Abstract
Dysregulation of oncogenes by translocation to an IgH (14q32) or IgL (κ, 2p11 or λ, 22q11) locus is a frequent event in the pathogenesis of B-cell tumors. Translocations involving an IgH locus and a diverse but nonrandom array of chromosomal loci occur in most multiple myeloma (MM) tumors even though the translocations often are not detected by conventional cytogenetic analysis. In a continuing analysis of translocations in 21 MM lines, we show that the novel, karyotypically silent t(14;16)(q32.3;q23) translocation is present in 5 MM lines, with cloned breakpoints from 4 lines dispersed over an approximately 500-kb region centromeric to the c-maf proto-oncogene at 16q23. Another line has a t(16;22)(q23;q11), with the breakpoint telomeric to c-maf, so that the translocation breakpoints in these 6 lines bracket c-maf. Only these 6 lines overexpress c-mafmRNA. As predicted for dysregulation of c-maf by translocation, there is selective expression of one c-maf allele in 2 informative lines with translocations. This is the first human tumor in which the basic zipper c-maf transcription factor is shown to function as an oncogene.
DYSREGULATION OF oncogenes by translocation to an IgH (14q32) or IgL (κ, 2p11 or λ, 22q11) locus is a seminal event in the pathogenesis of B-cell tumors.1We and others have shown recently that translocations to the IgH locus occur in most multiple myeloma (MM) tumors even though the translocations often are not detected by conventional karyotypes.2,3 Many nonrandom chromosomal partners (and oncogenes) are involved in these translocations.3-9 Two loci (cyclin D1 at 11q13 and FGFR3 at 4p16.3) are involved frequently, with each occuring at an incidence of about 20% to 25%. Several other loci are involved recurrently but at a much lower frequency (eg, IRF4 at 6p25, c-myc at 8q24, andbcl-2 at 18q21), and 10 or more other loci have been identified only once. However, in the majority of tumors that have an apparent IgH translocation by conventional karyotypic analysis, the partner locus has not been identified (14q+). We report here karyotypic and molecular characterization of a novel t(14;16)(q32;q23) translocation that causes overexpression of the c-maf oncogene and appears to occur at an incidence of about 25% in MM.
MATERIALS AND METHODS
Cell culture.
Translocation breakpoint fragments.
The cloned chromosome 16 translocation breakpoint fragments in λZAP Express (Stratagene, La Jolla, CA) included 6.9-kbEcoRI (JJN3) and 3.5-kb EcoRI (ANBL6) fragments detected with a 3′Sμ probe, 3.5-kb (KMS11) and 7.7-kb (MM.1)HindIII fragments detected with a 3′ Sγ probe, and 4.1-kb Bgl II-EcoRI (ANBL6) and 4.9-kb HindIII (MM.1) fragments detected with a 5′Sμ probe.
Isolation and characterization of overlapping YAC, BAC and P1 clones.
A number of YAC and BAC clones were obtained from N. Doggett, C.M. Aldaz, and A. Bednareck. Additional P1 and BAC human genomic library clones (Genome Systems, St Louis, MO) were identified with PCR reactions using primers corresponding to sequences determined from the translocation breakpoint fragments, from the ends of BAC or P1 genomic clones, and from c-maf.
Somatic cell hybrid mapping.
Non-Ig sequences from translocation breakpoint clones were mapped to specific human chromosomes using hamster hybrids containing one or a limited number of human chromosomes, as described previously.3 In addition the Stanford high resolution TNG3 radiation hybrid panels (Research Genetics, Huntsville, AL) were used for more precise mapping of polymerase chain reaction (PCR) markers to specific chromosome regions.
Primers and probes.
Switch region probes upstream (eg, 5′Sμ) and downstream (eg, 3′Sμ) of the repetitive sequences in each switch region were as described previously.3 5 The c-maf probe used in the Northern blot assay was a 1.2-kb fragment that contained 5′ untranslated and coding sequences that were PCR-amplified from cDNA. The primers used to amplify the Mnl I polymorphism are 5′-CTTCAGTTCATGAACTGGTGT and 5′-GTTTGCCAGGTTAAATGTGTA. Information regarding the oligonucleotides used for other PCR reactions is available by request.
Fluorescence in situ hybridization (FISH).
Preparation of metaphase chromosomes, chromosome 14 and 22 painting probes, c-maf and JJN3 breakpoint P1 clone probes, and hybridization and detection protocols are described elsewhere.5
Other procedures.
Genomic library construction and screening, isolation and sequencing of recombinant clones, and Southern and Northern blot analysis were described elsewhere.3 An 8226 oligo-dT primed cDNA library was constructed with EcoRI linkers and cloned into λ ZAPII (Stratagene), and a directional H929 oligo-dT primed cDNA library was prepared in UniZAP (Stratagene).
GenBank accession numbers for c-maf and translocation breakpoint sequences.
The GenBank accession no. for JJN3 breakpoint sequence is U73670; for KMS11 3′Sγ, AF055379; for ANBL6 5′Sμ and 3′Sμ breakpoint sequences, AF055380 and AF055381, respectively; for MM.1 5′Sμ and 3′Sμ, AF055382 and AF055383, respectively; for human c-maf, AF055376; for the long form of c-maf,AF055377; and for the intronic sequence separating the two exons of the long form of c-maf, AF055378.
RESULTS
As described elsewhere, our Southern blot assay identifies candidate translocation breakpoints as rearranged restriction enzyme fragments that hybridize with either a 5′ or a 3′ probe directed against sequences that flank IgH switch regions.3Figure 1a shows how this assay identifies the derivative chromosomes resulting from 2 patterns of IgH switch translocation in MM. In 1 case, there is a translocation involving only Sμ, so that the 5′Sμ probe detects the der(16) breakpoint and the 3′Sμ detects the der(14) breakpoint. In the other case, the translocation involves Sμ and Sγ, so that the 5′Sμ probe again detects the der(16) breakpoint, whereas a 3′Sγ probe detects the der(14) breakpoint. Figure 1B shows translocation breakpoint fragments for both the first kind (ANBL6) and the second kind (MM.1) of IgH switch translocation. Using this approach, we cloned 6 translocation breakpoint fragments involving chromosome 16 from 4 MM cell lines: (1) a 3′Sμ fragment from JJN3; (2) 5′ and 3′Sμ fragments from ANBL6; (3) 5′Sμ and 3′Sγ fragments from MM.1; and (4) a 3′Sγ fragment from KMS11. For each MM line, the non-Ig sequences at one end of each breakpoint fragment identify chromosome 16 in a panel of somatic cell hybrids. A P1 clone that includes the chromosome 16 sequences from the JJN3 breakpoint maps to 16q23 by FISH analysis of normal metaphase chromosomes (see Fig 4A). Noting that the c-maf proto-oncogene also maps to 16q23,11 we isolated a P1 clone containing c-maf and demonstrated colocalization of c-maf and the JJN3 chromosome 16 breakpoint sequences by two-color FISH experiments analyzing normal metaphase chromosome spreads and interphase nuclei (see Fig 4A). We also localized c-maf on the der(14) by FISH analysis of metaphase chromosomes from JJN3 cells (not shown), thus showing that c-maf is telomeric to the JJN3 chromosome 16 breakpoint sequences. Mapping of P1, BAC, and YAC clones demonstrated physical linkage at 16q23 of c-maf with the t(14;16) breakpoints in the 4 MM lines (Fig 1C). The ANBL6 and JJN3 chromosome 16 breakpoint sequences are present within a single BAC. The KMS11 and MM.1 breakpoints are more centromeric, with all of the cloned t(14;16) translocation breakpoints dispersed over an approximately 500-kb region. As shown in Fig 1C, c-maf and the chromosome 16 breakpoints from the 4 MM lines map in the order: cen—KMS11—MM.1—JJN3—ANBL6—c-maf—tel.
Identification and characterization of t(14;16)(q32;q23) translocation breakpoints. (A) Diagram of der(14) and der(16) breakpoints from translocations involving Sμ and Sγ. The centromere is to the left. Structural elements include enhancers (3′E and 5′E), switch region (S), and coding segments (rectangles). Thick horizontal lines depict hybridization probes. Vertical lines represent restriction enzyme sites. H, HindIII; Ba, BamHI; Bg,Bgl II. (B) Southern blots of MM cell line genomic DNAs digested with HindIII. Probes flanking switch regions are indicated at the bottom of each lane. For the ANBL6 line, 17-kb and 16.5-kb rearranged fragments are detected with the 5′ and 3′Sμ probes, respectively. For the MM.1 line, 4.9-kb and 7.7-kb rearranged fragments are detected with the 5′Sμ and 3′Sγ probes, respectively. The MM.1 4.0-kb fragment(s) that cohybridizes with 5′Sγ and 3′Sγ probes represents an unrearranged, germline fragment containing a γ switch region. (C) Map of region at 16q23 that contains c-maf and sequences present in the cloned t(14;16) breakpoint fragments. For the BAC clones (designated by number within box), the T7 (T) and SP6 (S) ends are shown when the orientation has been defined. Mapping was performed by using PCR reactions to detect sequences derived from the ends of BAC/P1 clones, translocation breakpoint fragments, and c-maf, plus 5 other chromosome 16 markers28. The composite map is not to scale but is fully consistent with analysis of additional clones and markers that are not shown. The dashed arrow indicates that the t(16;22) breakpoint in 8226 is telomeric to c-maf by FISH analysis.
Identification and characterization of t(14;16)(q32;q23) translocation breakpoints. (A) Diagram of der(14) and der(16) breakpoints from translocations involving Sμ and Sγ. The centromere is to the left. Structural elements include enhancers (3′E and 5′E), switch region (S), and coding segments (rectangles). Thick horizontal lines depict hybridization probes. Vertical lines represent restriction enzyme sites. H, HindIII; Ba, BamHI; Bg,Bgl II. (B) Southern blots of MM cell line genomic DNAs digested with HindIII. Probes flanking switch regions are indicated at the bottom of each lane. For the ANBL6 line, 17-kb and 16.5-kb rearranged fragments are detected with the 5′ and 3′Sμ probes, respectively. For the MM.1 line, 4.9-kb and 7.7-kb rearranged fragments are detected with the 5′Sμ and 3′Sγ probes, respectively. The MM.1 4.0-kb fragment(s) that cohybridizes with 5′Sγ and 3′Sγ probes represents an unrearranged, germline fragment containing a γ switch region. (C) Map of region at 16q23 that contains c-maf and sequences present in the cloned t(14;16) breakpoint fragments. For the BAC clones (designated by number within box), the T7 (T) and SP6 (S) ends are shown when the orientation has been defined. Mapping was performed by using PCR reactions to detect sequences derived from the ends of BAC/P1 clones, translocation breakpoint fragments, and c-maf, plus 5 other chromosome 16 markers28. The composite map is not to scale but is fully consistent with analysis of additional clones and markers that are not shown. The dashed arrow indicates that the t(16;22) breakpoint in 8226 is telomeric to c-maf by FISH analysis.
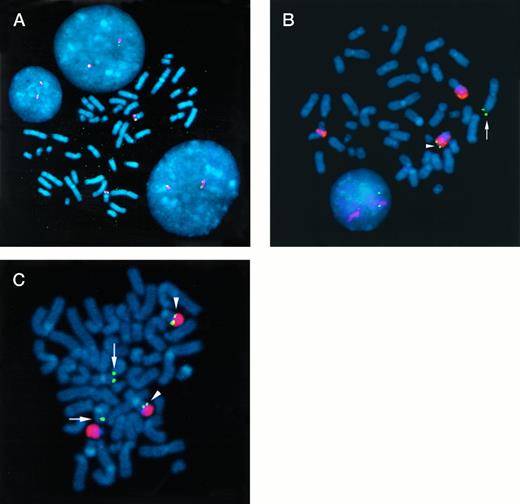
Two-color FISH analysis of normal lymphocytes and 2 MM cell lines. (A) P1 clones containing the c-maf gene (red signal) and chromosome 16 sequences from the JJN3 MM t(14;16) breakpoint (green signal) were hybridized to metaphase chromosomes (counterstained blue) and interphase nuclei from normal lymphocytes. The signals colocalize at chromosome band 16q23. (B) A chromosome 14 painting probe (red signal) and a c-maf P1 clone (green signal) were hybridized to metaphase chromosomes from the OCI-My5 MM cell line. There are no normal copies of chromosome 14 or 16. One copy of c-maf (arrowhead) has been translocated to the telomeric end of chromosome 14 at band q32, consistent with a t(14;16)(q32;q23) translocation, involving the IgH locus and c-maf, respectively. A second copy of c-maf (arrow) is translocated to a structurally altered, unidentified chromosome. (C) A chromosome 22 painting probe (red signal) and a c-maf P1 clone (green signal) were hybridized to metaphase chromosomes from the 8226 MM cell line. There are 4 copies of the c-maf gene, 2 of which have chromosome 22 sequences translocated telomeric to c-maf (arrowhead), whereas 2 are on a normal chromosome 16 (arrow). The breakpoint on chromosome 22 is consistent with a t(16;22)(q23;q11) translocation near the c-maf and IgL, λ loci, respectively.
Two-color FISH analysis of normal lymphocytes and 2 MM cell lines. (A) P1 clones containing the c-maf gene (red signal) and chromosome 16 sequences from the JJN3 MM t(14;16) breakpoint (green signal) were hybridized to metaphase chromosomes (counterstained blue) and interphase nuclei from normal lymphocytes. The signals colocalize at chromosome band 16q23. (B) A chromosome 14 painting probe (red signal) and a c-maf P1 clone (green signal) were hybridized to metaphase chromosomes from the OCI-My5 MM cell line. There are no normal copies of chromosome 14 or 16. One copy of c-maf (arrowhead) has been translocated to the telomeric end of chromosome 14 at band q32, consistent with a t(14;16)(q32;q23) translocation, involving the IgH locus and c-maf, respectively. A second copy of c-maf (arrow) is translocated to a structurally altered, unidentified chromosome. (C) A chromosome 22 painting probe (red signal) and a c-maf P1 clone (green signal) were hybridized to metaphase chromosomes from the 8226 MM cell line. There are 4 copies of the c-maf gene, 2 of which have chromosome 22 sequences translocated telomeric to c-maf (arrowhead), whereas 2 are on a normal chromosome 16 (arrow). The breakpoint on chromosome 22 is consistent with a t(16;22)(q23;q11) translocation near the c-maf and IgL, λ loci, respectively.
Hypothesizing that c-maf is the oncogene dysregulated by the t(14;16)(q32;q23) translocation, we screened our panel of 21 MM lines for c-maf expression using a reverse transcription-PCR (RT-PCR) assay. We found high levels of expression in the 4 MM lines with t(14;16) (MM.1, JJN3, KMS11, and ANBL6) plus 2 additional lines (OCI-My5 and 8226) and lower levels in several other lines after a limited number of PCR cycles (data not shown). To obtain a more quantitative estimate of c-maf mRNA expression, we did a Northern blot using total RNA from the 14 lines for which expression was detected by the RT-PCR assay. As shown in Fig 2A, there is a high level of expression of 4.4- and 2.3-kb c-maf mRNAs in the 6 lines mentioned above. The other lines show either no detectable expression or a level of expression that is approximately 10-fold lower. The same 4.4-and 2.3-kb mRNAs are expressed in most human tissues, some examples of which are shown in Fig 2B. In view of the unexplained finding of two sizes of mRNA for c-maf and the fact that human c-maf had not been well characterized, we decided to look more carefully at the structure of human c-maf. Previously, using a subtractive cDNA approach, we identified c-maf expression in the H929 and 8226 MM lines, whereas c-maf expression was not detected in the pair of lymphoblastoid cell lines used for the subtraction.12Using sequence analysis of c-maf cDNAs isolated from 8226 and H929, we determined the full sequence of alternatively processed human c-maf mRNAs (Fig 3). The 4.4-kb mRNA species encodes a predicted c-maf basic zipper transcription factor protein of 373 amino acids that is highly homologous to reported amino acid sequences for avian (86% identity) and murine (95% identity) c-maf.13 The 2.3-kb mRNA encodes a predicted c-maf protein of 403 amino acids, although the 5′ untranslated and coding sequence is identical to the 4.4-kb mRNA through amino acid 372. Noting a potential donor splice site at codon 373 in the 4.4-kb mRNA (Fig 3B), we proceeded to PCR-amplify from genomic DNA a 2.1-kb fragment that started just upstream of the polyadenylation site in the 4.4-kb mRNA species and ended in the unique coding region in the 2.3-kb mRNA species. Sequence analysis of the downstream end of this fragment demonstrated the presence of an acceptor splice signal within codon 373 of the 2.3-kb mRNA (Fig 3B), indicating that the 2.3-kb mRNA results from the removal of an approximately 4-kb intron that includes the 3′ untranslated region of the 4.4-kb mRNA species. The additional 30 amino acids in the predicted 403 residue protein contain no known sequence motifs and have no obvious homologies to other proteins, so that the significance of alternatively spliced forms of c-maf is presently unclear.
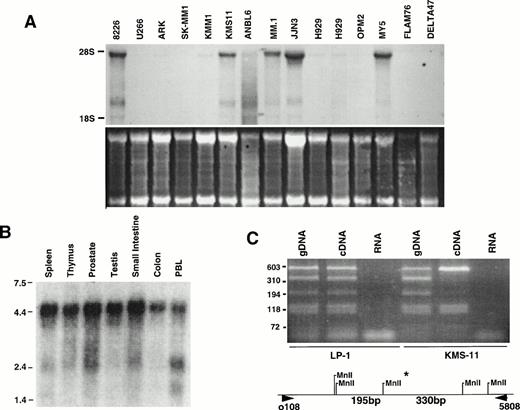
Expression of c-maf mRNA in myeloma cell lines and some normal human tissues. (A) A Northern blot containing 15 μg of total RNA from each of 14 MM lines was probed for c-maf, with the horizontal lines indicating the position of 5.0- and 2.0-kb ribosomal RNAs. Ethidium bromide staining is shown in the lower panel. (B) A Northern blot containing 2 μg of poly (A)+ RNA from each of several normal tissues was assessed for c-maf expression. (C) Genomic DNA, cDNA, and RNA from 2 MM cell lines was subjected to PCR amplification using appropriate oligonucleotides from the 3′ untranslated region of c-maf. The amplified products were digested with Mnl I and fractionated by electrophoresis on a 2% acrylamide gel. The positions of Mnl I sites, including the polymorphic (*) Mnl I site, in the amplified fragments are shown.
Expression of c-maf mRNA in myeloma cell lines and some normal human tissues. (A) A Northern blot containing 15 μg of total RNA from each of 14 MM lines was probed for c-maf, with the horizontal lines indicating the position of 5.0- and 2.0-kb ribosomal RNAs. Ethidium bromide staining is shown in the lower panel. (B) A Northern blot containing 2 μg of poly (A)+ RNA from each of several normal tissues was assessed for c-maf expression. (C) Genomic DNA, cDNA, and RNA from 2 MM cell lines was subjected to PCR amplification using appropriate oligonucleotides from the 3′ untranslated region of c-maf. The amplified products were digested with Mnl I and fractionated by electrophoresis on a 2% acrylamide gel. The positions of Mnl I sites, including the polymorphic (*) Mnl I site, in the amplified fragments are shown.
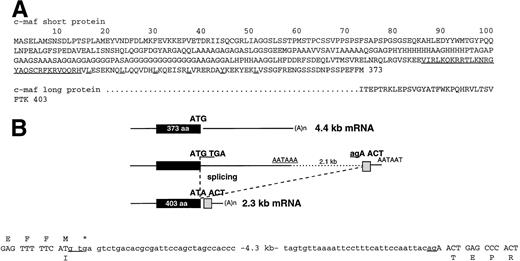
Sequence of short and long c-maf proteins generated by alternative RNA processing of transcripts from the c-maf gene. (A) A single letter code indicates the two sequences, with the long form identical to residues 1 through 372 of the short form. The basic region is underlined as a block, and individual residues that contribute to a presumptive leucine zipper are individually underlined. (B) This diagram summarizes the genome organization and codon sequences that account for the alternative RNA processing that generates the 2 forms of c-maf mRNA. The exon unique to the long form of the c-maf protein is located about 2.1 kb downstream of the polyadenylation signal used to generate the 4.4-kb mRNA that encodes the short form of the c-maf protein. The sequences of the exon-intron boundaries for the short mRNA are indicated, with the exon sequences in capital letters. The homology of the 4.4-kb mRNA to the avian c-maf genomic sequence indicates that the coding region and 3′ untranslated region are contained within a single exon.
Sequence of short and long c-maf proteins generated by alternative RNA processing of transcripts from the c-maf gene. (A) A single letter code indicates the two sequences, with the long form identical to residues 1 through 372 of the short form. The basic region is underlined as a block, and individual residues that contribute to a presumptive leucine zipper are individually underlined. (B) This diagram summarizes the genome organization and codon sequences that account for the alternative RNA processing that generates the 2 forms of c-maf mRNA. The exon unique to the long form of the c-maf protein is located about 2.1 kb downstream of the polyadenylation signal used to generate the 4.4-kb mRNA that encodes the short form of the c-maf protein. The sequences of the exon-intron boundaries for the short mRNA are indicated, with the exon sequences in capital letters. The homology of the 4.4-kb mRNA to the avian c-maf genomic sequence indicates that the coding region and 3′ untranslated region are contained within a single exon.
Two MM lines (8226 and OCI-My5) that overexpress c-maf had no candidate IgH switch translocation breakpoint fragments by our Southern blot assay, which could represent a failure of the assay to detect a switch-mediated translocation or could represent an example of a VDJ recombinase-mediated translocation into or near a JH or JL segment. The 8226 MM line has a t(1;14)(p13;q32) translocation as determined by conventional cytogenetic analysis,14 which we confirmed by two-color FISH analysis of metaphase chromosomes with chromosome 1 and 14 painting probes (data not shown), whereas there is no published karyotype for the OCI-My5 line. A metaphase FISH analysis with a c-maf probe and a chromosome 14 painting probe demonstrated that c-maf has been translocated to the telomere of the long arm of 14, ie, a t(14;16 )(q32;q23) translocation in OCI-My5 (Fig 4B) but not in 8226 (not shown). Another FISH analysis on metaphase chromosomes from line 8226 using the c-maf probe and a chromosome 22 painting probe showed translocation of chromosome 22 telomeric to c-maf on chromosome 16 (Fig 4C). The position of the breakpoint on chromosome 22 is consistent with a t(16;22)(q23;q11) translocation that would dysregulate c-maf by juxtaposition to the IgL λ locus.
A chromosomal translocation involving 1 of the Ig loci typically results in cis dysregulation of an oncogene located on the chromosomal partner involved in the translocation, whereas the allelic oncogene on the chromosome not involved in the translocation is not dysregulated. By comparison of our c-maf sequences and expressed sequence tags deposited in GenBank, we identified an apparent Mnl I polymorphism in the 3′ untranslated region of c-maf and confirmed that two MM lines contain c-maf alleles distinguished by the polymorphism at this site (Fig 2C). As predicted, the KMS11 line, which has a t(14;16) translocation, selectively expresses 1 of the 2 c-maf alleles. By contrast, the LP1 MM line, which has a t(4;14) translocation (unpublished data), expresses a very low level of c-maf, with equal levels of expression from each allele. In addition, we screened other lines for polymorphic markers in c-maf and identified two c-maf alleles distinguished by a T>C polymorphism at nucleotide 3626 in the 3′ untranslated region in the 8226 MM line. Again, one c-maf allele is selectively expressed (data not shown), consistent with the variant t(16;22) translocation causing dysregulation and overexpression of the c-maf allele involved in the translocation.
DISCUSSION
Although the translocation t(14;16)(q32.3;q23) has not been identified by conventional cytogenetic analysis, presumably because of the telomeric positions of both loci, our finding that 16q23 is translocated to an Ig locus in 6 of 21 MM lines suggests that translocation of 16q23 to 1 of the Ig loci occurs with an incidence of about 25% in MM, ie, an incidence similar to what we have observed for t(4;14) (FGFR3) and t(11;14) (cyclin D1). This translocation is not restricted to cell lines. First, tumor cells from the 1 cell line (JJN3) for which we were able to examine the primary tumor cells contained the translocation breakpoint as determined by Southern blot.3 Second, two labs reported recently that FISH analysis detects a t(14;16)(q32;q23) translocation in primary MM tumors.2 15 Finally, 7 of 22 primary intramedullary MM tumor samples express c-maf mRNA at a level comparable to the lines documented here to have a t(14;16) or t(16;22) translocation, whereas 4 samples from patients with monoclonal gammopathy of undetermined significance (MGUS) and the other tumor samples express low or nondetectable levels of c-maf (data not shown).
We cannot exclude the possibility that other genes are dysregulated by the t(14;16) translocation in MM, but we have identified the c-maf proto-oncogene as at least one oncogene that is dysregulated by these translocations involving 16q23. First, the breakpoints are dispersed over an approximately 500-kb region centromeric to c-maf, so that it is located on the der(14) chromosome that contains the strong 3′ IgH enhancer(s) but not the weaker intronic enhancer (Fig 1A). This dispersion of breakpoints hundreds of kilobases centromeric to an oncogene is similar to the situation for cyclin D1 and FGFR3, which are dysregulated in mantle cell lymphoma and MM when widely dispersed translocation breakpoints involving the IgH locus are localized as far as several hundred kilobases centromeric to these genes.4,5,16,17Second, the translocation breakpoint for a variant (IgL) t(16;22) translocation is telomeric to c-maf. It is well established for c-myc, bcl-2, and cyclin D1 that IgH translocation breakpoints and variant (IgL) translocation breakpoints, which usually are located centromeric or telomeric, respectively, bracket the oncogene.18-20 Third, c-maf is overexpressed only in the 6 MM lines for which we have identified a translocation involving 16q23. Fourth, 2 informative MM lines (KMS11 and 8226) contain 2 genetically distinguishable c-maf alleles, but each selectively expresses 1 allele, as predicted if the translocation is responsible for dysregulation of c-maf. By contrast, another MM line (LP1) without an apparent t(14;16) translocation expresses both genetically distinguishable alleles to a similar extent. Finally, it is important to emphasize that v-maf is a classical oncogene identified in an avian transforming virus21 and that studies by others have shown that overexpression of wild type c-mafis capable of contributing to transformation of fibroblasts in a model system.22 As indicated above, c-maf is a member of a large family of basic zipper transcription factors, eg, jun, fos, NF-IL6, and small maf proteins, many of which form heterodimers with one another. This family of transcription factors is involved in many basic cellular processes, including proliferation, differentiation, and responsiveness to interleukin-6, a cytokine that has a central role in normal plasma cell differentiation and in pathogenesis of MM.23
As stated at the outset, it is clear that translocations involving 1 of the Ig loci are present in most, if not all, MM tumors, including at least 20 lines in our panel of 21 MM lines. Curiously, in 2 of our MM lines with translocations involving 16q23, there is a second translocation involving an IgH locus, ie, a t(1;14)(p13;q32) translocation (but lacking an identified oncogene at present) in 8226 and a t(4;14)(p16.3;q32.3) translocation that dysregulatesFGFR3 in KMS11. Despite the difficulty of identifying translocations from conventional cytogenetic analysis of MM tumors and cell lines, others have also reported the coincidence of 2 IgH translocations, or an IgH translocation plus a variant translocation, in a number of MM lines and tumors.2,8,24-26 Obviously, the presence of 2 independent translocations in a single MM tumor is not a rare event. We note a recent report suggesting a similar incidence of IgH translocations in premalignant MGUS and frankly malignant MM.2 Thus, it seems likely that translocations in MGUS and MM occur during normal plasma cell development as a consequence of errors resulting from reactivated VDJ recombination in germinal centers27 or perhaps more often as a consequence of errors in physiological IgH switch recombination. Tumor progression beyond 1 or 2 translocations clearly involves a variety of additional genetic changes, including activating mutations in ras orFGFR3, the latter occurring in MM tumors with a t(4;14)(p16.3;q32.3) translocation, including the KMS11 cell line5 that also has a t(14;16)(q32.3;q23) translocation and overexpresses c-maf.
ACKNOWLEDGMENT
The authors thank Norman Doggett for providing probes and YAC clones, plus the initial mapping of the JJN3 breakpoint and c-mafsequences to his YAC clones. In addition, we are grateful to C. Marcelo Aldaz and Andrezj Bednareck for generously providing us with BAC and YAC DNAs at 16q23.
Address reprint requests to W. Michael Kuehl, MD, NCI Medicine Branch, Genetics Department, Naval Hospital, Bldg 8, Room 5101, Bethesda, MD 20889-5105; e-mail: wmk@helix.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal