viable cells recovered by centrifugation on Ficoll hypaque. These cells were cultured at a concentration of 2.5 × 105 cells/mL in culture medium supplemented with 10 μg/mL of control murine IgG1 (control), 3 ng/mL of IL-6 (IL-6), or 10 μg/mL of a mixture of the B1 and I2 anti-gp130 MoAbs (5 μg each; anti-gp130 antibodies). Every 5 days, cells were counted and diluted at the initial cell concentration with fresh culture medium and the initial concentration of cytokine or antibodies. Results are the mean cumulative numbers of cells determined on six different culture wells.
Graft of patients' myeloma cells.
Myeloma cells from 5 patients with MM were purified, imbedded with matrigel and agonist anti-gp130 MoAb, and implanted in vivo in SCID mice pretreated with agonist anti-gp130 and further injected every fortnight with these anti-gp130 antibodies.
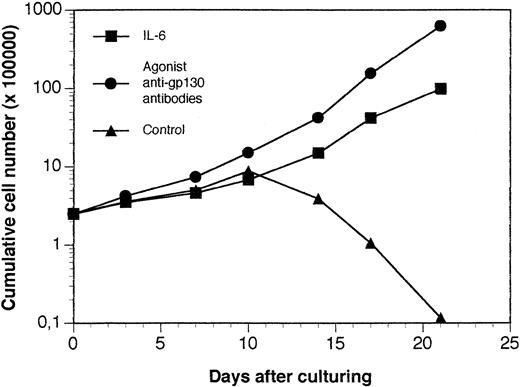
A tumor was obtained in a SCID mouse transplanted with the myeloma cells from a patient with plasma cell leukemia. The growth pattern of this solid tumor was similar to those reported for the XG-1 IL-6–dependent myeloma cell line. In particular, no human CD45+ cells could be detected in bone marrow or blood. Cells from this SCID tumor were human CD45+ cells that expressed no CD3 or CD19 antigens but expressed cytoplasmic λ Ig chain, syndecan-1, CD38, and CD56, the same as the donor patient's myeloma cells (Fig 9). They also expressed HLA class I but failed to express HLA class II antigens (Fig 9). Cytological examination indicated that these cells were plasmablastic cells. At the time of tumor detection, a large concentration of circulating human Ig was detected in the blood of the mice (150 μg/mL; Table 2). When cultured in vitro, tumoral cells could grow only in the presence of agonist anti-gp130 antibodies or IL-6 (Fig 10). This cell line was termed XG-13.
Phenotype of tumor cells developing in SCID mice grafted with patient's myeloma cells. The tumors growing in SCID mice grafted with a patient's myeloma cells and injected with agonist anti-gp130 MoAbs were harvested and put in suspension. Viable cells were recovered by centrifugation on a Ficoll-hypaque density medium and cells were labeled with MoAbs to various antigens or control murine antibodies. Fluorescence was analyzed with a FACSCAN apparatus. The dashed line represents the fluorescence profile with a control antibody and the solid line is with MoAbs to specific antigens. The percentages of cells labeled with the different MoAbs are indicated in the panels.
Phenotype of tumor cells developing in SCID mice grafted with patient's myeloma cells. The tumors growing in SCID mice grafted with a patient's myeloma cells and injected with agonist anti-gp130 MoAbs were harvested and put in suspension. Viable cells were recovered by centrifugation on a Ficoll-hypaque density medium and cells were labeled with MoAbs to various antigens or control murine antibodies. Fluorescence was analyzed with a FACSCAN apparatus. The dashed line represents the fluorescence profile with a control antibody and the solid line is with MoAbs to specific antigens. The percentages of cells labeled with the different MoAbs are indicated in the panels.
Implantation of Patients' Myeloma Cells in SCID Mice
| . | Control Mice . | Patient No. 1 . | Patient No. 2 . | Patient No. 3 . | Patient No. 4 . | Patient No. 5 . |
|---|---|---|---|---|---|---|
| Patient's disease | − | PCL | MM | MM | MM | MM |
| Tumor | + | − | − | − | − | − |
| Human Ig (μg/mL) | <1 | 150 | 8.1 | 10.7 | 8.8 | 11.9 |
| Soluble human IL-6R (ng/mL) | <20 | 78 | 41 | 33 | <20 | <20 |
| Soluble human gp130 (ng/mL) | <5 | 125 | <5 | <5 | <5 | <5 |
| Tumoral infiltration* | +++ | + | + | + | + | + |
| . | Control Mice . | Patient No. 1 . | Patient No. 2 . | Patient No. 3 . | Patient No. 4 . | Patient No. 5 . |
|---|---|---|---|---|---|---|
| Patient's disease | − | PCL | MM | MM | MM | MM |
| Tumor | + | − | − | − | − | − |
| Human Ig (μg/mL) | <1 | 150 | 8.1 | 10.7 | 8.8 | 11.9 |
| Soluble human IL-6R (ng/mL) | <20 | 78 | 41 | 33 | <20 | <20 |
| Soluble human gp130 (ng/mL) | <5 | 125 | <5 | <5 | <5 | <5 |
| Tumoral infiltration* | +++ | + | + | + | + | + |
Freshly explanted myeloma cells (50 × 106) from 1 patient with PCL and 4 with MM were imbedded in 150 μL of soft matrigel together with 3 μg of anti-gp130 MoAb mixture (1.5 μg each), and the gel was implanted surgically IP in SCID mice. These mice were injected IP 2 days before with 100 μg of B-S12 + B-P8 anti-gp130 MoAbs. Then, 50 μg of B-S12 + B-P8 anti-gp130 MoAbs were injected IP every fortnight. Mice either developed tumors (patient no. 1) or were killed at 360 days to look for human myeloma cells in the bone marrow, spleen, blood, liver, pancreas, or mesenteric adipose tissue. The levels of circulating human Ig, sIL-6R, and sgp130 were also assayed. Five mice receiving no human myeloma cells but injected with anti-gp130 MoAbs were used as controls.
Abbreviation: PCL, plasma cell leukemia.
Myeloma cells from the 4 patients with MM were detected in mesenteric adipose tissue.
In vitro growth of tumor cells harvested from SCID mice grafted with a patient's myeloma cells. The tumor growing in SCID mice grafted with myeloma cells from a patient with MM were put in suspension. Cells were cultured at a concentration of 5 × 105 cells/mL in culture medium supplemented with 10 μg/mL of control murine IgG1 (control), 3 ng/mL of IL-6 (IL-6), or 10 μg/mL of a mixture of the B-S12 and B-P8 anti-gp130 MoAbs (5 μg each; anti-gp130 antibodies). Every 5 days, cells were counted and diluted at the cell concentration with fresh culture medium and the initial concentration of cytokine or antibodies. Results are the mean cumulative numbers of cells determined on six different culture wells.
In vitro growth of tumor cells harvested from SCID mice grafted with a patient's myeloma cells. The tumor growing in SCID mice grafted with myeloma cells from a patient with MM were put in suspension. Cells were cultured at a concentration of 5 × 105 cells/mL in culture medium supplemented with 10 μg/mL of control murine IgG1 (control), 3 ng/mL of IL-6 (IL-6), or 10 μg/mL of a mixture of the B-S12 and B-P8 anti-gp130 MoAbs (5 μg each; anti-gp130 antibodies). Every 5 days, cells were counted and diluted at the cell concentration with fresh culture medium and the initial concentration of cytokine or antibodies. Results are the mean cumulative numbers of cells determined on six different culture wells.
Circulating human Ig were detected in the plasma of mice grafted with tumoral cells from the 4 patients with MM. The concentration ranged from 8.1 to 11.9 μg/mL (Table 2). SIL-6R was detected in the mice grafted with myeloma cells from 2 patients, unlike sgp130 (Table 2). No solid tumors were detected 360 days after transplantation. No human syndecan-1 cells or CD45 cells were detected in the bone marrow, blood, spleen, and liver. Immunohistological examination showed the presence of human myeloma cells in the mesenteric adipose tissue. These data indicated that a minority of myeloma cells survived in these mice.
DISCUSSION
We describe here a model that, in SCID mice, makes it possible to grow human myeloma cells whose survival and growth is dependent on gp130 transducer activation in vitro. This SCID model has several advantages. First, we use agonist antihuman gp130 MoAbs that have a long half-life (2 weeks) in vivo and do not activate murine gp130. Then, we avoid a daily injection of human IL-6 that has a short half-life in vivo (20 to 60 minutes)10,11 and is likely to have all the toxicities reported for IL-6 in vivo as human IL-6 binds to murine IL-6R.12-14 Another advantage is that we have to target only the gp130 transducer and avoid a cascade of activation of IL-6R and then of gp130 transducer by IL-6/IL-6R complexes.28This is likely to be important for in vivo models, because large levels of circulating agonist IL-6R circulate in the human (ie, 200 ng/mL in patients with MM) and may replace the need for membrane IL-6R.29,30 In the early phases after tumoral implantation, it is possibly very important to provide an optimal gp130 activation to prevent tumor cells from dying, as is the case with gp130 antibodies. It is noteworthy that one group has reported the possibility of growing in vitro human myeloma cells with a combination of IL-6 and sIL-6R but not with IL-6 alone, which is probably due to a too weak membrane IL-6R expression on myeloma cells.31
By using this optimal strategy to provide human gp130 transducer activation in SCID mice, we have been able to grow IL-6–dependent human myeloma cell lines and freshly explanted myeloma cells from 1 patient with extramedullary proliferation. In addition, we have observed the persistence of circulating human Ig for more than 1 year with freshly explanted myeloma cells from patients with medullary involvement. For one cell line (XG-2), tumors were obtained only in mice injected with anti-gp130 MoAbs, whereas for the XG-1 cell line, tumors were obtained both with and without anti-gp130 MoAbs. However, tumors without anti-gp130 MoAbs occurred 20 weeks after tumoral cell inoculation, 16 weeks later than in the presence of anti-gp130 MoAbs. This again emphasized than an optimal gp130 transducer activation in the early days after tumor cell injection is likely to be important to promote an optimal tumoral cell survival. The occurrence of XG-1 tumoral growth without anti-gp130 MoAbs in SCID mice was not surprising, because this cell line produced a weak amount of autocrine IL-6, and autonomously growing subclones can be obtained in vitro by culturing cells at a high cell density or with myeloma cell survival factors such as interferon-α.24 27
The tumors developed as a solid tumor along the peritoneal membrane and were enveloped in peritoneal epithelial cells. Tumors were fully vascularized. In the late stages of tumoral invasion, tumoral infiltration of the liver, pancreas, and spleen were found. Tumor infiltration of bone marrow was found for the XG-2, unlike the XG-1 myeloma cells, suggesting that the murine bone marrow environment is a suitable one for certain populations of human myeloma cells. Tumor cells retained all the phenotypical characteristics of the inoculated myeloma cells and produced human Ig, sIL-6R, and sgp130 that circulated in the mouse blood. This made it possible to monitor tumoral cell mass and growth by assaying the concentration of circulating human sIL-6R and human Ig.
The tumors growing in SCID mice also retained the dependence on gp130 activation to survive and grow in vitro, as did the inoculated myeloma cells. These findings again emphasize the usefulness of the agonist antibodies to provide a continous human gp130 activation in SCID mice. The tumor uptake was increased when myeloma cells were imbedded in matrigel together with agonist anti-gp130 antibodies before being implanted. In particular, no XG-1 tumor occurred spontaneously in mice injected with XG-1 cells not imbedded in matrigel. The improvement of tumor uptake through using matrigel is in agreement with previous reports showing that matrigel favored angiogenesis,26 thus, the feeding of myeloma cells by various blood nutrients, in particular by the circulating agonist anti-gp130 antibodies.
The fact that we have been able to get tumors from IL-6–dependent cell lines or from freshly explanted myeloma cells from a patient with extramedullary proliferation, unlike those with medullary involvement, is in agreement with in vitro data and like the pattern of disease growth in patients. In vitro, by using IL-6 or these agonist antibodies, we, and others, have been able to obtain IL-6–dependent cell lines from myeloma cells from patients with extramedullary proliferation only.7,23 Myeloma cells from patients with medullary involvement survive for several weeks with the agonist anti-gp130 antibodies but are blocked in the G1 phase of the cell cycle (unpublished results). For 2 patients, we obtained a survival lasting for several months without cell proliferation. For these patients' cells, activation of the gp130 transducer was a necessary signal to promote myeloma cell survival but not sufficient to promote G1 to S phase transition and cell cycling (unpublished results). When inoculated in SCID mice with agonist anti-gp130 antibodies, these myeloma cells could also survive for more than 1 year, as assayed by the detection of circulating human Ig, and they did not proliferate. In patients with medullary involvement, the situation is likely to be very similar, because only a minor fraction of myeloma cells proliferate in vivo.32 In these patients with medullary involvement, beside gp130 transducer activation, an additional signal might be delivered by stromal cells that make it possible for a minor fraction to enter the cell cycle. One of these signals might be linked to the FGF/FGR3 signalling. Indeed, t(4;14) translocations involving the FGR3 gene have been shown to occur in myeloma cell lines and in freshly explanted cells from some patients.33 In addition, FGR3 mutations similar to those found in dwarnish syndrome and leading to constitutive activation of the receptor have been shown in some cell lines.34 The recent demonstration that fetal bone favors the homing of myeloma cell lines in SCID mice may help to identify this cosignal.5
In patients with extramedullary proliferation, activation of gp130 transducer induces G1 to S phase transition,35,36suggesting that genetic disregulations contribute to this transition. In these patients, most of the abnormalities have been linked to gene coding for proteins regulating the apoptosis and the G1 to S phase transition: hypermethylation of P16 gene,37 deletion of Rb,38,39 mutations in P53 gene,40,41translocation involving the cyclin D1 gene,42,43 or the MUM1/IRF4 gene.44
This SCID mouse model of MM should be interesting as an assay for various therapeutical strategies, including immunotherapies and chemical agents. Because it takes into account the gp130 dependence on myeloma cell survival and proliferation,24 inhibitors of the gp130 transduction pathway could be assayed. For example, tyrphostin AG490 that blocks JAK 2 activation was recently shown to inhibit the growth of human leukemic cells in SCID mice without affecting mouse viability.45 We are now testing to find out whether these inhibitors may block JAK2 activation in myeloma cells as well as their in vitro survival and growth. If positive results are obtained, it would be useful to look for an inhibition of the gp130-dependent myeloma growth in SCID mice. However, because the agonist activity of gp130 antibodies is not affected by anti–IL-6 or IL-6R antibodies, one limitation of this model is that inhibitors of IL-6, IL-6 production, or IL-6R cannot be assayed, contrary to recently published models.6 In addition, the overproduction of IL-6 in MM is associated with accompanying symptoms related to IL-6 toxicities46-49 that cannot be observed in the present SCID model due to the lack of biological activity of anti-gp130 antibodies on murine cells.
In conclusion, the SCID model we present here is a unique model making it possible to obtain gp130-dependent survival and growth of myeloma cells from cell lines or from patients with active disease. This model should be useful to study various agents able to prevent tumor survival and growth in vivo.
Supported by grants from ARC (Paris, France), LFNC (Paris, France), and DRC (CHU Montpellier, France).
Address reprint requests to Bernard Klein, PhD, INSERM U475, 99 Rue Puech Villa, 34197 Montpellier Cedex 5, France; e-mail:klein@montp.inserm.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal