Abstract
Chemokine receptors (CR), which can mediate migration of immune cells to the site of inflammation, also function as coreceptors for human immunodeficiency virus (HIV) entry into CD4+ T lymphocytes and antigen-presenting cells. We demonstrate here that interferon-γ (IFN-γ) increases the expression of chemokine receptors CCR1, CCR3, and CCR5 in monocytoid U937 cells as detected by cell surface molecule labeling and mRNA expression, as well as by intracellular calcium mobilization and cell migration in response to specific ligands. The increased expression of these chemokine receptors also results in an enhanced HIV-1 entry into cells. Our data provide evidence for a relationship of cellular pathways that are induced by IFN-γ with those that regulate chemokine receptor expression.
INTERFERON-γ (IFN-γ), a cytokine preferentially secreted by activated T lymphocytes and natural killer (NK) cells, is involved in the modulation of a variety of immunological and inflammatory responses. Whether IFN-γ exerts cell proliferative,1 differentiating,2,3inhibitory/anergic,4 or apoptotic function5critically depends on the particular conditions in a given situation at the local site of action. Hematopoietic, antineoplastic, or antiviral effects of IFN-γ are well established. Signaling through IFN receptors transfers into cells in a manner that includes Jak/STAT pathways.6
Important players in inflammation and immune response are chemokines (some of which, like IP10 or MIG, are differentially expressed in response to IFN-γ) and their appropriate receptors.7 Chemokine receptors (CR) mediate signals that regulate leukocyte trafficking, infiltration/inflammation, hematopoiesis, and signals that can influence tumorigenesis.8 Upregulation of CCR1 and CCR2 by interleukin-2 (IL-2)9 or upregulation of CCR5 by granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4,10 or IL-10,11 for example, may involve Jak/STAT signaling events as well. This kind of activation provides yet another level of regulation of leukocyte trafficking and migration, because resting (inactive) immune cells may not be competent to migrate even in the presence of high concentrations of chemokines. Thus, cells have to first be primed and driven into a more (immune) competent effector stage.
Because of the interesting overlapping and/or integrating features in cellular modulation, we investigated the potential link between IFN-γ–induced cellular signaling and the expression of CRs to find out whether IFN-γ is involved in the effector cell priming process that relates to CR mediated signals. We used an in vitro model in which the majority of the conditions are standardized and the data can be reproduced without the variability associated with primary cells. Thus, we analyzed the monocytoid (CD14+, CD4+, HLA-DR+) cell line U937, a well-established model for antigen-presenting cells (APCs) and related signaling12 and assessed the expression and function of CRs on these cells. We used several independent assays to prove the principal connection between a cell's activation by IFN-γ and the resulting increase in CR expression, after we had obtained phenomenological evidence in primary human cells.
MATERIALS AND METHODS
Cell separation, culture, media, and supplements.
Peripheral blood samples were obtained from healthy volunteers after informed consent was received from these individuals according to the protocols approved by the Institutional Review Board of the National Heart, Lung and Blood Institute. Peripheral blood mononuclear cells (PBMCs) were isolated by automated Ficoll/Hypaque density-gradient centrifugation using a CS-3000 Plus Blood Cell Separator (Fenwal Division, Baxter Healthcare Corp, Deerfield, IL). Monocytes were isolated from PBMCs using counterflow-centrifugal elutriation using a Beckman JE-5.0 rotor and a type A chamber (Beckman Instruments, Inc, Palo Alto, CA). Purity of the separated monocyte fraction was approximately 98% as determined by cytomorphology in Pappenheim stain and approximately 96% as determined by the expression of CD14 antigen (LeuM3; Becton Dickinson, Mountain View, CA) and measured by flow cytometry. U937 cells were obtained from ATCC (Rockville, MD). Cells were cultured in complete medium (RPMI 1640, containing 10% heat-inactivated fetal calf serum [FCS], low endotoxin grade; all GIBCO BRL, Gaithersburg, MD) at a concentration of 0.8 × 106 cells/mL. IFN-γ (R&D Systems Inc, Minneapolis, MN) was added to the cultures in varying concentrations so as to assess dose-dependent effects. A concentration of 30 ng/mL was determined to be sufficient for mediating effects on CR expression without adverse (toxic) side effects and was therefore used in most of the experiments. Aliquots of differently treated cells were collected for mRNA analysis, calcium flux assays, cell surface staining and FACS analysis, or migration assays. Infection of monocyte-derived cells with human immunodeficiency virus (HIV)-1(BaL) and infection of U937 cells with HIV-1(IIIB) was performed essentially as previously described.13 Before infection, cells were cultured in the presence of IFN-γ for 6 days. Aliquots of cells (2 × 106 cells) were infected with HIV-1(BaL) or HIV-1(IIIB) at concentrations of 2 ng/mL p24 viral antigen for 90 minutes at 37°C. Both viral strains, Bal and IIIB, were grown and subsequently titered in primary mononuclear phagocytes and purified phytohemagglutinin (PHA)-activated PBMCs, respectively. Excess of virus was removed from exposed cultures by repeated washing steps. Aliquots of the differently treated cell cultures were collected at 10 hours and 20 hours after HIV-1 exposure. Cell viability was assessed by trypan blue exclusion. An equal number of viable cells were infected and subsequently analyzed by standardized polymerase chain reaction (PCR) for LTR-LTR circular forms of HIV proviral DNA.
Transient calcium flux measurement.
A method developed by J.M.B. was applied.14Briefly, aliquots of 106 cells were incubated for 20 minutes at 37°C with Fluo-3 and reconstituted with Pluronic F-127 (Molecular Probes, Eugene, OR) following the manufacturer's directions. After incubation, the samples were washed once with RPMI 1640 (without phenol red, without sodium carbonate, containg 25 mmol/L HEPES) and finally resuspended in 1 mL of the same medium. All samples were kept at 20°C until 45 seconds before analysis, at which time the sample tube was placed in a 37°C water bath. Data were acquired with a FACScalibur (Becton Dickinson), with excitation at 488 nm. Cells were gated by forward and side scatter properties. Calcium mobilization is determined by a two-parameter density plot, measuring linear emission at 530 nm in the FL-1 window for the gated cell population over time. After 20 seconds of acquisition, the appropriate chemokine (RANTES, MIP-1α, MIP-1β, or SDF-1α; all from R&D Systems Inc) was added by using a magnetic device to a final concentration of 25 ng/mL. PHA-activated PBMCs were used as positive controls for calcium flux.
Cell migration assays.
Cell migration assays were performed as previously described.15 Briefly, 15 × 104 U937 cells in 150 μL of RPMI 1640 medium containing 0.25% human serum albumin were transmigrated through 5-μm pore-size bare filter Transwell inserts (Costar, Cambridge, MA) for 6 hours. Migrated cells were counted by FACS analysis scatter-gating on lymphocytes. Chemotaxis was performed in the presence of optimized ligand concentrations (RANTES [75 ng/mL]; R&D Systems Inc). Three independent experiments were performed.
Semiquantitative mRNA assays.
Semiquantitative mRNA assays were performed using end-point dilution and direct comparison of partner samples as it is recommended under circumstances when no internal control/competitor is available. Aliquots of differently treated cells were collected at days 2 and 5. Total cellular RNA was isolated by TRIzol LS reagent (GIBCO BRL) following the manufacturer's protocol. Samples of RNA were treated by DNAse I (amplification grade; GIBCO BRL). Synthesis of first-strand cDNA was performed in 20 μL of reaction mix (50 mmol/L Tris-HCI, pH 8.3, 75 mmol/L KCI, 3 mmol/L MgCI, 10 mmol/L dithiothreitol [DTT], 500 μmol/L of each dNTP, 2.5 μmol/L random hexamer primer; PE Applied Biosystems, Branchburg, NJ), 1.5 μg RNA, and 200 U SuperScript II RT (GIBCO BRL) for 1 hour at 42°C, followed by heating for 5 minutes at 99°C. Amplification was performed with 2 μL of RT-mixture in a total volume of 20 μL, using 2 μmol/L dNTPs, 8 pmol primers, and 2.5 U AmpliTaq Gold polymerase (PE Applied Biosystems) for 35 cycles (94°C for 45 seconds, 60°C for 1.30 minutes, and 72°C for 2 minutes) after an initial 9 minutes of denaturation at 94°C. The resulting PCR products were separated on 2% SeaKem GTG agarose gel (FMC BioProducts, Rockland, ME). As a control for DNA contamination, equal amounts of RNA extraction products were used for each sample assessed and PCR amplification was performed without the addition of RT to the first-strand synthesis. The conditions for the semiquantitative mRNA assay were specifically defined so as to exclude the possibility of amplifying contaminating genomic DNA. The following sets of primers were used: for CCR1: sense, 5′-GCAACTCCGTGCCAGAAGGTGA-3′, and antisense, 5′CCAAATGATGATGCTGGTGATGACACCA-3′, yielding a PCR product of 411 bp; for CCR2b: sense, 5′-CAACATGCTGGTCGTCCTCATC-3′, and antisense, 5′-AGAAGCAAACACAGCCACCAACC-3′, yielding a PCR product of 340 bp; for CCR3: sense, 5′-CAGGGAGAAGTGAAATGACAACCTC-3′, and antisense, 5′-TCCTCTGGGTAAAGAGCACTGCAA-3′, yielding a PCR product of 583 bp; for CCR4: sense, 5′-AAATGAACCCCACGGATATAGCAG-3′, and antisense, 5′-GAAAACACGAAGAGCAGATCCGAGA-3′, yielding a PCR product of 273 bp; for CCR5: sense, 5′-GGTGGTGACAAGTGTGATCACTTGG-3′, and antisense, 5′-TCGGGAGCCTCTTGCTGGAAA-3′, yielding a PCR product of 564 bp; and for CXCR4: sense, 5′-AATCTTCCTGCCCACCATCTACTCC-3′, and antisense, 5′-GCGGTCACAGATATATCTGTCATCTGCC-3′, yielding a PCR product of 430 bp.
HIV entrance assay.
The method as described by Weichold et al13 was followed. Briefly, 2 × 106 cells were resuspended in 100 μL of a buffer containing 0.5% NP-40, 0.5% Tween 20, 2.5 mmol/L MgCl2, 10 mmol/L Tris, pH 8.3, 50 mmol/L KCl, and 120 μg/mL Proteinase K (Boehringer Mannheim, Indianapolis, IN) and incubated for 2 hours at 56°C. Proteinase K was inactivated at 98°C for 30 minutes. Samples were stored at −70°C. Aliquots (5 μL) were mixed with PCR reaction buffers (final volume, 50 μL) containing 10 mmol/L Tris, pH 8.3, 1.5 mmol/L MgCl2, 200 nmol/L each dNTPs, 0.5 μmol/L of each primers, and 2.5 U AmpliTaq Gold. PCR reactions were performed in a Robocycler (Stratagene, La Jolla, CA) using the following conditions: 1 cycle of 94°C for 9 minutes, 94°C for 45 seconds, 60°C for 90 seconds, and 72°C for 120 seconds; 35 cycles were performed with an additional extension time of 7 minutes at 72°C. The sequence of primers (5′-3′) used for the direct PCR is as follows: sense, CTACAAGGGACTTTCCGCTGG; and antisense, TCTTATCTGGCTCAACTGGTACTAGC.
Subsequently, an aliquot (5 μL) was used to perform nested PCR. The composition of PCR buffer and the amplification conditions were the same as the direct PCR. The sequence of primers (5′-3′) used for the nested PCR are as follows: sense, GACTTTCCGCTGGGGACTTTCC; and antisense, ATCTGGCTCAACTGGTACTAGCTTGT.
Flow cytometry.
For quantitative determination of cells bearing CR and to estimate the receptor density for MIP-1α or MIP-1β, a Fluorokine staining kit (R&D Systems Inc) was used following the manufacturer's recommendations, and flow cytometric analyses were performed. In some experiments, PHA-activated PBMCs were used as a positive control for the staining. Phycoerythrin (PE)-conjugated monoclonal antibodies to CD4 and CD14 as well as appropriate isotype control antibodies were obtained from Becton Dickinson. Flow cytometry was performed by incubating cells at 4°C in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 5 mmol/L EDTA for 30 minutes with antibodies at concentrations recommended by the manufacturer after cells had been washed and incubated with 10% normal goat serum in PBS to block unoccupied sites when indicated. Antibodies to CXCR4 (mIgG2a, clone 12G5; obtained from AIDS Research and Reference Reagents Program, Rockville, MD) were developed with anti-IgG2a (goat-antimouse, fluorescein isothiocyanate [FITC]-labeled, human-adsorbed; Caltag Laboratories, Burlingame, CA). The mouse IgG2a isotype control antibodies were obtained from Coulter (Hialeah, FL).
RESULTS
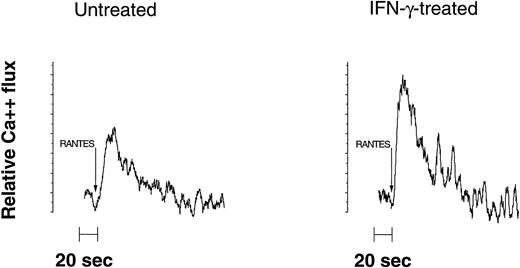
Because CRs are 7-transmembrane G-coupled proteins that mediate intracellular calcium mobilization,16 cell subpopulations were analyzed for their ability to respond to the chemokines RANTES, MIP-1α, MIP-1β, and SDF-1α using an intracellular calcium flux assay.14 Only a small proportion (<5%) of unprimed U937 cells responded minimally to RANTES and MIP-1α, whereas no response was observed with MIP-1β. After IFN-γ treatment, a marked increase in transient intracellular calcium flux response was observed to both RANTES and MIP-1α, but not to MIP-1β in about 50% of the cells (Fig 1). This effect was dose-dependent and was reduced to baseline levels when neutralizing (polyclonal) anti–IFN-γ antibodies were added during the preincubation time (not shown). Comparable to the observations made with primary monocytes (Fig 2, response to RANTES shown), the increase of calcium flux responses to RANTES and MIP-1α in U937 cells over time was notably increasing, beginning at day 2 and most pronounced 5 days after priming with IFN-γ (Fig 1, compare left with right panel). It is important to note that flux responses to SDF-1, a specific ligand for CXCR4, were not altered by IFN-γ, indicating a rather selective effect on CCR1, CCR3, CCR5, and possibly CCR4 (Fig 1).
IFN-γ–mediated increase of transient calcium flux in response to RANTES (ligand to CRs, including CCR1, CCR3, CCR4, and CCR5), MIP-1α (ligand to CRs, including CCR1, CCR4, and CCR5), MIP1-β (a ligand to CCR5), and SDF-1 (a ligand to CXCR4) as assessed in U937 cells. Fluorescence of bound versus unbound intracellular calcium in response to chemokines as depicted was analyzed by flow cytometry14 3 (left panel) and 5 days (right panel) after treatment with IFN-γ, as compared with untreated control cells (representative of 5 independent experiments).
IFN-γ–mediated increase of transient calcium flux in response to RANTES (ligand to CRs, including CCR1, CCR3, CCR4, and CCR5), MIP-1α (ligand to CRs, including CCR1, CCR4, and CCR5), MIP1-β (a ligand to CCR5), and SDF-1 (a ligand to CXCR4) as assessed in U937 cells. Fluorescence of bound versus unbound intracellular calcium in response to chemokines as depicted was analyzed by flow cytometry14 3 (left panel) and 5 days (right panel) after treatment with IFN-γ, as compared with untreated control cells (representative of 5 independent experiments).
Flow cytometrical detection of IFN-γ–mediated increased transient calcium flux response to RANTES in cells derived from elutriated monocytes from a healthy, HIV-seronegative donor. Untreated cells (depicted in the left panel) were compared with IFN-γ pretreated cells (right panel) in their response to 50 ng/mL RANTES (R&D Systems Inc). Fluorescence of bound versus unbound intracellular calcium in response to the chemokine was detected 5 days after treatment. The pattern of response is representative for analysis of monocytes derived from 6 subjects.
Flow cytometrical detection of IFN-γ–mediated increased transient calcium flux response to RANTES in cells derived from elutriated monocytes from a healthy, HIV-seronegative donor. Untreated cells (depicted in the left panel) were compared with IFN-γ pretreated cells (right panel) in their response to 50 ng/mL RANTES (R&D Systems Inc). Fluorescence of bound versus unbound intracellular calcium in response to the chemokine was detected 5 days after treatment. The pattern of response is representative for analysis of monocytes derived from 6 subjects.
A cell migration assay15 was used to determine whether treatment with IFN-γ enhances chemotaxis in response to RANTES. In this assay, the ability of U937 cells to migrate in culture through a membrane in response to different stimuli is determined by enumerating the cells in both sides of the culture chamber after 6 hours. IFN-γ pretreatment (30 ng/mL for 4 days) induced 64% ± 11% more cells to migrate in response to RANTES (75 ng/mL) as compared with controls.
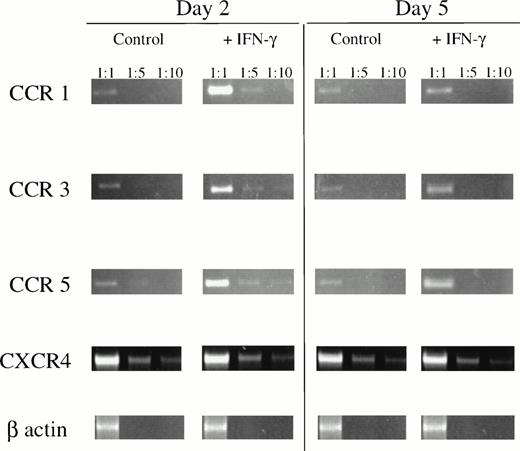
To determine if increased intracellular calcium mobilization and enhanced migration ability correlate with CR expression, mRNA levels in IFN-treated U937 cells were assessed over a time course of 6 days. Application of a standardized RT-PCR method showed differential modulation of CRs by IFN-γ. IFN-γ induced the highest increase in CCR1, CCR3, and CCR5 mRNA levels 3 days after treatment (at least 5-fold increase as compared with untreated controls; Fig 3). In the context of the calcium flux data, these results demonstrate that the peak of mRNA expression increase precedes CR expression by at least 48 hours. However, expression of CCR2b and CCR4 remained undetectable (data not shown), whereas constitutive levels of CXCR-4 mRNA were not altered by treatment with IFN-γ (Fig 3). Undetectable mRNA for CCR4 excludes with a high probability the involvement of this receptor in the observed chemokine binding and signaling in our system. Constitutive expression of CXCR4 mRNA, together with unchanged calcium flux in response to SDF-1, indicates that IFN-γ–induced changes in CR expression patterns are not the result of an overall change in cellular activation but more specifically related to only some and distinct cellular pathways.
IFN-γ enhances CR mRNA levels in U937 cells as determined by standardized RT-PCR. Samples containing similar a amount of viable cells as determined by trypan blue exclusion were analyzed at days 2 and 5 after treatment with IFN-γ. To determine the increase in mRNA expression upon treatment, samples were amplified using different dilutions (as indicated) and β-globin amplification was used to confirm comparability of the samples. The influence of possible contamination by genomic DNA in RT-PCR reactions was excluded by control amplifications in the absence of RT, because no signals were obtained under these conditions (not shown). Results are representative of 3 independent experiments.
IFN-γ enhances CR mRNA levels in U937 cells as determined by standardized RT-PCR. Samples containing similar a amount of viable cells as determined by trypan blue exclusion were analyzed at days 2 and 5 after treatment with IFN-γ. To determine the increase in mRNA expression upon treatment, samples were amplified using different dilutions (as indicated) and β-globin amplification was used to confirm comparability of the samples. The influence of possible contamination by genomic DNA in RT-PCR reactions was excluded by control amplifications in the absence of RT, because no signals were obtained under these conditions (not shown). Results are representative of 3 independent experiments.
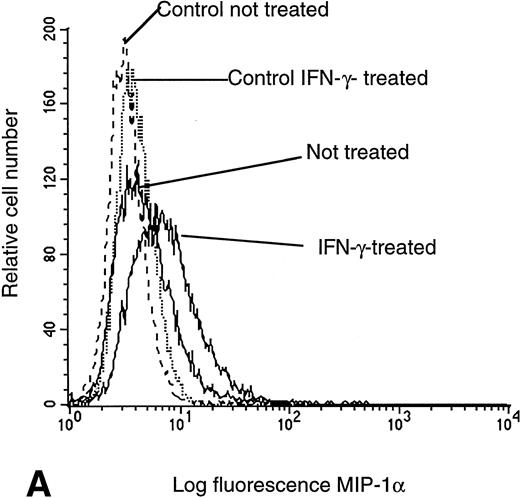
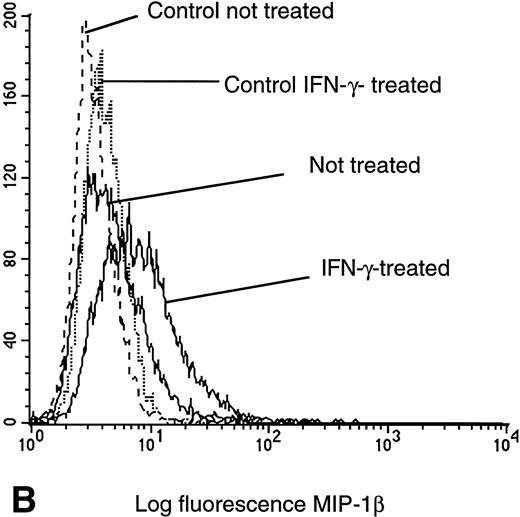
Because calcium flux response, migration, and specific mRNA expression provide functional but indirect measures of cell surface CR density, we stained IFN-γ–pretreated U937 cells with the specific biotinylated ligands MIP-1α and MIP-1β and compared these samples with untreated cells by flow cytometry using a ligand-biotin/avidin-FITC detection system. In confirmation of calcium flux, cell migration, and mRNA data, a significant increase in fluorescence intensity was observed in cells labeled with MIP-1α (Fig 4A) or MIP-1β (Fig 4B), indicating a higher surface receptor density of CCR1 and CCR5 after IFN-γ treatment. Results from direct cell surface molecule staining for CXCR4 with specific monoclonal antibodies confirmed that previous calcium flux and mRNA data in that the density of cell surface CXCR4 were not altered by pretreatment of IFN-γ (Fig 4C). These results exclude a nonspecific action on a cell's calcium flux apparatus by IFN-γ and indicate a relative specific and distinct cellular activation and enhanced expression of some CR subsets. It is worth noting that the levels of cell surface CD4 expression were assessed by flow cytometry and found to be unchanged under different treatment conditions (Fig 4D).
Flow cytometry assessment of HIV receptor cell surface expression on U937 cells. Cell surface CR expression was increased after treatment with IFN-γ as determined by labeling with MIP-1α. Enhanced binding of MIP-1β was detected on cells pretreated with IFN-γ, as compared with untreated controls. CXCR4 expression remained unchanged, independent of pretreatment, as assessed with specific anti-CXCR4 monoclonal antibodies. CD4 expression on U937 cells was not altered by exposure to IFN-γ.
Flow cytometry assessment of HIV receptor cell surface expression on U937 cells. Cell surface CR expression was increased after treatment with IFN-γ as determined by labeling with MIP-1α. Enhanced binding of MIP-1β was detected on cells pretreated with IFN-γ, as compared with untreated controls. CXCR4 expression remained unchanged, independent of pretreatment, as assessed with specific anti-CXCR4 monoclonal antibodies. CD4 expression on U937 cells was not altered by exposure to IFN-γ.
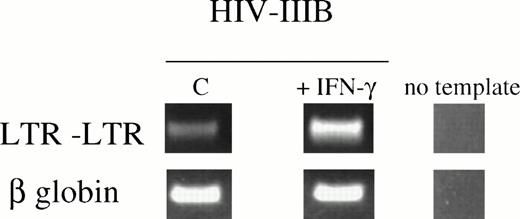
The demonstration that β-chemokines can inhibit HIV-1 entry into PBMCs17 and the subsequent identification of CRs as coreceptors for HIV infection16,18 have provided important pieces of the acquired immunodeficiency syndrome (AIDS) pathogenesis puzzle. Different HIV strains can now be functionally characterized and divided into 3 major groups: those that mainly use CCR5 as coreceptor (preferentially macrophage tropic), those that predominantly use CXCR4 as coreceptor (T lymphotropic), and dualtropic (using CCR3, CCR5, and CXCR4) strains.19 The density of these CRs on the surface of the APC represents one of the most important factors determining a cell's susceptibility to HIV infection.20 To evaluate whether the increased responsiveness to RANTES and MIP-1α and the enhanced expression of HIV coreceptors correlate with a higher susceptibility to HIV-1 infection, IFN-pretreated U937 cells were exposed to HIV-1(IIIB), a viral strain that uses CCR3 and CCR5 in addition to CXCR4. HIV-1 cellular entry and replication were assessed using a PCR technique to detect LTR-LTR circular forms,21 a convenient marker for newly synthesized viral DNA. After 5 days of pretreatment with IFN-γ, increased susceptibility to HIV-1(IIIB) infection was observed (Fig5). Convergent to this data, we detected at least fivefold increases in infection of IFN-γ–primed primary monocytes with HIV-1(BaL) as compared with untreated controls, using the same detection assay (not shown). The levels of cell surface CD4 expression that were found to be unchanged under different treatment conditions (shown for U937 in Fig 3D) indicate that differences in susceptibility to HIV-1 infection of monocytoid cells were unrelated to the expression of the HIV high-affinity receptor.
IFN-γ increases susceptibility to HIV-1IIIB infection in U937 cells. Standardized PCR amplification of LTR(U3)-(U5)LTR circular forms of HIV-1 DNA was applied to assess de novo viral replication,23 20 hours after exposure. Samples were normalized by enumerating viable cells using trypan blue exclusion. β-Globin amplification was applied to confirm comparability of the samples. Results are representative of 4 independent experiments.
IFN-γ increases susceptibility to HIV-1IIIB infection in U937 cells. Standardized PCR amplification of LTR(U3)-(U5)LTR circular forms of HIV-1 DNA was applied to assess de novo viral replication,23 20 hours after exposure. Samples were normalized by enumerating viable cells using trypan blue exclusion. β-Globin amplification was applied to confirm comparability of the samples. Results are representative of 4 independent experiments.
DISCUSSION
Although inhibitory effects of IFN-γ on HIV-gene expression and viral replication are well established, previous work demonstrated that infection of primary mononuclear phagocytes with a dualtropic strain of HIV-1 (JR-FL) was enhanced by treatment of the cells with IFN-γ before infection.22 Similar to our observations, CD4 expression as determined by flow cytometry was not altered by treatment with IFN-γ. The increased susceptibility of monocyte-derived cells was generally thought by the investigators to be linked to changes in activation and differentiation mediated by IFN-γ, although the mechanism was not specifically defined. We reproduced these observations in assessing the susceptibility of primary monocyte-derived cells to HIV-1(BaL) infection. Viral entrance was found to be increased in IFN-γ–pretreated cells as compared with controls. In addition, we have measured calcium flux responses to chemokines with and without IFN-γ pretreatment in normal donor-derived monocytoid cell populations and observed a reproducible and consistent response pattern similar to that in U937, excluding a cell line restricted artifact. However, as commonly observed when primary cells derived from different donors are used for experiments, a high degree of variability in these assays cannot be avoided. Consequently, we used a well-accepted APC model, the U937 cell line, that allowed us to characterize the observed phenomenon more in detail.
We now have established that IFN-γ increases CCR1, CCR3, and CCR5 mRNA expression on U937 cells and that this event correlates with a cell's increased susceptibility to HIV-1 entrance. Coreceptor function of CCR3 and CCR5, but not of CCR1, was previously demonstrated for different T-cell–tropic HIV-1 strains, using an HIV-envelope–dependent cell-cell fusion model.23Compatibly, our data indicate that CCR3, CCR5, and CXCR4 can act cooperatively in mediating infection of cells by T-cell–tropic HIV-1 strains (O. Barabitskaja, manuscript in preparation). Further evidence for links between IFN-γ, CCR3, and CCR5 in vivo is provided by the fact that elevated levels of IFN-γ were found in the cerebrospinal fluid (CSF) of HIV-1 seropositive individuals24 and that HIV-1 infection of microglial cells is mediated through CCR3 and CCR5.25 High levels of IFN-γ may thus increase the susceptibility of brain macrophages to HIV infection, an event considered to be important in the onset of AIDS-associated dementia.26
It is interesting to note that CCR5 receptor expression as detected with biotinylated MIP-1β increased after IFN-γ treatment in U937 cells, in accordance with the mRNA data, whereas no calcium flux induction by MIP-1β (the specific ligand for CCR5) was detectable. We thus speculate that U937 has a specific defect that involves CCR5 signaling, possibly at the level of receptor trafficking that includes surface receptor internalization and recycling.
Usually, during the course of HIV-1 infection, a viral evolution due to mutation-related shifts of receptor usage from predominantly M-tropic (CCR5), via dual-tropic, to T-tropic (CXCR4) strains or quasi-species is observed.19 These events, especially the evolving predominance of T-cell–tropic strains within an organism, tend to correlate with HIV disease progression.27 The driving forces for the selection and establishment of viral quasispecies within the organism are neither clearly defined nor understood. The ability of receptors such as CCR3 to support and enhance viral entry independently from the predominant tropism of HIV-1 strains may represent an important key for the switch from CCR5 to CXCR4 receptor usage and hence for disease progression.
Based on our observations, we hypothesize that, during the course of HIV-infection, elevated levels of IFN-γ may act by differentially upregulating CRs on APCs. As a possible consequence, a larger population of cells becomes attracted to the site of inflammation and develops into effector APC. Developing APC express relatively high levels of CRs and become highly susceptible to HIV infection. This may lead to the generation of a long-lasting reservoir of infected cells as well as to HIV-mediated depletion of APC subsets. Under these conditions, when the balance of appropriate immune cell interaction is altered, excessive production of IFN-γ by (inappropriately) stimulated T cells will result in a vicious cycle of enhanced activation, recruitment, and infection, leading over time to the depletion of immune cells observed in AIDS.
Activation patterns of T cells have been categorized, based on the cytokines predominantly produced, into T-helper cell type 1 (Th1) response (mainly IL-2 and IFN-γ production) and T-helper type 2 (Th2) response (mainly IL4, IL-5, and IL-10 production). During the course of HIV disease, a shift from Th1 to an antivirally less effective Th2 pattern has been proposed.28 Although the in vivo relevance of this model to explain altered immune responses in AIDS is debatable, because in situ analysis failed to produce evidence for a switch in cytokine phenotypes from Th1 to Th2 during disease progression,29 the increased production of IFN-γ was found to be consistently eminent. Cytokines (such as IFNs) that display a comparatively short half-life mainly function within a tightly regulated (or disregulated), locally restricted environment. Thus, IFN-γ produced in a microenvironment as part of a first line of response (proinflammatory) would be responsible for enhancing the invasion of susceptible immune cells and possibly prime more T cells into a Th1 phenotype. Subsequently, Th1 type cytokines may affect APCs, induce their maturation and, upon their interaction with T cells, a shift toward a Th2 cytokine phenotype would be mediated within this particular microenvironment.30 Based on our observation, it appears that it is predominantly during the Th1 cytokine phase of a local immune response when elevated production of IFN-γ facilitates recruitment of HIV target cells as well as HIV infection.
The newly identified regulatory function of IFN-γ on subsets of APCs may help to understand the complex patho-physiologic role of this cytokine, not only during hematopoiesis and in homeostasis of blood cells. It may also provide new clues for alternative iatrogen control of inflammation and neoplasia and contribute to the development of alternative immune-modulating therapeutic approaches for AIDS.
Supported in part by IRCCS Policlinico S. Matteo, Ricerca Corrente No. 820RCR97/01.
Address reprint requests to Frank F. Weichold, MD, PhD, Institute of Human Virology, University of Maryland, 725 W Lombard St, Baltimore, MD 21201; e-mail: weichold@umbi.umd.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal