Abstract
Thirteen cell lines with different levels of Pgp and MRP expression were used to assess the ability of calcein acetoxymethyl ester (calcein-AM) uptake and calcein efflux to measure Pgp and MRP functions, respectively. There was a good correlation between MRP expression and the modulatory effect of probenecid (a specific modulator of MRP) on the calcein efflux (r = .91, P= .0003) and between Pgp expression and the modulatory effect of CsA on calcein-AM uptake (r = .96, P < .0001). In light of the high correlations for both proteins, we tested calcein-AM uptake and efflux in fresh myeloid leukemic cells. In 53 acute myeloid leukemia (AML) patients, there was also a good correlation between MRP expression (measured by reverse transcription-polymerase chain reaction and by MRPm6 expression by flow cytometry) and the modulatory effect of probenecid on the calcein fluorescence (r = .92, P < .0001) and between Pgp expression as measured by UIC2 antibody binding on flow cytometry and the modulatory effect of cyclosporin A on calcein-AM uptake (r = .83,P < .0001). Pgp activity was higher in CD34+leukemia than in CD34− leukemia (2.26 ± 1.50 v1.46 ± 1.21, respectively; P = .003), and MRP activity was higher in CD34− leukemia than in CD34+leukemia (1.77 ± 0.40 v 1.4 ± 0.29, respectively; P= .004). Pgp expression and activity (P = .004 andP = .01, respectively) and MRP activity (P = .03) but not MRP expression were prognostic factors for achievement of complete remission. These results suggest that functional testing (with calcein-AM ± modulators) for the presence of both MRP and Pgp activities is of prognostic value and that MRP contributes to drug resistance in AML.
MULTIDRUG RESISTANCE (MDR) of some human cancers, particularly acute myeloid leukemia (AML), remains a major obstacle to successful chemotherapy. The best characterized resistance mechanism in AML is the one mediated by the MDR1 gene. MDR1 gene expression has been extensively studied in AML and has been shown to be associated with poorer outcome.1-4 MDR1 gene expression has been also correlated with functional parameters (dye and drug uptake/efflux) measured by flow cytometry in AML. Several dyes (Rhodamine 123 [Rh123] and DiOC2) and drugs (daunorubicin and doxorubicin) may be used to assess Pgp function. These functional tests correlate also with treatment outcome.3 However, in several studies, discrepant cases were reported, with increased efflux and no significant MDR1 expression.5,6 This suggests that alternative proteins, such as the more recently recognized multidrug resistance associated protein (MRP)7 or the lung resistance protein (LRP),8 may contribute to the MDR phenotype. But the role and functionality of these two proteins are still discussed and unclear in AML.9 10 This emphasizes also that functional tests play a major part in the understanding of the MDR phenotype. But, until then, only MDR1 functional tests have been provided for AML.
Cells exposed to calcein acetoxymethyl ester (calcein-AM) become fluorescent after the cleavage of calcein-AM by cellular esterases that produces a fluorescent derivate calcein. Pgp, the product of the multidrug transporter MDR1 gene, actively extrudes the calcein-AM, but not the fluorescent calcein.11 On the other hand, fluorescent calcein and calcein-AM are extruded by the multidrug transporter MRP.12 Therefore, calcein-AM uptake (with specific modulator of Pgp) can be used to assess whether MDR1 is functional and calcein efflux can explore MRP activity. Calcein-AM uptake and efflux have been studied in cell lines,13 but not in fresh cells of AML patients, except for one publication with few patients studied (14 patients for calcein-AM).14 With the calcein-AM functional assay, the role and relative importance of Pgp and MRP can be clarified in AML.
We have studied the relative importance of Pgp and MRP in AML using calcein-AM functional test. In addition, the correlation between this fluorescence-based flow cytometric functional assay and MDR proteins expression as well as the correlations between this functional assay and clinical or biological parameters were analyzed.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
The present study used 13 cell lines with different levels of MDR1, MRP, and LRP: A549 (given by S. Chevillard, Institut Curie, Paris, France), a lung adenocarcinoma expressing a spontaneously high level of MRP; K562, a human erythroleukemia (gift from B.I. Sickic, Stanford, CA); and K562/HHT30, K562/HHT100, K562/HHT200, K562/HHT300, and K562/HHT400, sublines of K562 developed in our laboratory that have been selected with 30, 100, 200, 300, and 400 ng/mL of homoharringtonine (HHT).15 The other cell lines were HL60, a human promyelocytic leukemia; HL60/Pgp and HL60/MRP sublines of HL60, resistant to daunorubicin (gift by F. Lacombe [Bordeaux, France] and F. Calvo [Hôpital Saint-Louis, Paris, France], respectively); and the T-lymphoblastic cell lines CEM and CEM/VLB (gift by F. Calvo) selected with 50 ng/mL of vinblastine.
All cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 UI penicillin, and 100 ng/mL streptomycin at 37°C in a humid atmosphere with 5% CO2. All experiments were performed in triplicate and at three different times. Exponentially growing cells were used for all experiments.
Patients
Fifty-three consecutive AML patients were analyzed. Diagnosis was based on French-American-British (FAB) criteria.16 17Immunophenotyping was performed by using flow cytometry. For each patient, several clinical (age, sex, and World Health Organization [WHO] performance status) and biological (white blood cell count [WBC], cytogenetic, and CD34) characteristics at diagnosis were analyzed, as well as their response to treatment administered. Unfavorable karyotypes were defined as t(9;22) or abnormalities of chromosomes 5 or 7, abnormalities of chromosome 11q2.3, and complex abnormalities. Samples were considered positive for CD34 when more than 20% of the viable cells were stained with CD34 antibody (HPCA2 clone; Becton Dickinson, Grenoble, France) in excess of the negative control. We also quantified CD34 expression as a continuous D value function using the Kolmorogov-Smirnov (KS) test. Patients were included in the EORTC Leukemia Cooperative Group protocols (AML10 or AML13 for younger or older patients, respectively; AML10: 100 mg/m2/d AraC from d1 through d10; 150 mg/m2/d etoposide from d1 through d5 and one of these anthracyclins [50 mg/m2/d daunorubicin or 10 mg/m2/d idarubicin or 12 mg/m2/d mitoxantrone on d1, d3, and d5]; AML13: 7 mg/m2/d mitoxantrone d1, d3, and d5; 100 mg/m2/d AraC from d1 through d7; and 100 mg/m2/d etoposide from d1 through d3). Complete remission (CR) to induction treatment was defined as a normal marrow cellularity with less than 5% of blast cells and near-normal peripheral blood counts during a 1-month period.
Fresh Leukemic Cells
Peripheral blood (31 patients, when blood samples contained >70% of blasts, before mononuclear cell isolation) or bone marrow (22 patients, when blood samples contained <70% of blasts, before mononuclear cell isolation) were collected in heparinized glass tubes after patients had given informed consent. Mononuclear cells were isolated on a ficoll density gradient by centrifugation for 20 minutes at 2,000 rpm. Interphase cells were washed and resuspended in RPMI1640 medium, buffered with 20 mmol/L HEPES, pH 7.4 (without phenol red), and supplemented with 10% fetal calf serum. All samples contained at least 70% of blasts before mononuclear cells isolation. Samples were analyzed for proteins expression and function on the same day, within 6 hours. In the analysis of the fresh leukemic samples, the expression and function of MDR proteins were performed with selected cells by CD34 antibody (2-color assays) or other markers (eg, CD33/CD7, CD33/CD2, CD33/CD19, or CD33/CD22 by 3-color assays), if possible, or with physical characteristics only if blast cells had no characteristic marker.
MRP, MDR1, and LRP mRNA Expression Measured by Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA from cells (107) was extracted according to the acid guanidinium-phenol-chloroform technique.18 cDNA was synthesized as described previously.9 The resulting cDNA was stored at −20°C until used. The expression of MDR1 and MRP by RT-PCR was described elsewhere.9,19 For LRP, the primers used were 5′-ACA ACT ACT GCG TGA TTC TC-3′ (LRP sense strand) and 5′-TCA GCA TGT AGG TGC TTC CA-3′ (LRP antisense strand). They amplified a 390-bp LRP fragment corresponding to nucleotides 941 to 1330 of the LRP cDNA sequence.20 The specificity of these primers was proved by the fact that there was no nonspecific band amplified by PCR. PCR was performed as described previously.21 The achieved reaction mixture was heated at 95°C for 3 minutes. Amplification was performed in sequential cycles of 95°C for 30 seconds, 53°C for 30 seconds, and 72°C for 45 seconds. After 28 cycles of amplification, all samples were incubated for an additional 8 minutes at 72°C. Variations between samples in the quantity of cDNA synthesis were normalized by the quantity of β2 microglobulin (β2m) in each sample. The results were calculated as the ratio of quantities of MDR mRNA product to β2m mRNA product.
MRP, MDR1, and LRP Proteins Expression Measured by Flow Cytometry
Cells were permeabilized in 15% (vol/vol) lysing solution G (Becton Dickinson) in H2O and incubated for 15 minutes in phosphate-buffered saline/bovine serum albumin (PBS/BSA) containing 1% (vol/vol) normal goat serum. Cells (5 × 105) were incubated for 1 hour at 4°C in 100 μL PSB/BSA 5% containing either the monoclonal antibody (MoAb) (UIC2 [1 μg/mL; IgG2a; Immunotech] or MRPm6 [2 μg/mL; IgG1] or LRP56 [2 μg/mL; IgG2b; given by R.J. Scheper, Amsterdam, The Netherlands]) or the mouse isotype-matched control MoAbs. Antibody binding was detected with R-phycoerythrin–labeled goat antimouse Igs (Immunotech [Marseille, France] and Becton Dickinson) in accordance with the consensus recommendations of Beck et al.22 Fluorescence was analyzed on a FACSORT flow cytometer (Becton Dickinson). For each sample, 5,000 events were collected. Protein values were expressed by two methods: first, as adjusted for control, ie, as ratio of arithmetic mean fluorescence of UIC2/IgG2A control or MRPm6/IgG1 control or LRP56/IgG2b control,23 and second, protein staining of gated leukemic blasts was compared with the one of a control cells by the means of the KS test. This statistic, denoted D, measures the difference between two distribution functions and generates a value ranging from −1.0 to 1.0. These two methods accurately identify small differences in fluorescence and are useful in detection of low-level protein expression, which frequently occurs in patient samples.5These two methods were strongly correlated (r = .83, P< .0001 for MRP ; r = .70, P < .0001 for LRP; andr = .77, P < .0001 for MDR1). In this study, we used the ratio of MDR MoAbs fluorescence divided by control MoAbs fluorescence. Correlations between MDR proteins expression and clinical, biological, and clinical outcome were performed using MDR proteins expression, as a continuous variable in accordance with the consensual recommendations of Beck et al.22
Functional Tests in Cell Lines and Fresh Leukemic Cells Using Rh123, Daunorubicin, and Calcein-AM
Rh123.
Cells (5 × 105) were stained with 200 ng/mL of Rh123 for 20 minutes at 37°C in RPMI medium. The cells were washed twice in PBS and resuspended in Rh123-free medium and allowed to efflux for 60 minutes at 37°C, either with or without modulators of MDR1 (2 μmol/L cyclosporin A [CsA]) or MRP (2 mmol/L probenecid).23-25 At the indicated times, 1 × 104 cells were taken for flow cytometry analysis. Samples were analyzed on a FACSORT flow cytometer (Becton Dickinson). Cells from each subline that had not been exposed to Rh123 were used as controls.
Daunorubicin.
The same technique was used for anthracyclin and Rh123. Briefly, cells were stained with 10−6 mol/L of DNR for 30 minutes at 37°C and then resuspended in anthracyclin-free medium for 1 hour at 37°C, either with or without modulators.
Calcein-AM.
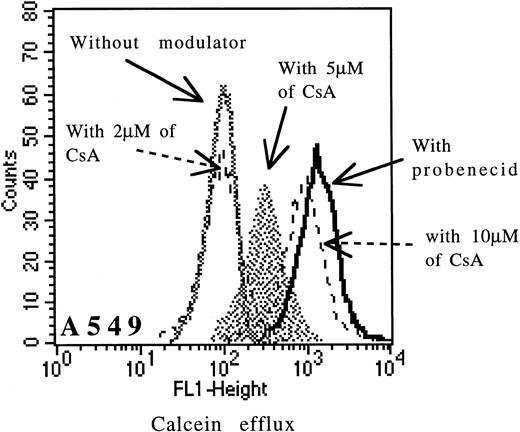
Cells were incubated with 0.1 μmol/L of calcein-AM for 15 minutes at 37°C in RPMI medium with or without modulators. Cells were washed twice in cold PBS and samples were analyzed on FACSORT flow cytometer (uptake of calcein-AM with CsA for MDR1 analysis). Cells were resuspended in calcein-AM–free medium and allowed to efflux for 90 minutes at 37°C either with or without modulators (for MRP analysis). All samples were analyzed without fixation. When we measured nonfluorescent calcein-AM uptake with CsA (Pgp function), we assessed the amount of fluorescent calcein that had been converted from nonfluorescent calcein-AM. Clearly, when the Pgp pump was active, less calcein-AM was retained and less was converted to fluorescent calcein. Similary, once converted, we measured the amount extruded during the efflux assay (MRP function). In our experience, the specificity of CsA was dose-dependent. The specificity of CsA for Pgp was good between 1 and 2 μmol/L and then decreased until 10 μmol/L (Fig 1) for cell lines and fresh leukemic samples. At concentrations greater than 5 μmol/L, CsA might in fact be able to inhibit also MRP function. In the study, we have used 2 μmol/L CsA (a specific concentration of Pgp function) for cell lines and leukemic samples.
Effect of increasing doses of CsA (from 2 μmol/L to 10 μmol/L) on calcein efflux in the A549 cell line.
Effect of increasing doses of CsA (from 2 μmol/L to 10 μmol/L) on calcein efflux in the A549 cell line.
All the data were calculated as the ratio of drug fluorescence with modulator divided by drug fluorescence without modulator after subtraction of the fluorescence of the control. Dead cells were gated out following scatter characteristics.26
Statistical Analysis
Clinical and biological factors were investigated for their influence on remission rate by the χ2 or Fisher's exact tests for binary variables and by the Mann Whitney U test for continuous values. Correlations among levels of expression of continuous values were estimated using the Spearman rank coefficient.
RESULTS
Correlations for 13 Cell Lines Between RT-PCR and Flow Cytometry for MRP, MDR1, and LRP Expression
Results of MDR1, MRP, and LRP expression measured by RT-PCR and flow cytometry are shown in Table 1. Correlation between RT-PCR and flow cytometry was good for MRP and MDR1 genes expression (r = .94, P = .0005 for MRP and r = .96, P < .0001 for MDR1), but not for LRP (r = .57, not significant).
MDR mRNA and MDR Proteins Expression (Measured by RT-PCR and by Flow Cytometry, Respectively), Effect of Probenecid on Calcein Efflux, and Effect of CsA on Calcein-AM Uptake for 13 Cell Lines
| Cell Lines . | mRNA Expression Measured by RT/PCR* (mean ± SD)-151 . | Protein Expression Measured by Flow Cytometry-152 (mean ± SD)-151 . | Effect of Probenecid on Calcein Efflux-153 (mean ± SD)-151 . | Effect of CsA on Calcein-AM Uptake-153 (mean ± SD)-151 . | ||||
|---|---|---|---|---|---|---|---|---|
| MDR1 . | MRP . | LRP . | Pgp . | MRP . | LRP . | |||
| A549 | 0 ± 0 | 1.11 ± 0.39 | 1.25 ± 0.33 | 1 ± 0.03 | 2.88 ± 0.24 | 13.2 ± 6.7 | 1.51 ± 0.08 | 1 ± 0 |
| K562 | 0 ± 0.01 | 0.1 ± 0.03 | 0.12 ± 0.02 | 1 ± 0.06 | 1.73 ± 0.24 | 15.1 ± 3 | 1.31 ± 0.05 | 1 ± 0 |
| HHT30 | 0.64 ± 0.53 | 0.46 ± 0.17 | 0.36 ± 0.02 | 1.4 ± 0.1 | 1.86 ± 0.14 | 12.5 ± 1.6 | 1.33 ± 0.04 | 1 ± 0 |
| HHT100 | 0.93 ± 0.68 | 0.08 ± 0.07 | 1.11 ± 0.14 | 6.8 ± 1.5 | 1.65 ± 0.11 | 11 ± 2.8 | 1.23 ± 0.08 | 4.9 ± 1 |
| HHT200 | 1.32 ± 0.6 | 0.21 ± 0.1 | 0.59 ± 0.31 | 17.2 ± 2.5 | 1.56 ± 0.1 | 11 ± 3.3 | 1.17 ± 0.05 | 16.3 ± 6.1 |
| HHT300 | 2.65 ± 1.06 | 0.33 ± 0.39 | 0.9 ± 0.26 | 71.3 ± 7.2 | 1.84 ± 0.18 | 19.3 ± 4.4 | 1.25 ± 0.11 | 39 ± 7.5 |
| HHT400 | 2.3 ± 0.79 | 0.38 ± 0.1 | 0.11 ± 0.01 | 40.8 ± 8.5 | 1.89 ± 0.19 | 24.2 ± 5.3 | 1.29 ± 0.06 | 24.5 ± 4.2 |
| HL60 | 0 ± 0.01 | 0.59 ± 0.11 | 0.24 ± 0.11 | 1.1 ± 0.2 | 2.18 ± 0.28 | 29.7 ± 7.5 | 1.32 ± 0.15 | 1 ± 0.1 |
| HL60 Pgp | 8.74 ± 2.16 | 0.38 ± 0.03 | 0.32 ± 0.06 | 125.3 ± 18.9 | 1.49 ± 0.22 | 13.8 ± 3.5 | 1.2 ± 0.05 | 46.7 ± 9.9 |
| HL60 MRP | 0.4 ± 0.51 | 2.14 ± 0.24 | 0.02 ± 0.04 | 2.5 ± 0.6 | 5 ± 2.4 | 1.1 ± 0.3 | 1.85 ± 0.13 | 10.9 ± 8.1 |
| CEM | 0.5 ± 0.24 | 0.21 ± 0.05 | 0.01 ± 0.01 | 2.2 ± 0.5 | 1.58 ± 0.4 | 1.2 ± 0.4 | 1.28 ± 0.04 | 1 ± 0.2 |
| CEM/VLB | 3.21 ± 0.97 | 0.26 ± 0.05 | 0.1 ± 0.02 | 52.8 ± 10.4 | 1.65 ± 0.21 | 1.3 ± 0.2 | 1.27 ± 0.06 | 18.5 ± 4.5 |
| U937 | 0 ± 0.02 | 0 ± 0.03 | 0.74 ± 0.27 | 1.2 ± 0.4 | 1.07 ± 0.13 | 12.6 ± 5.9 | 1.17 ± 0.02 | 1 ± 0.1 |
| Cell Lines . | mRNA Expression Measured by RT/PCR* (mean ± SD)-151 . | Protein Expression Measured by Flow Cytometry-152 (mean ± SD)-151 . | Effect of Probenecid on Calcein Efflux-153 (mean ± SD)-151 . | Effect of CsA on Calcein-AM Uptake-153 (mean ± SD)-151 . | ||||
|---|---|---|---|---|---|---|---|---|
| MDR1 . | MRP . | LRP . | Pgp . | MRP . | LRP . | |||
| A549 | 0 ± 0 | 1.11 ± 0.39 | 1.25 ± 0.33 | 1 ± 0.03 | 2.88 ± 0.24 | 13.2 ± 6.7 | 1.51 ± 0.08 | 1 ± 0 |
| K562 | 0 ± 0.01 | 0.1 ± 0.03 | 0.12 ± 0.02 | 1 ± 0.06 | 1.73 ± 0.24 | 15.1 ± 3 | 1.31 ± 0.05 | 1 ± 0 |
| HHT30 | 0.64 ± 0.53 | 0.46 ± 0.17 | 0.36 ± 0.02 | 1.4 ± 0.1 | 1.86 ± 0.14 | 12.5 ± 1.6 | 1.33 ± 0.04 | 1 ± 0 |
| HHT100 | 0.93 ± 0.68 | 0.08 ± 0.07 | 1.11 ± 0.14 | 6.8 ± 1.5 | 1.65 ± 0.11 | 11 ± 2.8 | 1.23 ± 0.08 | 4.9 ± 1 |
| HHT200 | 1.32 ± 0.6 | 0.21 ± 0.1 | 0.59 ± 0.31 | 17.2 ± 2.5 | 1.56 ± 0.1 | 11 ± 3.3 | 1.17 ± 0.05 | 16.3 ± 6.1 |
| HHT300 | 2.65 ± 1.06 | 0.33 ± 0.39 | 0.9 ± 0.26 | 71.3 ± 7.2 | 1.84 ± 0.18 | 19.3 ± 4.4 | 1.25 ± 0.11 | 39 ± 7.5 |
| HHT400 | 2.3 ± 0.79 | 0.38 ± 0.1 | 0.11 ± 0.01 | 40.8 ± 8.5 | 1.89 ± 0.19 | 24.2 ± 5.3 | 1.29 ± 0.06 | 24.5 ± 4.2 |
| HL60 | 0 ± 0.01 | 0.59 ± 0.11 | 0.24 ± 0.11 | 1.1 ± 0.2 | 2.18 ± 0.28 | 29.7 ± 7.5 | 1.32 ± 0.15 | 1 ± 0.1 |
| HL60 Pgp | 8.74 ± 2.16 | 0.38 ± 0.03 | 0.32 ± 0.06 | 125.3 ± 18.9 | 1.49 ± 0.22 | 13.8 ± 3.5 | 1.2 ± 0.05 | 46.7 ± 9.9 |
| HL60 MRP | 0.4 ± 0.51 | 2.14 ± 0.24 | 0.02 ± 0.04 | 2.5 ± 0.6 | 5 ± 2.4 | 1.1 ± 0.3 | 1.85 ± 0.13 | 10.9 ± 8.1 |
| CEM | 0.5 ± 0.24 | 0.21 ± 0.05 | 0.01 ± 0.01 | 2.2 ± 0.5 | 1.58 ± 0.4 | 1.2 ± 0.4 | 1.28 ± 0.04 | 1 ± 0.2 |
| CEM/VLB | 3.21 ± 0.97 | 0.26 ± 0.05 | 0.1 ± 0.02 | 52.8 ± 10.4 | 1.65 ± 0.21 | 1.3 ± 0.2 | 1.27 ± 0.06 | 18.5 ± 4.5 |
| U937 | 0 ± 0.02 | 0 ± 0.03 | 0.74 ± 0.27 | 1.2 ± 0.4 | 1.07 ± 0.13 | 12.6 ± 5.9 | 1.17 ± 0.02 | 1 ± 0.1 |
*The results were given as the ratio of quantities of MDR mRNA product/β2m mRNA product.
The experiments were performed in triplicate, and at three different times.
Protein values were expressed as adjusted for control, ie, as ratio of arithmetic mean fluorescence of UIC2/IgG2A control or MRPm6/IgG1 control or LRP56/IgG2b control.
All the data are ratios of drug fluorescence with modulator divided by drug fluorescence without modulator after subtraction of the fluorescence of the control.
Correlations for 13 Cell Lines Between MRP, MDR1, and LRP Expression and Functional Tests Using Rh123, Daunorubicin, and Calcein-AM
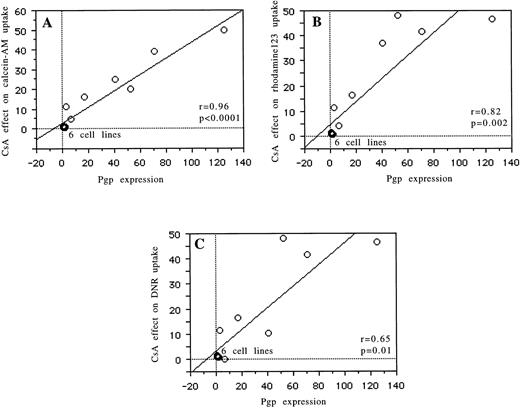
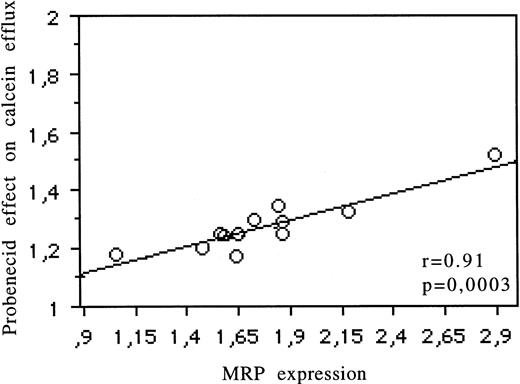
Relations between Pgp expression (UIC2) and the modulatory effects of CsA on the Rh123 (r = .82, P = .002), daunorubicin (r = .65, P = .01), and calcein-AM (r = .96,P < .0001) uptake are reported in Fig 2 and Table 1. The relation between MRP expression (MRPm6) and the modulatory effects of probenecid on calcein efflux is shown in Fig 3 (r = .91,P = .0003) and Table 1. There was no correlation between MRP expression and the effects of probenecid on Rh123 and DNR uptake or efflux or between Pgp expression and the modulatory effect of probenecid on the three probes used. An example is shown in Fig 4. There was no correlation between LRP expression (measured by RT-PCR or flow cytometry) and the effect of one of the modulators with Rh123, DNR, or calcein-AM.
Correlations for 13 cell lines between Pgp expression (measured by flow cytometry) and the modulatory effect of CsA on calcein-AM uptake (A), between Pgp expression and the modulatory effect of CsA on Rh123 uptake (B), and between Pgp expression and the modulatory effect of CsA on daunorubicin uptake (C).
Correlations for 13 cell lines between Pgp expression (measured by flow cytometry) and the modulatory effect of CsA on calcein-AM uptake (A), between Pgp expression and the modulatory effect of CsA on Rh123 uptake (B), and between Pgp expression and the modulatory effect of CsA on daunorubicin uptake (C).
Correlation for 13 cell lines between MRP protein expression (measured by flow cytometry) and the modulatory effect of probenecid on calcein efflux.
Correlation for 13 cell lines between MRP protein expression (measured by flow cytometry) and the modulatory effect of probenecid on calcein efflux.
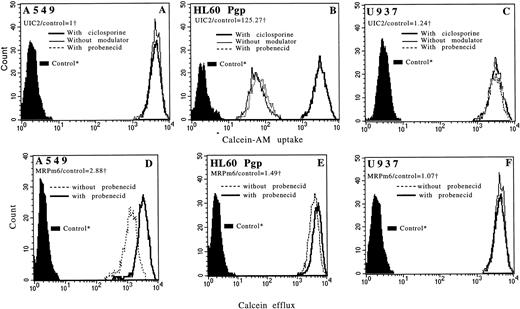
Flow cytometric histograms showing functional incorporation of calcein-AM in A549 (A), in HL60 Pgp (B), and in U937 (C) cell lines with CsA (bold line), with probenecid (dotted line), and without modulator (thin line). For A549 and U937 cell lines, the three histograms are superimposed. Flow cytometric histograms showing functional efflux of fluorescent calcein in A549 (D), in HL60 Pgp (E), and in U937 (F) cell lines with probenecid (bold line) and without probenecid (dotted line). For the U937 cell line, the two histograms are superimposed. Pgp and MRP expressions (measured by flow cytometry) of each cell line are noted. *Controls: autofluorescence of the cells that were not exposed to calcein-AM. †We used the ratio of MDR MoAbs (UIC2 or MRPm6) fluorescence divided by control MoAbs (mouse isotype-matched control MoAbs, IgG2A for UIC2 and IgG1 for MRPm6) fluorescence.
Flow cytometric histograms showing functional incorporation of calcein-AM in A549 (A), in HL60 Pgp (B), and in U937 (C) cell lines with CsA (bold line), with probenecid (dotted line), and without modulator (thin line). For A549 and U937 cell lines, the three histograms are superimposed. Flow cytometric histograms showing functional efflux of fluorescent calcein in A549 (D), in HL60 Pgp (E), and in U937 (F) cell lines with probenecid (bold line) and without probenecid (dotted line). For the U937 cell line, the two histograms are superimposed. Pgp and MRP expressions (measured by flow cytometry) of each cell line are noted. *Controls: autofluorescence of the cells that were not exposed to calcein-AM. †We used the ratio of MDR MoAbs (UIC2 or MRPm6) fluorescence divided by control MoAbs (mouse isotype-matched control MoAbs, IgG2A for UIC2 and IgG1 for MRPm6) fluorescence.
Correlations for Fresh Leukemic Cells Between MRP, MDR1, and LRP Expression and Functional Tests Using Rh123, Daunorubicin, and Calcein-AM
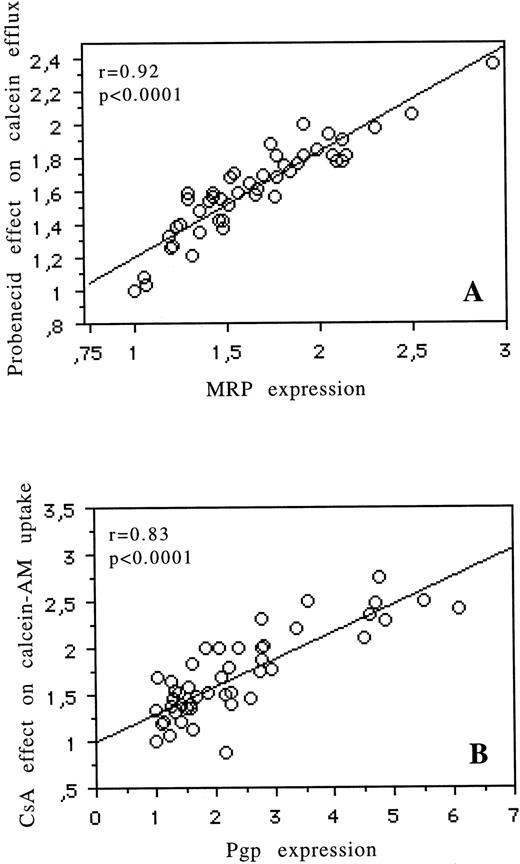
Figure 5A illustrates the relation between MRP expression measured by flow cytometry and the modulatory effect of probenecid on calcein efflux (r = .92, P < .0001). Figure 5B shows the relation of Pgp expression, as measured with UIC2 antibody, with the modulatory effect of CsA on calcein-AM uptake (r = .83, P < .0001). See four examples in Fig 6. For MDR1, we found a correlation between expression of Pgp (measured by flow cytometry) and the modulatory effect of CsA on Rh123 and DNR uptake (r = .77,P = .005 and r = .65, P = .01, respectively). There was no correlation between MRP expression (measured by flow cytometry) and the modulatory effect of probenecid on Rh123 or DNR uptake or efflux (data not shown). There was no correlation between LRP expression (measured by RT-PCR and flow cytometry) and any of the functional tests used (data not shown).
Correlation for fresh leukemic cells between MRP expression (measured by flow cytometry) and the effect of probenecid on calcein efflux (A). Correlation for fresh leukemic cells between Pgp expression (measured by flow cytometry) and the effect of CsA on calcein-AM uptake (B).
Correlation for fresh leukemic cells between MRP expression (measured by flow cytometry) and the effect of probenecid on calcein efflux (A). Correlation for fresh leukemic cells between Pgp expression (measured by flow cytometry) and the effect of CsA on calcein-AM uptake (B).
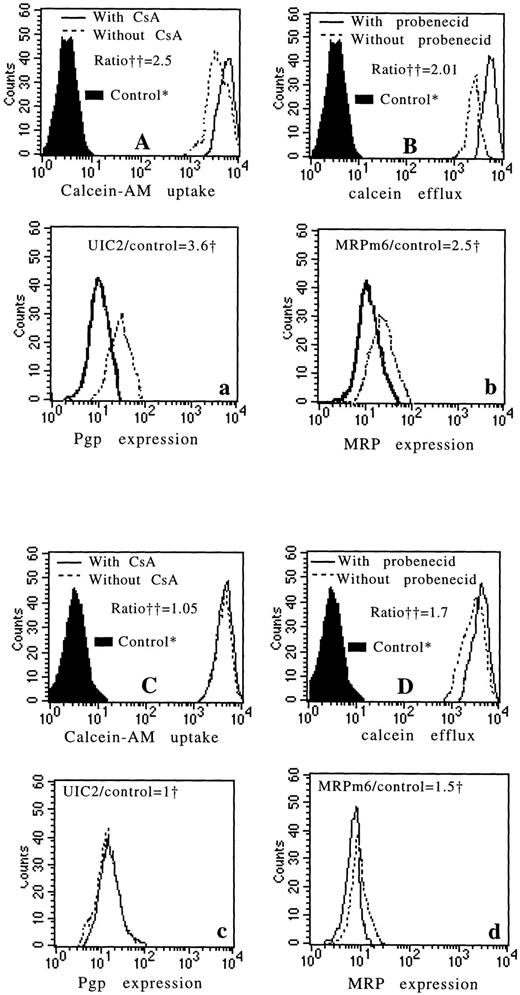
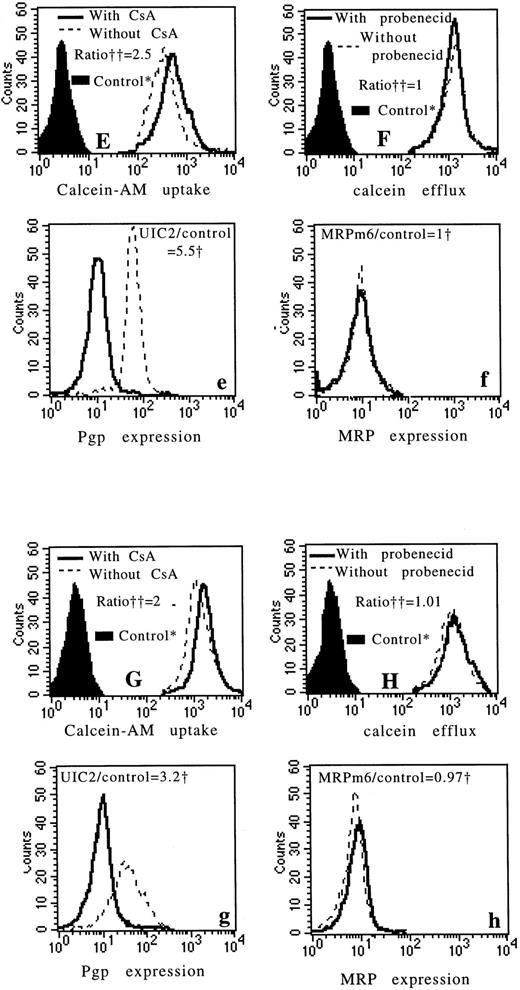
Four examples of fresh leukemic samples. The first example is a sample with high Pgp expression (a) and with high MRP expression (b) (measured by flow cytometry). There is an important modulatory effect of CsA on calcein-AM uptake (A) and an important effect of probenecid on calcein efflux (B). The second example is a sample with no Pgp expression (c) and with a weak MRP expression (d). There is no modulatory effect of CsA on calcein-AM uptake (C) and a little modulatory effect of probenecid on calcein efflux (D). The third and fourth examples are samples with high Pgp expression (e and g) and no MRP expression (f and h). In these two examples, there is an important modulatory effect of CsA on calcein-AM uptake (E and G) and no modulatory effect of probenecid on calcein efflux (F and H). *Controls: autofluorescence of the cells that were not exposed to calcein-AM. †We used the ratio of MDR MoAbs (UIC2 or MRPm6) fluorescence divided by control MoAbs (mouse isotype-matched control MoAbs, IgG2A for UIC2 and IgG1 for MRPm6) fluorescence. ††All the data were calculated as the ratio of drug fluorescence with modulator divided by drug fluorescence without modulator after subtraction of the fluorescence of the control.
Four examples of fresh leukemic samples. The first example is a sample with high Pgp expression (a) and with high MRP expression (b) (measured by flow cytometry). There is an important modulatory effect of CsA on calcein-AM uptake (A) and an important effect of probenecid on calcein efflux (B). The second example is a sample with no Pgp expression (c) and with a weak MRP expression (d). There is no modulatory effect of CsA on calcein-AM uptake (C) and a little modulatory effect of probenecid on calcein efflux (D). The third and fourth examples are samples with high Pgp expression (e and g) and no MRP expression (f and h). In these two examples, there is an important modulatory effect of CsA on calcein-AM uptake (E and G) and no modulatory effect of probenecid on calcein efflux (F and H). *Controls: autofluorescence of the cells that were not exposed to calcein-AM. †We used the ratio of MDR MoAbs (UIC2 or MRPm6) fluorescence divided by control MoAbs (mouse isotype-matched control MoAbs, IgG2A for UIC2 and IgG1 for MRPm6) fluorescence. ††All the data were calculated as the ratio of drug fluorescence with modulator divided by drug fluorescence without modulator after subtraction of the fluorescence of the control.
MRP, MDR1, and LRP Expression in Fresh Leukemic Samples and Comparison With Clinical and Biological Parameters
There was no correlation between the expression of the three proteins studied, except a weak correlation between LRP and MRP proteins expression (r = .4, P = .01); but there was no correlation between LRP, MRP, and MDR1 when RT-PCR was used.
CD34+ leukemic cells expressed more Pgp and less MRP than CD34− leukemic cells (CD34+ [2.26 ± 0.79] v CD34− [1.46 ± 0.53], P= .03 for Pgp; CD34+ [1.4 ± 0.29] vCD34− [1.77 ± 0.40], P = .004 for MRP). We found no correlation between LRP and CD34. In the same way, the modulatory effect of CsA on calcein-AM uptake (which measures Pgp function) was more important in CD34+ leukemia than in CD34− leukemia (2.01 ± 0.49 v 1.69 ± 0.41, P = .02, respectively). On the other hand, the modulatory effect of probenecid on calcein efflux (which measures MRP function) was more important in CD34− leukemia than in CD34+ leukemia (1.70 ± 0.27 v 1.26 ± 0.22, P = .02, respectively). The same correlations were observed when we measured CD34 expression by a continuous D value function (data not shown). Patients older than 60 years of age expressed more Pgp, but not more MRP and LRP than younger patients (2.21 ± 0.45 v 1.47 ± 1.19 for Pgp).
Prognostic Factors for Response to Therapy
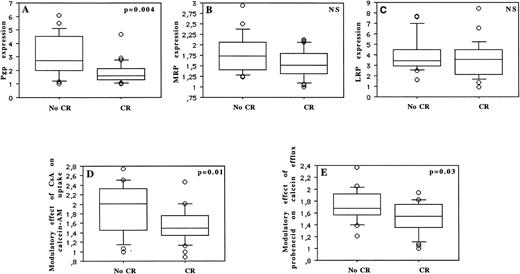
Thirty-two (60%) of 53 patients achieved a CR. Patients were treated with two similar induction treatment protocols (see the Materials and Methods). The prognostic factors for achievement of CR are summarized in Table 2 and Fig 7. Age was the only predictive clinical parameter for achievement of CR (P = .05). Among laboratory parameters, CR rate was significantly associated with CD34 and Pgp expression, with cytogenetic, with functional uptake of calcein-AM, and with functional efflux of calcein. CR rate significantly decreased with increasing Pgp expression (P = .004), with increasing expression of CD34 (P = .01), and with unfavorable cytogenetics (P = .02). But CR rate was not associated with MRP (P = .07) or LRP (P = .39) expression. CR rate was also significantly worse in patients with an important modulatory effect of CsA on calcein-AM uptake (which measures Pgp function) and with an important modulatory effect of probenecid on calcein efflux (which analyzes MRP function) (P = .01 and P = .03, respectively).
Prognostic Factors for Achievement of CR for 53 AML Patients
| Parameters . | Patients Who Achieved CR (32 patients) . | Patients Who Did Not Achieve CR (21 patients) . | P Value . |
|---|---|---|---|
| MDR parameters | |||
| Pgp (measured by flow cytometry) | 1.81 ± 0.79 | 3.01 ± 1.51 | .004 |
| Modulatory effect of CsA on calcein-AM uptake | 1.53 ± 0.34 | 1.90 ± 0.51 | .01 |
| Modulatory effect of CsA on Rh 123 uptake* | 1.51 ± 0.95 | 2.21 ± 1.12 | .02 |
| Modulatory effect of CsA on daunorubicin uptake* | 1.10 ± 0.52 | 1.98 ± 0.85 | .04 |
| MRP (measured by flow cytometry) | 1.55 ± 0.33 | 1.78 ± 0.45 | NS |
| Modulatory effect of probenecid on calcein efflux | 1.53 ± 0.25 | 1.73 ± 0.27 | .03 |
| LRP (measured by flow cytometry) | 3.54 ± 1.71 | 4.05 ± 1.66 | NS |
| Other parameters | |||
| Age (yr) | 52 ± 19 | 59 ± 19 | .05 |
| CD34+ patients (%)† | 52% | 85% | .01 |
| Unfavorable cytogenetic | 2% | 28% | .02 |
| Parameters . | Patients Who Achieved CR (32 patients) . | Patients Who Did Not Achieve CR (21 patients) . | P Value . |
|---|---|---|---|
| MDR parameters | |||
| Pgp (measured by flow cytometry) | 1.81 ± 0.79 | 3.01 ± 1.51 | .004 |
| Modulatory effect of CsA on calcein-AM uptake | 1.53 ± 0.34 | 1.90 ± 0.51 | .01 |
| Modulatory effect of CsA on Rh 123 uptake* | 1.51 ± 0.95 | 2.21 ± 1.12 | .02 |
| Modulatory effect of CsA on daunorubicin uptake* | 1.10 ± 0.52 | 1.98 ± 0.85 | .04 |
| MRP (measured by flow cytometry) | 1.55 ± 0.33 | 1.78 ± 0.45 | NS |
| Modulatory effect of probenecid on calcein efflux | 1.53 ± 0.25 | 1.73 ± 0.27 | .03 |
| LRP (measured by flow cytometry) | 3.54 ± 1.71 | 4.05 ± 1.66 | NS |
| Other parameters | |||
| Age (yr) | 52 ± 19 | 59 ± 19 | .05 |
| CD34+ patients (%)† | 52% | 85% | .01 |
| Unfavorable cytogenetic | 2% | 28% | .02 |
*Only 49 patients were tested.
Samples were considered positive for CD34 when more than 20% of viable cells were stained with CD34 antibody in excess of the negative control.
MDR prognostic factors with or without influence on the achievement of CR: Pgp expression (A), MRP expression (B), and LRP expression (C) (measured by flow cytometry), modulatory effect of CsA on calcein-AM uptake (D), and modulatory effect of probenecid on calcein efflux (E).
MDR prognostic factors with or without influence on the achievement of CR: Pgp expression (A), MRP expression (B), and LRP expression (C) (measured by flow cytometry), modulatory effect of CsA on calcein-AM uptake (D), and modulatory effect of probenecid on calcein efflux (E).
DISCUSSION
In different tumour cell lines with various levels of MDR proteins (MRP, MDR1, and LRP), we have confirmed that calcein-AM was a specific and sensitive probe for MRP and Pgp functions.11,13,27-29Probenecid, a specific and effective chemosensitizer of MRP,25 was used to modulate the calcein efflux and CsA was used to modulate the calcein-AM uptake. Although calcein-AM was actively extruded by MRP and Pgp, calcein-AM with or without CsA (at 2 μmol/L) provided in cell lines a functional test as specific and sensitive as Rh123 with or without CsA, the most specific and sensitive Pgp functional test.14 22 All our results concerning calcein-AM uptake were calculated as the ratio of calcein-AM uptake with CsA (a specific modulator of Pgp, at 2 μmol/L) divided by calcein-AM uptake without CsA. For that reason, we analyzed only Pgp function and no MRP function. These results encouraged us to test calcein-AM in fresh myeloid leukemic cells.
In myeloid leukemic cells, we showed that calcein-AM (with or without CsA at 2 μmol/L) provided also a sensitive and specific functional test that was strongly correlated with Pgp expression. This test was as specific and sensitive as functional tests using Rh123 and DNR in AML samples. Pgp expression and the modulatory effect of CsA on calcein-AM uptake were correlated with achievement of CR, the presence of CD34 and age. These findings, and particularly the fact that Pgp might limit the effectiveness of chemotherapy in CD34+ AML patients, were already noted.5 30
The functional test using calcein-AM (efflux of calcein and effect of probenecid, a specific modulator of MRP, on calcein efflux) was strongly correlated with MRP expression in AML. Therefore, calcein-AM after cleavage by cellular esterase to fluorescent calcein was a specific and sensitive MRP probe. So, calcein-AM might be used to probe specifically both MRP and Pgp activities. Singularly, expression of MRP measured by RT-PCR and flow cytometry was not a prognostic factor for achievement of CR, but probenecid effect on calcein efflux (which measures MRP function) was predictive for achievement of CR in our data. Several studies support the hypothesis that MRP functions as a glutathione S-conjugate carrier.31,32 Therefore, the MRP function is dependent on glutathione level. This may explain a dissociation between MRP expression, which was not a prognostic factor, and MRP function, which was a prognostic factor for achievement of CR. Nevertheless, in our study, there was a striking correlation between these two parameters (MRP expression and function). A few patients with high expression of MRP had a weak effect of probenecid on calcein efflux. Perhaps these results may explain this small difference between clinical outcome and MRP expression correlation (P = .07) and between clinical outcome and MRP function correlation (P = .03). This emphasizes also the facts that the functional test (with calcein-AM with or without probenecid) is essential in the understanding of the MRP role and that MRP contributes to MDR mechanism in AML. As for Pgp, it is important to look for dissociation between protein and function.6 In addition to inhibition of calcein efflux, probenecid may be associated with an increased accumulation of daunorubicin and vincristine and with the correction of the altered distribution of daunorubicin.25 The concentrations of probenecid (from 0.01 to 2 mmol/L) that reverse MRP function are clinically achievable in vivo,25 without major toxicity. These results suggest that probenecid is a good modulator for MRP activity and a potential candidate for clinical use to reverse MDR-associated MRP.
MRP was overexpressed in CD34− AML when compared with CD34+ AML. These findings indicate that the MRP phenotype may limit the effectiveness of chemotherapy in CD34−AML and not in CD34+ AML. In accordance with this result, we and others have shown that the level of MRP expression in CD34+ normal hematopoietic cells and CD34+leukemic cells are similar to those observed in sensitive cell lines,19,33 but that MRP is overexpressed in more mature cells.19 33
In our study, LRP expression (measured by RT-PCR and flow cytometry) was not predictive for achievement of CR. In another study,10 LRP was found to be a prognostic factor for achievement of CR, but they used only one technique for LRP detection (immunocytochemistry). This could be not enough, compared with the recommendation given for Pgp/MDR1 by the Memphis Consensus meeting22 asking for two different techniques. This rule probably applies to other MDR mechanisms. We have found no correlation between LRP expression (measured by RT-PCR or flow cytometry) and the three functional tests used. Another study34 has shown an intracellular decrease of daunorubicin in LRP-positive AML (without MDR1 or MRP expression), but the responsibility of LRP in intracellular decrease of daunorubicin was not proved, because there were no LRP-specific modulators used. In our study, no significant associations were observed between LRP and other clinical or biological parameters (age, cytogenetic, CD34, and WBC).
In conclusion, (1) calcein-AM may be used in fresh leukemic cells to probe specifically both MRP and Pgp activities. (2) With these functional assays the role of Pgp have been confirmed in AML. (3) Overexpression of MRP mRNA or protein is not a prognostic factor in AML, but the activity of MRP is a prognostic factor for achievement of CR. (4) Therefore, a functional test (with calcein-AM with or without probenecid) is an essential tool for one to understand the MRP role in AML.
ACKNOWLEDGMENT
The authors are grateful to Hélène Simon for the linguistic review of our manuscript.
Supported in part by a grant from ARC (Grant No. 6078).
Address reprint requests to Ollivier Legrand, MD, Hôpital Hôtel-Dieu, 1 place du Parvis Notre Dame, Service d'Hématologie Clinique, Professeur Zittoun, 75 181 Paris Cedex 04, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal