Abstract
We have studied the cellular and molecular responses to long-term hydroxyurea (HU) treatment in 29 severely affected young patients with sickle cell disease (mean age, 10.9 ± 4.1 years). Patients received HU at 20 mg/kg/d on 4 consecutive days per week initially, with a monthly escalated dose avoiding marrow-toxicity (mean steady-state dose, 34.2 ± 4.6 mg/kg/d) for 12 to 36 months (mean duration, 22 months). The studied parameters were hemoglobin F (HbF), F reticulocytes (F retics), F cells, the amount of HbF per F cell (F/F cell), polymer tendency at 40% and 70% oxygen saturation, and hemolysis. Initial HbF (Fi) was dispersed (from 0.85% to 13.9%). HbF increased in all patients but 1. HbF at maximal response (Fmax) reached a sustained level varying from a 1.5-fold to a 16-fold Fi after a variable delay (6 to 24 months). Fmax was not related to HU dosage, but ▵F (Fmax − Fi) was strongly correlated to ▵MCV (MCVmax − MCVi). HbF increase resulted from the increase of both F cells and F/F cell. In this rather short series, Fi and Fmax were not significantly associated with age, gender, or β-globin haplotype. Neither Fmax nor ▵F was related to bone marrow reserve, as measured by baseline reticulocyte or neutrophil counts. However, Fmax was highly dependent on Fi. When patients are individualized into three groups according to Fmax (group 1, Fmax >20% [12 patients]; group 2, 10% < Fmax < 20% [11 patients]; group 3, Fmax <10% [5 patients]), Fi is significantly different between groups, being the highest in group 1. In addition, the best responders (group 1) were significantly different from patients in the two other groups with higher levels of total hemoglobin, decreased bilirubin, and decreased polymer tendency.
SICKLING OF RED BLOOD cells in sickle cell disease (SCD) is the consequence of a single amino acid substitution in the β-globin chain (β6Glu→Val) that is responsible for the polymerization of the abnormal hemoglobin S (HbS) upon deoxygenation.1-3 The extent of polymer formation at any oxygen saturation is primarily dependent on the total intracellular hemoglobin concentration and on the respective percentages of S and non-S hemoglobins within the cell.4,5 Non-S hemoglobins, such as hemoglobins A (HbA), A2 (HbA2), or F (HbF), influence the polymerization process, because they reduce the intracellular HbS concentration and because mixed hybrids with HbS and HbF (or A2) do not enter the polymer.5-9 This last property makes HbF the most potent inhibitor of deoxyHbS polymerization.
Accordingly, many approaches to develop therapies for SCD10have focused on preventing polymerization by the use of pharmacological agents that increase the production of HbF.11 Among the various drugs proposed within the last years to improve the clinical course of SCD, hydroxyurea (HU)12 seems to be the most effective and has now been tried in large multicentric series of adult patients.13 After a double-blind trial enrolling 299 adults patients, it appeared that HU significantly reduced the frequency of painful crises, acute chest syndrome episodes, and blood transfusion requirements. These promising results in adult patients, associated with the absence of toxic effects or malignancies observed during long-term HU administration in a series of young patients with cyanotic congenital heart disease,14 encouraged us to investigate this treatment in young SCD patients. We present here data concerning a phase II therapeutic assay performed over 3 years in a cohort of 29 young SCD patients to appreciate if HU could stimulate HbF production without inducing clinical or hematological toxicity. This cohort included a large majority of children and teenagers belonging to a first generation of African immigrants, usually of homogeneous ethnic background. Beside the clinical follow-up, which supported the efficacy of HU in reducing painful events,15 attention was focused on the evolution of HbF expression and the related cellular parameters, F reticulocytes (F retics) and F cells, measured at 3-month intervals, and the expected effects of those variables on polymer formation and rate of hemolysis. In addition, we studied different factors that might predict the response to HU to guide this therapy.
MATERIALS AND METHODS
Patients
Twenty-nine young homozygous SCD patients (21 males and 8 females) were selected for this study from the clinics of centers belonging to the French Study Group on Sickle Cell Disease. The age range was 4 to 19 years (mean age, 10.9 ± 4.1 years). One child was less than 5 years of age, 10 were between 5 and 10 years of age, 10 were between 10 and 15 years of age, and 8 were more than 15 years of age. Diagnosis of SCD was established for each individual on the basis of hemoglobin electrophoresis and family studies. To be eligible, patients had to have reported at least three painful crises necessitating an hospitalization in the precedent year. Exclusion criteria were renal insufficiency (creatinine clearance <120 mL/min/1.73 m2), hepatic insufficiency (ALAT >5N, or chronic hepatic disease), iron deficiency or current iron supplementation, hypersplenism, human immunodeficiency virus infection, or a past history of frequent and severe infections. Patients for whom a monthly follow-up seemed difficult to ensure were excluded. The necessity to avoid pregnancy was explained to older girls, and contraceptive pills were prescribed when necessary. The storage of frozen sperm was proposed to mature boys. Patients were treated with HU for 12 to 36 months (mean duration of the treatment, 22 months) according to a previously published protocol16: 20 mg/kg/d on 4 consecutive days per week initially, with a monthly increase of 5 mg/kg/d in absence of myelotoxicity (maximum, 40 mg/kg/d). Myelotoxicity was defined as a 20% decrease in hemoglobin levels, less than 2.5 × 109 neutrophils/L, less than 150 × 109platelets/L, or less than 100 × 109 reticulocytes/L. Temporary cessation of treatment was prescribed if reticulocytes decreased below 50 × 109/L, neutrophils below 1.5 × 109/L, or platelets below 100 × 109/L, until normalization of the parameters occurred. Stopping treatment was prescribed if the ALAT value had increased twofold or if the creatinine value was more than 30% of the initial value. The protocol was approved by the Ethics Committee of the Hôpital Necker-Enfants Malades. Fully informed parents had to give their written consent. The patients were regularly observed and blood samples were obtained before the administration of HU and every month during the treatment to supervise the good tolerance to the treatment.
Laboratory Studies
The quarterly biological follow-up included a complete blood count and the determination of the erythrocyte indices using the H*1 hematology analyzer (Bayer Corp, Tarrytown, NY), a reticulocyte count performed after methylene blue supravital stain, and the determination of HbF, F retics, and F cells.
HbF was quantified by a high performance ion-exchange liquid chromatography (HPLC) procedure.17 F retics and F cells were determined by an immunofluorescent assay, as previously described.18 The amount of HbF per F cell (F/F cell) was calculated using the following ratio: (mean cell hemoglobin content × HbF)/F cells. The tendency of F cells and non-F cells toward intracellular polymerization was calculated from the total intracellular hemoglobin concentration and the percentages of HbS, HbF, and HbA2 at 40% and 70% oxygen saturation values, which correspond to the physiologically relevant region of oxygen saturation.19-21 Total bilirubin was measured. The β-globin gene cluster haplotype and the α-gene number were assessed once during the initial weeks according to previously reported methods.22
Statistical Analysis
Because the sample size was too small to apply parametric tests, nonparametric statistical procedures were used, allowing us to compare groups of patients without any assumption on the distribution of variables. Comparisons of parameters, before HU and at the time of HbF maximal response, were performed by using the Wilcoxon signed test, with a level of significance (P) set at .05 (two-tailed formulation). Comparisons between more than two groups of patients, either at baseline or at the time of HbF maximal response, were performed by using the Kruskal-Wallis test, with a level of significance (P) set at .05 (two-tailed formulation). Comparisons between males and females for pretreatment HbF and maximal HbF were performed using the Mann-Whitney test. Different factors reported to be important in determining HbF levels were analyzed by simple linear regression. Regression coefficients and P values are provided in the Results.
RESULTS
Patients
Follow-up varied from 12 to 36 months (mean duration, 22 months). Four cessations of treatment were observed. HU was discontinued in 2 children considered to have failed to respond to treatment: in the first case after 6 months and in the second case after 2 years, although improvement had been initially observed. One child moved from the area. A girl developed a systemic lupus syndrome 1 year after having been included in the protocol and HU therapy was stopped. In our series, 20 patients were homozygous for one of the three common β-globin haplotypes (Senegal [n = 3], CAR or Bantu [n = 8], and Benin [n = 9]), 3 were heterozygous CAR-Benin, 1 was atypical, and 5 were undetermined. Determination of α-globin genotype was performed in 19 patients: 14 had 4 α-genes, 4 had 3 α-genes, and 1 had 2 α-genes. Based on the initial F retics, 6 of the 8 girls could be classified as LL for the X-linked FCP locus23; only 1 was HL and 1 was HH. Initial F retics were available for 11 boys, and all were L.
Hematological Data
Table 1 shows the mean hematological response of the cohort before HU, yearly during the treatment, and at the time of HbF maximal response. At that time, an increase in hemoglobin level of more than 1 g/dL was observed in 11 patients. Overall, the increase of the mean hemoglobin level was significant, from 8.4 ± 1.2 before HU to 8.9 ± 1.1 g/dL (P= .03). This increase was associated with an increase in the mean cell volume (MCV) from 84.5 ± 10.1 to 101.4 ± 13.4 fL (P < .001). MCV values were not correlated to HU dosage. The reticulocyte count decreased significantly from 417 ± 214 to 229 ± 129 × 109/L (P < .001). A significant decrease was observed in the neutrophil count from 7.2 ± 2.9 to 4.2 ± 2.6 × 109/L (P < .0005). The platelet count also decreased from 393 ± 170 to 340 ± 114 × 109/L, but this decrease was not significant.
Laboratory Values During Treatment With HU
| Parameters . | Pretreatment Value (n = 29) . | HbF Maximal Response (n = 28) . | Year 1 (n = 28) . | Year 2 (n = 20) . | Year 3 (n = 8) . |
|---|---|---|---|---|---|
| Total hemoglobin (g/dL) | 8.4 ± 1.2 | 8.9 ± 1.1* | 9.0 ± 1.2 | 9.1 ± 0.9 | 9.2 ± 0.6 |
| MCV (fL) | 84.5 ± 10.1 | 101.4 ± 13.4* | 101.6 ± 13.6 | 101.8 ± 15.9 | 102.4 ± 14.8 |
| Neutrophils (×109/L) | 7.2 ± 2.9 | 4.2 ± 2.6* | 4.3 ± 2.9 | 5.2 ± 2.3 | 4.7 ± 2.9 |
| Platelets (×109/L) | 393 ± 170 | 340 ± 114 | 338 ± 117 | 293 ± 175 | 340 ± 109 |
| Reticulocytes (×109/L) | 417 ± 214 | 229 ± 129* | 235 ± 109 | 266 ± 140 | 192 ± 73 |
| HbF (%) | 4.0 ± 3.4 | 17.3 ± 8.5* | 13.9 ± 7.4 | 13.0 ± 9.4 | 22.5 ± 2.8 |
| F reticulocytes (%) | 7.1 ± 3.9 | 21.7 ± 10.7* | 24.6 ± 15.0 | 26.0 ± 16.9 | 32.7 ± 35.1 |
| F cells (%) | 24.4 ± 14.3 | 59.9 ± 19.4* | 57.0 ± 23.2 | 54.2 ± 22.1 | 55.5 ± 27.3 |
| F/F cell (pg) | 4.1 ± 1.3 | 8.7 ± 2.7* | 8.4 ± 2.3 | 6.9 ± 3.3 | 11.8 ± 1.7 |
| Parameters . | Pretreatment Value (n = 29) . | HbF Maximal Response (n = 28) . | Year 1 (n = 28) . | Year 2 (n = 20) . | Year 3 (n = 8) . |
|---|---|---|---|---|---|
| Total hemoglobin (g/dL) | 8.4 ± 1.2 | 8.9 ± 1.1* | 9.0 ± 1.2 | 9.1 ± 0.9 | 9.2 ± 0.6 |
| MCV (fL) | 84.5 ± 10.1 | 101.4 ± 13.4* | 101.6 ± 13.6 | 101.8 ± 15.9 | 102.4 ± 14.8 |
| Neutrophils (×109/L) | 7.2 ± 2.9 | 4.2 ± 2.6* | 4.3 ± 2.9 | 5.2 ± 2.3 | 4.7 ± 2.9 |
| Platelets (×109/L) | 393 ± 170 | 340 ± 114 | 338 ± 117 | 293 ± 175 | 340 ± 109 |
| Reticulocytes (×109/L) | 417 ± 214 | 229 ± 129* | 235 ± 109 | 266 ± 140 | 192 ± 73 |
| HbF (%) | 4.0 ± 3.4 | 17.3 ± 8.5* | 13.9 ± 7.4 | 13.0 ± 9.4 | 22.5 ± 2.8 |
| F reticulocytes (%) | 7.1 ± 3.9 | 21.7 ± 10.7* | 24.6 ± 15.0 | 26.0 ± 16.9 | 32.7 ± 35.1 |
| F cells (%) | 24.4 ± 14.3 | 59.9 ± 19.4* | 57.0 ± 23.2 | 54.2 ± 22.1 | 55.5 ± 27.3 |
| F/F cell (pg) | 4.1 ± 1.3 | 8.7 ± 2.7* | 8.4 ± 2.3 | 6.9 ± 3.3 | 11.8 ± 1.7 |
*P < .05 from Wilcoxon signed test, comparing the pretreatment values with values at HbF maximal response.
HbF Production
Variations in HbF.
Table 1 summarizes the mean HbF response of the cohort before HU, yearly during the treatment, and at the time of HbF maximal response.
Initial HbF levels (Fi) before treatment were dispersed (from 0.85% to 13.9%). Fi values were highly correlated to MCV (r = .57,P = .002). Female patients (n = 8) had a higher mean Fi than males (n = 21: 6.1% ± 5.1% v 3.5% ± 2.1%), but this difference was not significant. Mean Fi values for Senegalese, Benin, and CAR homozygous patients were 5.5% ± 0.8%, 3.3% ± 1.6%, and 4.3% ± 4.1%, respectively, but these differences were not significant. In this series, Fi was not correlated to age.
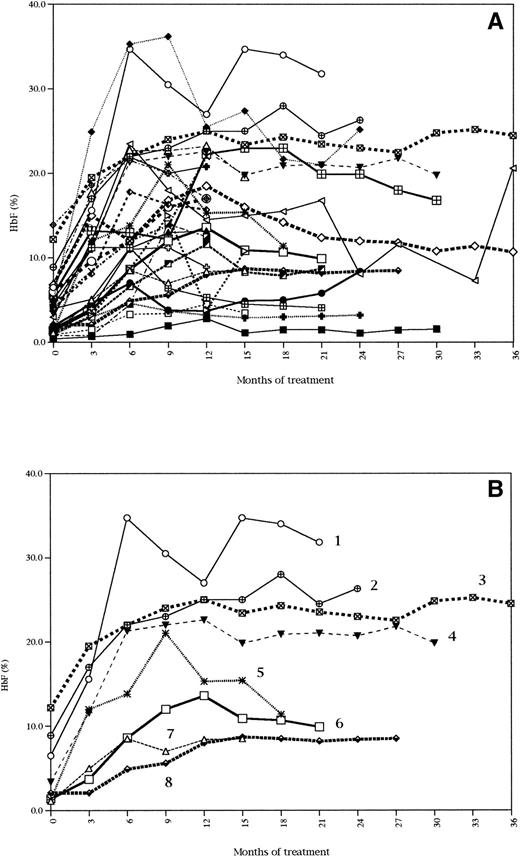
An increase in HbF was consistently observed in all the patients except 1 (treatment was stopped for this patient). The variations of HbF percentages are presented in Fig 1. Individual data for all the patients are represented in Fig 1A, which shows the great variability of response from patient to patient. To better illustrate the various types of response described below, eight of these curves were selected and are shown in Fig 1B.
HbF response during treatment with HU. Data for all the patients (A). Data for 8 selected patients representative of the various types of response to HU (B).
HbF response during treatment with HU. Data for all the patients (A). Data for 8 selected patients representative of the various types of response to HU (B).
At the time of the patients' HbF maximal response, HbF reached an absolute level (Fmax) varying from 3.3% to 36.2% (patient no. 1 in Fig 1B reached an Fmax of 34.7%). From patient to patient, this represented a variable increase of Fi, from 1.5-fold (patient no. 3 in Fig 1B) to 16-fold (patient no. 5 in Fig 1B). Eighteen patients achieved at least a twofold increase of Fi at 3 months and 8 patients at 6 months. Two patients achieved a twofold increase after 6 months, with their HbF increase being 1.5- and 1.8-fold, respectively, at 6 months (patient no. 3 in Fig 1B). The delay to reach Fmax was variable, from 6 months (patients no. 7 and 4 in Fig 1B) to 18 months (patient no. 2 in Fig 1B) and even 24 months (1 case) of treatment. For that matter, patients no. 7 and 8 are interesting to compare, because they reached the same sustained Fmax value, but at very different times (6 and 15 months, respectively). In some cases, HbF values reached a plateau after 6 months (patient no. 7 in Fig 1B) or were still increasing after 12 months (patient no. 2 in Fig 1B). The slope of HbF increase was not predictive of Fmax (see patients no. 6 and 7) and was not correlated to age. Once the peak of maximal HbF value was reached, most of the time, HbF stabilized at a lower level, except for 5 patients for whom the maximal HbF value was sustained (patients no. 8, 7, 4, 3, and 2 in Fig 1B).
Fmax was not correlated to the maximum HU dosage, but it was correlated to MCVmax (r = .56, P = .002). Similarly, ΔF (Fmax − Fi) was strongly correlated to ΔMCV (MCVmax − MCVi) (r = .62, P = .0007). Fmax was not related to reticulocyte, neutrophil, or platelet initial values, but it was highly correlated to initial F retics (r = .63, P = .003). It was not correlated to age, gender, or β-globin haplotypes. Because the vast majority of the patients belonged to the L (boys) or LL (girls) phenotype concerning the FCP locus, no conclusion can be drawn from our series as to the potential influence of this locus on HbF response. Similarly, the number of patients for whom the α-globin gene status was determined was too small to conclude on its eventual influence.
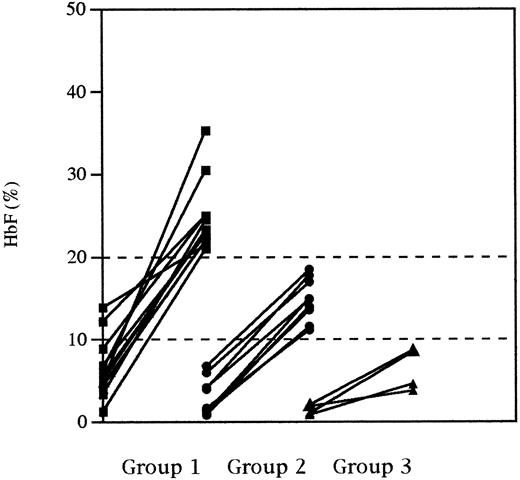
We chose arbitrarily 10% and 20% Fmax levels to individualize three groups of patients: group 1, whose Fmax were greater than 20%; group 2, whose Fmax were greater than 10% but lower than 20%; and group 3, whose Fmax remained less than 10%. HbF variation from Fi to Fmax for each individual in these three groups is shown in Fig 2. Noticeably, the 20% level (group 1) was reached by 12 patients in a delay varying from 6 to 15 months. Nine of them had a pretreatment HbF value greater than 4%. The 10% level (group 2) was reached by 11 patients. Five patients did not reach the 10% level (group 3) and 4 of them had an initial HbF value less than 2%. Comparisons between the three groups were performed for all the parameters that were found to be significantly different before HU and at the time of Fmax on the whole cohort analysis (Table 2). Pretreatment hemoglobin levels, MCV values, and reticulocyte counts were not significantly different from one group to another. However, Fi values were significantly different between the three groups (P = .005). At the time of HbF maximal response, significant differences were observed between the three groups for hemoglobin levels (P = .004) and MCV values (P = .03). From pretreatment to the time of HbF maximal response, the reticulocyte count decreased by 55%, 37%, and 25% for groups 1, 2, and 3, respectively, but these differences are not significant. Within each group, significant variations were observed from pretreatment to the time of HbF maximal response for total hemoglobin, MCV, reticulocytes, and HbF for group 1; MCV, reticulocytes, and HbF for group 2; and MCV and HbF for group 3 (see Table 2 for details). Considering the whole series, the average bilirubin level decreased from a pretreatment value of 40.4 ± 24.8 mg/L to 33.3 ± 20.2 mg/L at the time of HbF maximal response (P = .07). But when this parameter was analyzed within each of the three groups, a significant decrease was only observed for group 1 (Table 2).
HbF response (from initial HbF to maximal HbF) in patients divided in three groups, according to a 10% and 20% maximal HbF level.
HbF response (from initial HbF to maximal HbF) in patients divided in three groups, according to a 10% and 20% maximal HbF level.
Laboratory Values in Groups of Patients No. 1, 2, and 3
| Parameters . | Pretreatment Values . | P* . | Values at HbF Maximal Response . | P* . | ||||
|---|---|---|---|---|---|---|---|---|
| Group 1 (n = 12) . | Group 2 (n = 11) . | Group 3 (n = 5) . | Group 1 (n = 12) . | Group 2 (n = 11) . | Group 3 (n = 5) . | |||
| Hemoglobin g/dL | 8.4 ± 1.3 | 7.9 ± 1.0 | 8.9 ± 1.4 | NS | 9.7 ± 0.9† | 8.2 ± 0.7 | 8.7 ± 1.2 | .004 |
| MCV (fL) | 88.3 ± 12.3 | 82.9 ± 7.3 | 76.3 ± 9.7 | NS | 108.6 ± 7.7† | 100.7 ± 10.3† | 88.9 ± 15.5† | .03 |
| Neutrophils (×109/L) | 7.6 ± 2.4 | 7.0 ± 3.6 | 7.0 ± 2.9 | NS | 4.0 ± 2.5† | 3.6 ± 1.9† | 5.1 ± 2.4 | NS |
| Reticulocytes (×109/L) | 443 ± 213 | 450 ± 247 | 289 ± 132 | NS | 197 ± 89† | 284 ± 162† | 219 ± 68 | NS |
| HbF (%) | 6.3 ± 3.7 | 3.3 ± 2.1 | 1.4 ± 0.6 | .005 | 25.1 ± 5.0† | 14.4 ± 2.6† | 5.8 ± 2.6† | <.0001 |
| F reticulocytes (%) | 10.8 ± 6.8 | 5.3 ± 2.4 | 4.5 ± 1.0 | .01 | 34.6 ± 14.2† | 18.5 ± 5.3† | 10.6 ± 4.7 | .0005 |
| F cells (%) | 33.6 ± 14.5 | 20.1 ± 7.8 | 13.2 ± 6.1 | .01 | 76.2 ± 6.0† | 58.8 ± 10.4† | 30.3 ± 10.3 | <.0001 |
| F/F cell (pg) | 5.6 ± 1.6 | 3.8 ± 1.2 | 3.1 ± 0.9 | .005 | 11.1 ± 1.2† | 8.3 ± 1.2† | 5.4 ± 1.6 | <.0001 |
| P40 | 0.26 ± 0.06 | 0.30 ± 0.08 | 0.30 ± 0.07 | NS | 0.19 ± 0.02† | 0.19 ± 0.06 | 0.22 ± 0.03 | NS |
| P70 | 0.04 ± 0.04 | 0.07 ± 0.07 | 0.06 ± 0.06 | NS | 0.00 ± 0.01† | 0.02 ± 0.03 | 0.02 ± 0.03 | NS |
| Bilirubin (mg/L) | 47.5 ± 28.1 | 36.9 ± 24.6 | 30.2 ± 14.7 | NS | 26.0 ± 14.4† | 40.5 ± 28.8 | 35.7 ± 4.0 | NS |
| Parameters . | Pretreatment Values . | P* . | Values at HbF Maximal Response . | P* . | ||||
|---|---|---|---|---|---|---|---|---|
| Group 1 (n = 12) . | Group 2 (n = 11) . | Group 3 (n = 5) . | Group 1 (n = 12) . | Group 2 (n = 11) . | Group 3 (n = 5) . | |||
| Hemoglobin g/dL | 8.4 ± 1.3 | 7.9 ± 1.0 | 8.9 ± 1.4 | NS | 9.7 ± 0.9† | 8.2 ± 0.7 | 8.7 ± 1.2 | .004 |
| MCV (fL) | 88.3 ± 12.3 | 82.9 ± 7.3 | 76.3 ± 9.7 | NS | 108.6 ± 7.7† | 100.7 ± 10.3† | 88.9 ± 15.5† | .03 |
| Neutrophils (×109/L) | 7.6 ± 2.4 | 7.0 ± 3.6 | 7.0 ± 2.9 | NS | 4.0 ± 2.5† | 3.6 ± 1.9† | 5.1 ± 2.4 | NS |
| Reticulocytes (×109/L) | 443 ± 213 | 450 ± 247 | 289 ± 132 | NS | 197 ± 89† | 284 ± 162† | 219 ± 68 | NS |
| HbF (%) | 6.3 ± 3.7 | 3.3 ± 2.1 | 1.4 ± 0.6 | .005 | 25.1 ± 5.0† | 14.4 ± 2.6† | 5.8 ± 2.6† | <.0001 |
| F reticulocytes (%) | 10.8 ± 6.8 | 5.3 ± 2.4 | 4.5 ± 1.0 | .01 | 34.6 ± 14.2† | 18.5 ± 5.3† | 10.6 ± 4.7 | .0005 |
| F cells (%) | 33.6 ± 14.5 | 20.1 ± 7.8 | 13.2 ± 6.1 | .01 | 76.2 ± 6.0† | 58.8 ± 10.4† | 30.3 ± 10.3 | <.0001 |
| F/F cell (pg) | 5.6 ± 1.6 | 3.8 ± 1.2 | 3.1 ± 0.9 | .005 | 11.1 ± 1.2† | 8.3 ± 1.2† | 5.4 ± 1.6 | <.0001 |
| P40 | 0.26 ± 0.06 | 0.30 ± 0.08 | 0.30 ± 0.07 | NS | 0.19 ± 0.02† | 0.19 ± 0.06 | 0.22 ± 0.03 | NS |
| P70 | 0.04 ± 0.04 | 0.07 ± 0.07 | 0.06 ± 0.06 | NS | 0.00 ± 0.01† | 0.02 ± 0.03 | 0.02 ± 0.03 | NS |
| Bilirubin (mg/L) | 47.5 ± 28.1 | 36.9 ± 24.6 | 30.2 ± 14.7 | NS | 26.0 ± 14.4† | 40.5 ± 28.8 | 35.7 ± 4.0 | NS |
Abbreviations: NS, not significant, P > .05; P40 and P70, polymer tendency at 40% and 70% oxygen saturation in the F-cell population.
P values from Kruskal-Wallis test comparing the values of the three groups either before treatment or at the time of HbF maximal response.
P < .05 from Wilcoxon signed test within group categories, comparing the pretreatment values with values at HbF maximal response.
Variations in cellular parameters.
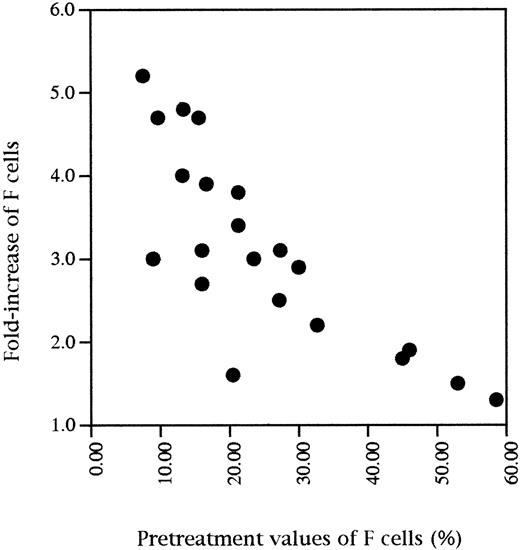
Table 1 shows the mean values of F cells, F/F cell, and F retics before HU, yearly during treatment, and at the time of HbF maximal response. An increase of these parameters was constant, but it was very variable from patient to patient both in terms of kinetics and of the maximal levels achieved. F cells exhibited a 1.2- to 5.1-fold increase of the pretreatment value and F retics a 1.5- to 7.4-fold increase. Reaching a plateau at 6 months was frequent but not constant. Statistical analysis showed that HbF levels were highly correlated to both F cells (r = .95, P < .0001) and F/F cells (r = .77,P < .0001) before treatment and also at the time of HbF maximal response with a similar level of significance. Considering F cells, the highest values at the time of HbF maximal response were observed for the patients with the lowest pretreatment values of F cells (Fig 3). Variations in cellular parameters were studied according to the β-globin haplotypes. The values were higher in Senegalese patients as compared with CAR and Benin patients, but in a nonsignificant way (data not shown).
Fold-increase of F cell percentages at the time of HbF maximal response as a function of initial values of F cells (F cells at HbF maximal response/initial F cells).
Fold-increase of F cell percentages at the time of HbF maximal response as a function of initial values of F cells (F cells at HbF maximal response/initial F cells).
The study of the cellular parameters before HU and at the time of HbF maximal response were studied in the three groups defined above (Table2). Because these groups have been defined on the value of Fmax, F cells, F/F cell, and F retics were understandably different between the three groups at the time of maximal HbF response. But a significant difference in the values of these three parameters was also observed between the groups before treatment, in agreement with the difference in Fi. Within each group, significant variations were observed from pretreatment to the time of HbF maximal response for F retics, F cells, and F/F cell for groups 1 and 2, but not for group 3 (see Table 2 for details).
Polymer Formation at Different Oxygen Saturations
In the F-cell population, the rate of polymer tendency decreased significantly during the treatment in all cases (except 1; Table 2). At 40% oxygen saturation, calculated values were 0.28 ± 0.07 (0.16 to 0.42) and 0.20 ± 0.04 (0.13 to 0.30) before HU and at the time of HbF maximal response respectively (P < .0003). At 70% oxygen saturation, calculated values were 0.05 ± 0.05 (0.00 to 0.17) and 0.01 ± 0.02 (0.00 to 0.07) before HU and at the time of HbF maximal response, respectively (P < .003). Considering groups 1, 2, and 3, neither pretreatment values nor values at the time of HbF maximal response were significantly different from one group to another (Table 2). A decrease in polymer tendency at 40% and 70% oxygen saturation during treatment occurred for all three groups of patients, but the decrease was significant only for group 1 (P = .01 and P = .02). In the non–F-cell population, no variation of polymer tendency was observed as predicted from the lack of changes in HbF levels.
DISCUSSION
Contrasting with the large body of data concerning the use of HU in adult patients, including the multicentric series,13 only a few pediatric trials have been reported to date in the United States,24 Belgium,25 and France,15respectively. These reports deal mostly with global HbF response, clinical benefit, and tolerance. We report data here concerning the cellular and molecular parameters of the response to HU, including variations of F retics, F cells, F/F cell, and polymer tendency in the F-cell population, in 29 patients of the French cohort.
Two parameters can be used to describe HbF response: (1) the rate of HbF increase and (2) the maximal value achieved. We find that both parameters are highly variable; thus, their accurate evaluation depends both on the size of the series and on the duration of follow-up. Here, 29 young patients have been observed for periods varying from 12 to 36 months (average, 22 months), as compared with 6 months of follow-up of 25 patients in the Belgian series25 and a 6 to 39 months (average, 24 months) of follow-up of 13 patients in the American series.24 We find that the maximal level of HbF is not reached at 6 months for 68% of the children and only after 12 months for 14% of them. In addition, we find that the HbF value at 6 months is not predictive of the maximal achieved value.
Once the peak of maximal HbF value is reached, HbF stabilized at a slightly lower level, except for 5 patients for whom the maximal HbF value was sustained. The plateauing of HbF at 2 or 3 years of HU contrasts with Steinberg's observation in adults,26 who shows that only half of patients have a sustained increase of HbF after 2 years of HU treatment. Whether HbF response under HU is better in children than in adults is an hypothesis that may be suggested on another issue: a fourfold increase of HbF level is observed in the two series that include the youngest children (Ferster's series [children 2 to 22 years of age]25 and our series [children 4 to 19 years of age]), whereas a twofold increase is only observed in Scott's series, which includes older children (children 10 to 17 years of age).24 Furthermore, a negative relationship was found between the age and the slope of HbF increase in de Montalembert's series,15 which includes the patients of the series studied here and also 6 additional children, most of them very young. However, comparison between series is difficult, because we used a different drug dosing than Scott and Ferster. We can nevertheless observe that our mean steady state dose was 34.2 ± 4.6 mg/kg administered 4 days/wk, equivalent to a weekly dose of about 136 mg/kg and thus very close to the steady-state weekly doses administered by Ferster (140 to 175 mg/kg) and Scott (160 mg/kg). Still, it is important to consider that HbF response to the treatment was highly variable. At the time of HbF maximal response, HbF increased 1.5- to 16-fold. We found no correlation between HU dosages and maximal HbF values. However, ΔF (Fmax − Fi) was strongly correlated to ΔMCV (MCVmax − MCVi), which might be a better index of the overall pharmacological effect of the HU treatment.
Alternatively, response variability might be related to other factors such as genetic background23,26 or bone marrow reserve. Females values were higher than males values, but in a nonsignificant way. The patients with SCD observed in France include a large majority of children belonging to a first generation of African immigrants, usually of homogeneous ethnic background and indeed most of our patients were homozygous for 1 of the 3 common β-globin haplotypes. Thus, it constitutes a population of choice to investigate a potential effect of the haplotype. In this series, we found that neither initial nor maximal HbF was correlated with β-globin haplotype. This may be in contradiction with some results of the literature,27 but the size of the series might be too small, most particularly for patients with the Senegal haplotype. Similarly, our series is not large enough to evaluate an eventual effect of the α-globin gene status on the response to HU. We did not find any correlation between ΔF (Fmax − Fi) and baseline reticulocyte or neutrophil counts, which have been proposed to reflect the bone marrow reserve, ie, the capacity of the marrow to withstand moderate doses of HU with acceptable myelotoxicity.27 The number of myelotoxic episodes in our series was clearly lower than that reported in Steinberg's series.27 It was actually limited to three episodes defined according to our criteria15: one was a neutropenic episode (neutrophils, 1.3 × 109/L), one a thrombopenia (platelets, 90 × 109/L), and the third one a reticulopenia (reticulocytes, 80 × 109/L). This may be related either to the modified schedule of administration that we used (4 days/wk instead of daily) or to a better hematological tolerance of the drug in children. In this short pediatric series, there was no correlation between myelotoxic events and HbF increase, suggesting that perhaps marrow reserve does not influence HbF response in children.
However, we found a clear relation between Fmax and Fi. Indeed, when we divide the patients in three groups according to the Fmax levels, based on the assumption that this parameter is the most relevant to the expected clinical benefit,28-32 then all the Fi-related parameters in the three groups are significantly different. In contrast, these three groups are indistinguishable on the bases of initial hematological and hemolysis parameters. This finding also contrasts with Steinberg's observation in adults,27 who found no association between HbF response and baseline HbF levels. At the time of HbF maximal response, group 1 (that with the maximal response, HbF >20%) is clearly singled out. All the HbF-related parameters are at the highest, including F retics. As a result, polymer tendency is reduced and so is total bilirubin. In accordance, total Hb is increased and reticulocyte count is low. This decrease in polymer tendency agrees with Bridges's results that HU increases HbS polymerization delay time.33
We find that Fi were correlated to the two parameters F cells and F/F cell. In agreement with the data of Charache et al,34 we find that HbF increase during HU treatment resulted both from the increase of F cells and F/F cell and thus might proceed from cellular as well as molecular mechanisms. When the factor of increase of F cells from the initial value is determined at the time of the patients' HbF maximal response, we find that the highest values are achieved for the patients exhibiting the lowest pretreatment values of F cells. This observation contrasts with our result that the best responders (group 1) are those with the highest Fi. However, this finding may be an indirect effect of the sensitivity of the method of detection. Indeed, when scoring F cells, cells containing amounts of HbF just below the threshold of detection are naturally scored with non-F cells and minor variations in HbF production during treatment may be sufficient to shift the distribution of these cells across the threshold.35,36In conclusion, our data tend to support the fact that response to HU treatment in young patients is better than in adults, because we observed only one nonresponder. It results in a HbF level that is sustained at a level slightly lower than the HbF maximal value. We find that the HbF response in children is dependent on the initial HbF value but not, as observed in the adults, on the bone marrow reserve. The best responders form a group that distinguishes clearly from the others, with higher Hb levels, decreased bilirubin, and decreased polymer tendency. Our study was focused on the parameters of HbF response to HU treatment. Given the fact that HU clearly has pleiotropic effects, other parameters will have to be studied before a clear correlation could be established between the clinical and biological response to treatment. These additional factors will be likely to provide further insights into the polygenic modulation of HU response and to generate more definitive predictive markers of the robustness of the response and of the ultimate clinical utility.
ACKNOWLEDGMENT
The authors thank Dr Dominique Labie for her critical reading of this manuscript and her constant support.
APPENDIX
Investigators of the French Study Group on Sickle Cell Disease: F. Bernaudin, Service de Pédiatrie, Centre Hospitalier Intercommunal, Créteil; M. Belloy and M. Benkerrou, Centre de la Drépanocytose, Hôpital Robert Debré, Paris; R. Mardini and N. Philippe, Service d'Hématologie, Hôpital Debrousse, Lyon; M. Hunault, Service d'Hématologie, Hôpital Hotel Dieu, Paris; F. Gouraud, Service d'Hématologie, Hôpital Trousseau, Paris; T. Cynober, Laboratoire d'Hématologie, Hôpital Bicêtre, Le Kremlin Bicêtre; D. Bachir, Centre de la Drépanocytose, Hôpital Henri Mondor, Créteil; J. Vedrenne, Service de Pédiatrie, Centre Hospitalier, Fontainebleau; J. Lorilloux, Service de Pédiatrie, Hôpital Delafontaine, Saint Denis; C. Olivier, Service de Pédiatrie, Hôpital Louis Mourier, Colombes.
Supported in part by Grants No. 494011 from the Institut National de la Santé et de la Recherche Médicale (INSERM), Grant No. 950075 from the Délégation à la Recherche Clinique de l'Assistance Publique-Hôpitaux de Paris, and Grant No. TS3*-CT93-0244 from the European Union.
Address reprint requests to Micheline Maier-Redelsperger, Service d'Hématologie Biologique, Hôpital Tenon, 4, rue de la Chine, 75970 Paris Cedex 20, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal