Abstract
Differentiation-dependent thymocyte migration in the thymus may be important for T lymphopoiesis and might be regulated by thymic chemoattractants. We examined modulation of chemotactic responsiveness of thymocyte subsets during their early to late stages of development in response to 2 thymus-expressed chemokines, SDF-1 and CKβ-11/MIP-3β/ELC. SDF-1 shows chemotactic preference for immature thymocytes (subsets of triple negative thymocytes and double positive [DP] subset) over mature single positive (SP) thymocytes. CKβ-11/MIP-3β/ELC shows low chemotactic activity on the immature thymocytes, but it strongly attracts mature SP thymocytes, effects opposite to that of SDF-1. SDF-1–dependent chemoattraction of immature thymocytes is not significantly desensitized by a negative concentration gradient of CKβ-11/MIP-3β/ELC, and chemoattraction of mature SP thymocytes to CKβ-11/MIP-3β/ELC is not antagonized by SDF-1, demonstrating that these two chemokines have different chemoattractant preferences for thymocyte subsets and would probably not inhibit each other's chemotaxis in the event of microenvironmental coexpression. The chemotactic responsiveness of thymocytes and mature T cells to the 2 chemokines is respectively enhanced after selection process and migration to the spleen. These studies demonstrate the presence of thymocyte chemoattractants with differential chemotactic preference for thymocytes, a possible mechanism for thymocyte migration in the thymus.
CHEMOKINES ARE members of a family of small proteins with molecular weight around 10 kD.1-5 There are at least 4 chemokine subfamilies (CXC, CC, C, and CX3C), depending on the number of cysteines and spacing between the first 2 cysteines. Chemokines bind G-protein–coupled receptors with 7-transmembrane domains.6-8 Although the apparent functions of chemokines are to chemoattract diverse cell types, it has been reported that these cytokines have many functions, such as inhibition of human immunodeficiency virus (HIV) infection, regulation of angiogenesis, regulation of immune reaction and hematopoiesis, and antitumor effects.3 SDF-1 is a CXC chemokine that is expressed ubiquitously in most tissues, including thymus.9 SDF-1 has efficacious chemotactic activity for hematopoietic progenitors,10,11 lymphocytes, and monocytes.12 Mice deficient in SDF-1 expression cannot survive and die around birth.13 SDF-1 specifically binds the CXCR4/fusin/LESTR/HUMSTR receptor and inhibits T-trophic HIV infection.14,15 CKβ-11/MIP-3β/ELC, a CC chemokine, is highly expressed in thymus16,17 and has chemotactic activity for T and B cells18 and a subset of hematopoietic progenitor cells (Kim and Broxmeyer, unpublished results). It specifically binds to the CCR7/EBI1/BLR2 receptor.17Both SDF-1 and CKβ-11/MIP-3β/ELC are divergent from other CXC and CC chemokines, showing low amino acid identities to other chemokines. The chromosomal location for the SDF-1 and CKβ-11/MIP-3β/ELC genes is 10 and 9, respectively, in humans,17 unlike most CXC and CC chemokines that have their genes, respectively, in human chromosomes 4 and 17.1 2
Stem cells or common lymphoid progenitor cells migrate from bone marrow to the fetal thymic primordium to seed thymic T-cell hematopoiesis.19 Immature thymocytes undergo a multistep differentiation pathway, forming an immune system able to distinguish self from nonself and respond to most pathogens.20 T-cell hematopoiesis in the thymus is highly organized. Immature double negative (DN) thymocytes are found in the outer cortex of thymus, whereas more mature double positive (DP) thymocytes reside in the remainder of the cortex. Mature single positive (SP) thymocytes are found in the medulla, where they migrate to the peripheral blood and become mature T cells.21,22 Specialized subcompartments of thymus have been identified based on epithelial cell distribution and accessory cell composition.21 It is likely that migration of thymocytes at different stages of differentiation is a regulated process.
To study the possibility that different chemokines attract thymocytes differentially and thus coordinate thymocyte migration within the thymus, we examined the specific chemotactic activity of 2 thymus-expressed chemokines. Our results indicate that the chemokines in thymus have different chemoattractant preferences for thymocyte subsets and may selectively control the migration of these cells in the thymus during T lymphopoiesis.
MATERIALS AND METHODS
Chemokines and antibodies.
SDF-1 was a kind gift from Dr Ian Clark-Lewis (University of British Columbia, Vancouver, British Columbia, Canada). Human CKβ-11/MIP-3β/ELC was expressed in Chinese hamster ovary (CHO) cells. Expression, purification, N-terminal analysis, and matrix-assisted laser desorption ionized (MALDI) mass spectrometry for recombinant human CKβ-11/MIP-3β/ELC were performed as previously described.18 This protein was previously demonstrated to be chemotactically active for mouse cells.18 For purification of lin− thymocytes, the following antibodies were used: anti–CD3e-biotin (clone 144-2C11), anti–CD4-biotin (clone L3T4), anti–CD8a-biotin (clone Ly-2), anti–Gr-1-biotin (clone RA3-6B2), anti–NK1.1-biotin (clone PK136), anti–Mac-1-biotin (clone M1/70), and anti–B220-biotin (clone RA3-6B2) (obtained from Pharmingen, San Diego, CA). For analysis of thymocyte migration, the following antibodies were used: anti–CD3-phycoerythrin (PE)1 (clone 144-2C11), anti–CD4-TriColor (clone L3T4), anti–CD8a-fluorescein isothiocyanate (FITC) (clone Ly-2), anti–CD25-PE (clone PC61), and anti–CD69-PE (H1.2F3) (Pharmingen). Anti–CD44-FITC (IM7.8.1) and Streptavidin-PE were purchased from Caltag Laboratories (South San Francisco, CA).
Animals and cell separation.
Four-week-old female BALB/C mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in the Indiana University Medical Center Laboratory Animal Resource Center in accordance with guidelines of the Committee on Animals of the Indiana University School of Medicine. Thymuses of 4- to 5-week-old female BALB/C mice were used to prepare single cell suspensions of thymocytes. Thymuses were crushed on an iron mesh. After debris, red blood cells (RBCs) and polymorphonuclear cells (PMNs) were removed by centrifugation on lympholyte-M (Cedarlane, Hornby, Canada), thymocytes were depleted of lin+ non-T cells by a magnetic bead depletion method using a cocktail of biotin-labeled antibodies to Gr-1, B220, Mac-1, and NK1.1 according to the manufacturer's recommendation (Pharmingen). Streptavidin-beads (Miltenyi Biotech, Auburn, CA) were used to deplete lin+ non-T cells. This depletion method routinely yielded non–T-cell–free thymocytes with purity greater than 97%. Triple negative (TN; CD3−CD4−CD8−) thymocytes were purified as described by others,23 except that Miltenyi Biotech streptavidin-beads were used to deplete antibody-coated cells in this study. Briefly, a cocktail of biotinylated antibodies (anti-CD3, anti-CD4, anti-CD8, anti-B220, anti–Gr-1, anti–Mac-1, and anti-NK1.1) was used to bind non-T cells and T-lineage cells. Streptavidin-bead (Miltenyi biotec) was used to deplete cells coated with the biotinylated antibodies. The purity of TN cells was routinely greater than 98%.
In vitro and organ chemotaxis assay.
Chemotaxis assays were performed using the Costar Transwell system (6.5-mm diameter, 5-μm pore size, polycarbonate membrane) as previously described.18 Briefly, purified thymocytes (5 × 105) were added to the upper chamber of each well, and chemokines were added to the upper and/or lower chamber at various concentrations. Cell migration was allowed to occur for 3 hours and cells migrating to the lower chamber were harvested and either directly counted by FACscan (Becton Dickinson, Mountain View, CA) for 30 seconds or counted after staining with fluorescent antibodies to CD4 and CD8, CD25 and CD44, or CD4, CD8, and CD69. Migrated cells were calculated as a percentage of the specifically phenotyped input cells. For organ chemotaxis, thymuses were carefully removed from 4- to 5-week-old female BALB/C mice, and 1 thymus was put into the upper chamber of the Transwell as a monolayer to cover the entire surface of the 6.5-mm membrane. Diluted chemokines (final volume, 600 μL) were added to the lower chamber, and chemotaxis medium (50 μL) without chemokines was added to the upper chamber to form a chemotactic gradient. Chemotaxis chambers were incubated for 4 to 5 hours at 37°C and 5% CO2 in a humidified incubator. Thymocytes migrating into the lower chamber were stained with anti–CD4-TriColor and anti–CD8-FITC. CD4+SP, CD4+8+DP, CD4−8− DN, and CD8+SP thymocyte populations were analyzed on the basis of CD4 and CD8 expression by FACscan (Becton Dickinson). Isolation and chemotaxis of human CD4+ T cells and CD8+ T cells were assayed as previously described.18 For pertussis toxin inhibition of chemotaxis, pertussis toxin (Sigma Chemical Co, St Louis, MO) at various concentrations (0, 10, 100, and 1,000 ng/mL) was used to pretreat thymocytes in the chemotaxis buffer (5 × 107 cells/mL) for 1 hour at 37°C and 5% CO2 in a humidified incubator before chemotaxis.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of CXCR4 and CCR7 mRNA distribution in different thymocyte subsets.
Thymocytes were stained with anti–CD4-TriColor, anti–CD8-FITC, and a cocktail of biotinylated antibodies (anti-B220, anti–Gr-1, anti–Mac-1, and anti-NK1.1), followed by second staining with streptavidin-PE. Non-T cells were excluded by gating only Lin (PE)-negative thymocytes. CD4+, CD4+8+, CD4−8−, and CD8+thymocytes (≅97% pure) were sorted using a FACStar plus (Becton Dickinson). Total RNA was isolated from the sorted cells (5 × 105) with Trizol solution (GIBCO-BRL/LifeTechnologies, Grand Island, NY) according to the manufacturer's instructions. Single-strand cDNA was made from the 0.5 μg total RNA with SuperScript Preamplification System for First Strand Synthesis (GIBCO-BRL/LifeTechnologies). Primers used to detect CXCR4 mRNA were 5′-GTT CGA ATT CAA CCA CCA CGG CTG TAG AG-3′ for a forward primer and 5′-GTC AGC CAT GGC ATC AAC TG-3′ for a reverse primer, which give PCR products of 349 bp. For CCR7 mRNA, 2 primers were used, ie, 5′-GTT CGA ATT CAT CAG CAT TGA CCG CTA CGT-3′ for forward primer and 5′-GCG TGC CTG GAG CAA GGT ACG-3′ for reverse primer, which give 318-bp PCR products. PCR reactions were performed in the presence of 0.1 μL of32PdCTP (Amersham, Arlington Heights, IL; 800 mCi/mmol/L/reaction) for 30 cycles (94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute). The PCR products were resolved on a 5% nondenaturing polyacrylamide gel. After drying, the filters were analyzed by Molecular Imager (Bio-Rad, Hercules, CA) and exposed on x-ray films. As an internal control, β-actin mRNA was amplified using two primers: 5′-ATG TTT GAG ACC TTC AAC AC-3′ and 5′-CACGTC ACA CTT CAT GAT GG-3′.
Statistical analysis.
The Student's t-test was used to analyze data for significance differences. P values less than .05 were regarded as significant differences.
RESULTS
SDF-1 and CKβ-11/MIP-3β/ELC are chemoattractants for thymocytes.
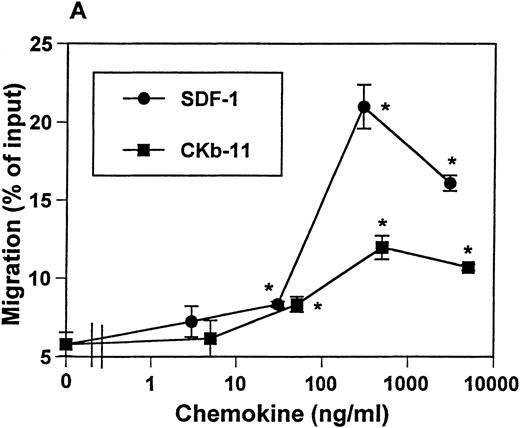
Expression of SDF-1 mRNA was previously reported to be ubiquitous, with a high level of mRNA expression detected in thymus.9 The mRNA of CKβ-11/MIP-3β/ELC is detected in thymus, lymph node, trachea, colon, small intestine, and lung.16 17Interestingly, thymus is the organ expressing the highest level of CKβ-11/MIP-3β/ELC mRNA. These data suggest the possible roles of these chemokines in thymocyte development and migration. We examined the chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for thymocytes. SDF-1 and CKβ-11/MIP-3β/ELC both attracted thymocytes depleted of non-T cells in a dose-dependent manner (Fig 1A). SDF-1 often provided a stronger chemoattractant signal for total thymocytes than CKβ-11/MIP-3β/ELC. Optimal chemokine concentrations for thymocyte chemotaxis were about 300 to 500 ng/mL for both SDF-1 and CKβ-11/MIP-3β/ELC.
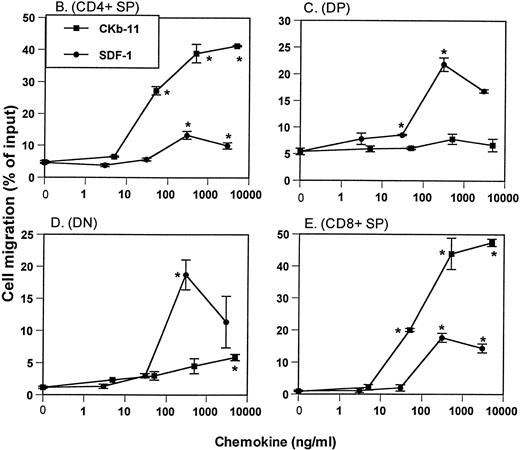
Chemotactic preferences of SDF-1 and CKβ-11/MIP-3β/ELC for thymocyte subsets. Effects on total thymocytes (A), CD4+ SP (B), DP (C), DN (D), and CD8+ SP (E) cells are shown. Thymocyte isolation and chemotaxis assays were performed as described in the Materials and Methods. Thymocytes depleted of non-T cells were used as input cells for chemotaxis. Numbers of cells migrating to the lower chamber were expressed as the percentage of input thymocytes in the upper chamber at the start time of chemotaxis. Data are expressed as the mean (±difference) of the percentage of cell migration obtained from duplicated experiments and are representative of 5 independent experiments. *Significant differences (P < .05) from background migration.
Chemotactic preferences of SDF-1 and CKβ-11/MIP-3β/ELC for thymocyte subsets. Effects on total thymocytes (A), CD4+ SP (B), DP (C), DN (D), and CD8+ SP (E) cells are shown. Thymocyte isolation and chemotaxis assays were performed as described in the Materials and Methods. Thymocytes depleted of non-T cells were used as input cells for chemotaxis. Numbers of cells migrating to the lower chamber were expressed as the percentage of input thymocytes in the upper chamber at the start time of chemotaxis. Data are expressed as the mean (±difference) of the percentage of cell migration obtained from duplicated experiments and are representative of 5 independent experiments. *Significant differences (P < .05) from background migration.
Differential chemotactic preference of SDF-1 and CKβ-11/MIP-3β/ELC.
Expression of CD4 and CD8 on thymocytes defines 4 populations of thymocytes: most immature CD4−CD8−DN thymocytes, immature CD4+CD8+ DP thymocytes, and mature CD4+ and CD8+ SP T cells. To examine the chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC on thymocyte subpopulations, we isolated thymocytes depleted of non-T cells, performed chemotaxis assays using the 2 chemokines at various concentrations, and stained input and migrated cells with anti–CD4-TriColor and anti–CD8-FITC. For the CD4+thymocyte population, CKβ-11/MIP-3β/ELC provided strong chemotactic activity, whereas SDF-1 demonstrated weak chemotactic activity (Fig1B). The range of net CD4+ SP cell chemotaxis above background level (shown as the percentage of input cell number) through 5 independent experiments was 6% to 16% for SDF-1 and 35% to 60% for CKβ-11/MIP-3β/ELC. For DP thymocytes, SDF-1 attracted these cells far better than CKβ-11/MIP-3β/ELC (Fig 1C). The range of net chemotaxis of DP thymocytes through 5 independent experiments was 10% to 19% for SDF-1 and 2% to 5% for CKβ-11/MIP-3β/ELC. SDF-1 also attracted DN thymocytes very well (17% to 21%; n = 5 experiments), whereas CKβ-11/MIP-3β/ELC demonstrated weak chemotactic activity (3% to 8%; n = 5 experiments) for this thymocyte population (Fig 1D). Although both SDF-1 and CKβ-11/MIP-3β/ELC attracted CD8+ thymocytes, the chemotactic activity of CKβ-11/MIP-3β/ELC was far greater than that of SDF-1 (Fig 1E). The range of net chemotaxis for CD8+ SP thymocytes through 5 independent experiments was 13% to 20% for SDF-1 and 33% to 47% for CKβ-11/MIP-3β/ELC. Thus, SDF-1 is more selective than CKβ-11/MIP-3β/ELC for chemotaxis of DP and DN thymocytes, and CKβ-11/MIP-3β/ELC is more selective than SDF-1 for the chemotaxis of CD4+ and CD8+ thymocytes.
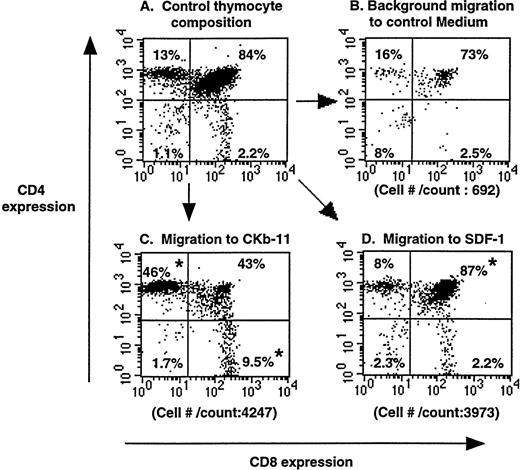
To examine the chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC in the context of the thymus, we set up chemotaxis experiments using whole thymuses. A whole murine thymus was placed on a polycarbonate membrane of the Transwell system so that the thymus tightly covered the entire membrane surface. Chemokines, at optimal concentrations, were added to the lower chamber and blank medium to the upper thymus-containing chamber forming a chemokine gradient from the lower through the thymus and to the upper chamber. In this setting, SDF-1 and CKβ-11/MIP-3β/ELC attracted thymocytes out of thymus in the upper chamber to the lower chamber (Fig 2). The composition and number of mobilized thymocytes in response to SDF-1 were different from that attracted to CKβ-11/MIP-3β/ELC or control medium (background migration). Although SDF-1 attracted all thymocyte subsets compared with control numbers, specific attraction of the DP subset was more notable than other subsets. Although, CKβ-11/MIP-3β/ELC attracted notable numbers of SP and DP thymocytes, it preferentially attracted more CD4+ and CD8+ SP subsets than DP subset. This organ chemotaxis assay supports the chemotaxis data shown in the Fig 1 for starting thymocytes in suspension culture.
Chemoattraction of thymocytes from thymuses (organ chemotaxis) by SDF-1 and CKβ-11/MIP-3β/ELC. Thymocytes in thymus before chemotaxis (A) and thymocytes migrating to control medium (B), CKβ-11/MIP-3β/ELC (C), and SDF-1 (D) are shown. Total cell numbers per count for 30 seconds by FACscan and the percentage of each subset in total thymocytes, representative of 4 experiments, are shown. Thymus organ chemotaxis assays were performed as described in the Materials and Methods. Migrated thymocytes were stained with anti–CD4-TriColor and anti–CD8-FITC, acquired for 30 seconds, and analyzed by FACscan. The mean composition (±SD) of the 4 thymocyte subsets from the 4 experiments is 12.1 ± 0.6 (CD4+ SP), 83.8 ± 2.8 (DP), 2.8 ± 0.6 (DN), and 2.6 ± 0.7 (CD8+SP) for control thymocyte suspension; 11.2 ± 1.9 (CD4+SP), 80.2 ± 2.8 (DP), 5.6 ± 2.3 (DN), and 3.0 ± 0.7 (CD8+ SP) for those attracted to SDF-1; and 50.1 ± 9.8 (CD4+ SP), 30.7± 8.8 (DP), 2.4 ± 0.8 (DN), and 16.9 ± 3.2 (CD8+ SP) for those attracted to CKβ-11/MIP-3β/ELC. *Significant differences (P < .05) from control thymocyte subset composition.
Chemoattraction of thymocytes from thymuses (organ chemotaxis) by SDF-1 and CKβ-11/MIP-3β/ELC. Thymocytes in thymus before chemotaxis (A) and thymocytes migrating to control medium (B), CKβ-11/MIP-3β/ELC (C), and SDF-1 (D) are shown. Total cell numbers per count for 30 seconds by FACscan and the percentage of each subset in total thymocytes, representative of 4 experiments, are shown. Thymus organ chemotaxis assays were performed as described in the Materials and Methods. Migrated thymocytes were stained with anti–CD4-TriColor and anti–CD8-FITC, acquired for 30 seconds, and analyzed by FACscan. The mean composition (±SD) of the 4 thymocyte subsets from the 4 experiments is 12.1 ± 0.6 (CD4+ SP), 83.8 ± 2.8 (DP), 2.8 ± 0.6 (DN), and 2.6 ± 0.7 (CD8+SP) for control thymocyte suspension; 11.2 ± 1.9 (CD4+SP), 80.2 ± 2.8 (DP), 5.6 ± 2.3 (DN), and 3.0 ± 0.7 (CD8+ SP) for those attracted to SDF-1; and 50.1 ± 9.8 (CD4+ SP), 30.7± 8.8 (DP), 2.4 ± 0.8 (DN), and 16.9 ± 3.2 (CD8+ SP) for those attracted to CKβ-11/MIP-3β/ELC. *Significant differences (P < .05) from control thymocyte subset composition.
We performed calcium mobilization assay for thymocytes to study differential signaling by SDF-1 and CKβ-11/MIP-3β/ELC. However, SDF-1 and CKβ-11/MIP-3β/ELC did not mobilize detectable levels of calcium in thymocytes at concentrations up to 300 nmol/L, suggesting chemotactic activity is not necessarily correlated with calcium-mobilizing activity in thymocytes (data not shown).
Expression of CXCR4 and CCR7 mRNA in different thymocyte subsets.
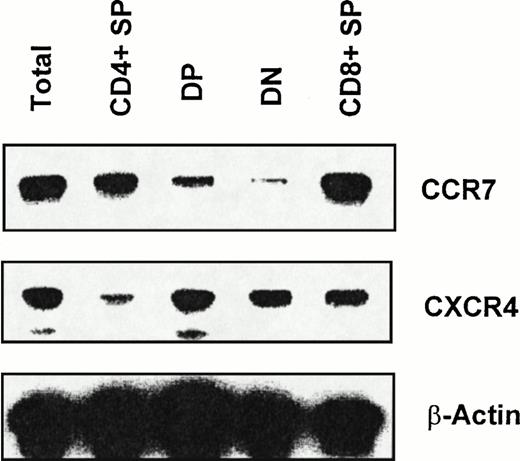
Chemokines induce chemotaxis by binding G-protein–coupled receptors with 7-transmembrane domains. We examined mRNA expression of CXCR4, a receptor for SDF-1, and CCR7, a receptor for CKβ-11/MIP-3β/ELC, in total thymocytes and thymocyte sub-populations. We sorted total thymocytes, CD4+ SP, DP, DN, and CD8+ SP thymocyte populations depleted of lin+ non-T cells for RT-PCR analysis of receptor mRNA expression. CXCR4 and CCR7 mRNA was detected not only in total thymocytes, but also in all 4 subpopulations of thymocytes (Fig 3; 1 representative of 3 independent experiments). However, we consistently observed that the mRNA expression level of CXCR4 in DP, DN, and CD8+ thymocytes was greater than in CD4+thymocytes (Fig 3). For CCR7 expression, the message level of CCR7 in CD4+ and CD8+ thymocytes was higher than in DP and DN thymocytes (Fig 3).
RT-PCR analysis of CXCR4 and CCR7 mRNA expression in thymocyte subsets. RT-PCR analyses were performed as described in the Materials and Methods using the same amount of total RNA obtained from each sorted thymocyte subset. Data shown are representative of 3 independent experiments. β-Actin was amplified as an internal control.
RT-PCR analysis of CXCR4 and CCR7 mRNA expression in thymocyte subsets. RT-PCR analyses were performed as described in the Materials and Methods using the same amount of total RNA obtained from each sorted thymocyte subset. Data shown are representative of 3 independent experiments. β-Actin was amplified as an internal control.
Migration behavior of total thymocytes in response to complex chemotactic environments formed by SDF-1 and CKβ-11/MIP-3β/ELC.
In the experiments examining chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC, only 1 chemoattractant had been added to the chemotaxis assay system. To examine the chemotactic specificity and selectivity of SDF-1 and CKβ-11/MIP-3β/ELC in a more complex chemotactic environment, we set up experiments by adding the 2 chemokines to the upper and/or lower chambers in a combinatorial manner. SDF-1 or CKβ-11/MIP-3β/ELC in the upper chamber respectively inhibited the chemotaxis by SDF-1 or CKβ-11/MIP-3β/ELC in the lower chamber, suggesting that there was homologous desensitization/inhibition of chemotaxis (Fig 4A, bar no. 2 v 4, bar no. 3v 5). The homologous inhibition by SDF-1 was more complete than that by CKβ-11/MIP-3β/ELC (Fig 4A, bar no. 5 v 4). The extent of cross or heterologous inhibition of chemotaxis between SDF-1 and CKβ-11/MIP-3β/ELC was less than the homologous inhibition (Fig4A, bar no. 4 v 7, bar no. 5 v 6). When SDF-1 and CKβ-11/MIP-3β/ELC were added to the lower chamber together, there was an additive effect in the net migration above background (Fig 4A, bar no. 8). Interestingly, either SDF-1 or CKβ-11/MIP-3β/ELC in the upper chamber inhibited the additive thymocyte migration by SDF-1 and CKβ-11/MIP-3β/ELC in a manner subtracting only the chemotactic effect of either chemokine in the lower chamber (Fig 4A, bars no. 9 and 10). Homologous desensitization of chemotaxis by the 2 chemokines was additive (Fig 4A, bar no. 11), but not complete enough to bring chemotaxis to background, implying that the incomplete homologous inhibition by CKβ-11/MIP-3β/ELC seen in bar no. 4 of Fig 4A contributed to this inhibition.
Migration behavior of total thymocytes (A) and thymocyte subsets, CD4+ SP (B), DP (C), DN (D), and CD8+SP (E) subsets, in chemotactic environments formed by SDF-1 and CKβ-11/MIP-3β/ELC. The 2 chemokines were added to upper or lower chambers as indicated in the Fig (+ and − denote the presence and absence of indicated chemokines, respectively) at optimal concentrations (500 ng/mL for CKβ-11/MIP-3β/ELC and 300 ng/mL for SDF-1). Thymocytes depleted of non-T cells were used as input cells for chemotaxis. Cell migration to lower chambers is expressed as the mean percentage of migration (±differences of duplicated experiments) of input thymocyte subsets added in the upper chamber at the start time of chemotaxis. Significant differences (P < .05) were observed between the following 2 bars: 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 2 and 8, 3 and 8, 8 and 10, and 9 and 11 (for [A]); 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 3 and 8, 8 and 9, and 10 and 11 (for [B]); 1 and 2, 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [C]); 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [D]); and 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 3 and 8, 8 and 9, 8 and 10, and 10 and 11 (for [E]).
Migration behavior of total thymocytes (A) and thymocyte subsets, CD4+ SP (B), DP (C), DN (D), and CD8+SP (E) subsets, in chemotactic environments formed by SDF-1 and CKβ-11/MIP-3β/ELC. The 2 chemokines were added to upper or lower chambers as indicated in the Fig (+ and − denote the presence and absence of indicated chemokines, respectively) at optimal concentrations (500 ng/mL for CKβ-11/MIP-3β/ELC and 300 ng/mL for SDF-1). Thymocytes depleted of non-T cells were used as input cells for chemotaxis. Cell migration to lower chambers is expressed as the mean percentage of migration (±differences of duplicated experiments) of input thymocyte subsets added in the upper chamber at the start time of chemotaxis. Significant differences (P < .05) were observed between the following 2 bars: 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 2 and 8, 3 and 8, 8 and 10, and 9 and 11 (for [A]); 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 3 and 8, 8 and 9, and 10 and 11 (for [B]); 1 and 2, 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [C]); 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [D]); and 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 3 and 8, 8 and 9, 8 and 10, and 10 and 11 (for [E]).
Chemotaxis of thymocyte subsets under complex chemotactic environments formed by SDF-1 and CKβ-11/MIP-3β/ELC.
The thymocyte migration pattern seen in Fig 4A describes the chemotaxis of total thymocytes in a chemotactic environment formed by SDF-1 and CKβ-11/MIP-3β/ELC. However, this is the mixed chemotactic behavior of many thymocyte subsets. To examine the chemotactic behavior of each thymocyte subset under these various chemotactic conditions, we tracked migration of the each thymocyte subset by staining the input and migrated cells with fluorescent anti-CD4 and anti-CD8 antibodies.
For the CD4+ SP thymocyte subset (Fig 4B), in addition to the typical homologous desensitization of chemotaxis by CKβ-11/MIP-3β/ELC (bar no. 2 v 4) or SDF-1 (bar no. 3v 5), there was heterologous desensitization of SDF-1–dependent chemotaxis by CKβ-11/MIP-3β/ELC (bars no. 6 and 7). However, the inhibition of CKβ-11/MIP-3β/ELC–dependent chemotaxis by SDF-1 was marginal, suggesting the dominance of CKβ-11/MIP-3β/ELC over SDF-1 on CD4+ thymocyte migration (bars no. 7 and 10). Although CKβ-11/MIP-3β/ELC–dependent homologous and heterologous desensitization was partial (bars no. 4, 9, and 11) in that it did not reduce the chemotaxis to the background level, it was the most influential factor in reducing the overall CD4+ SP cell chemotaxis.
In contrast to the CD4+ subset, SDF-1 was a dominant chemoattractant over CKβ-11/MIP-3β/ELC for the DP subset (Fig 4C). Efficient homologous desensitization was observed in SDF-1–dependent chemotaxis for the DP subset (bar no. 3 v 5), whereas the CKβ-11/MIP-3β/ELC–dependent chemotaxis was weak and homologous desensitization was not detectable (Fig 4C, bar no. 2 v 4). CKβ-11/MIP-3β/ELC in the upper chamber had no significant effect on SDF-1–dependent chemotaxis (bar no. 6). SDF-1 in the upper chamber in all conditions demonstrated a strong inhibitory effect on DP thymocyte migration (bars no. 10 and 11).
Chemotaxis of DN thymocytes (Fig 4D) was very similar to that of the DP thymocytes in that SDF-1 was a dominant chemoattractant for this subset. CKβ-11/MIP-3β/ELC showed a small but significant contribution not only in chemotaxis of DN thymocytes along with SDF-1 (bar no. 8), but also in homologous (bars no. 4 and 9) and heterologous desensitization (bars no. 6, 9, and 11) of the chemotaxis. SDF-1 was again dominant over CKβ-11/MIP-3β/ELC in the desensitization of chemotaxis (bar no. 9 v 10).
The effects in Fig 4E demonstrating the migration behavior of CD8+ SP thymocytes were similar to that of the CD4+ thymocytes in Fig 4B suggested the dominance of CKβ-11/MIP-3β/ELC over SDF-1 in induction of chemotaxis and inhibition of chemotaxis. Although the chemotactic activity of SDF-1 is weaker than that of CKβ-11/MIP-3β/ELC for this subset, it was significantly high, and SDF-1 showed homologous and heterologous desensitization of CD8+ thymocyte chemotaxis (bars no. 5, 7, 10,and 11).
Chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for TN thymocyte subsets.
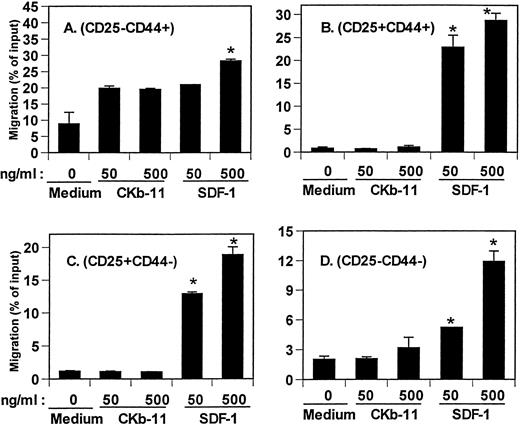
Lymphoid common progenitors migrate from bone marrow to fetal thymus to seed and repopulate the thymus. The earliest thymic lymphoid progenitors have a CD117+CD44+CD25−CD4lophenotype.24 This population is rare and represents only 0.05% of all thymocytes. It is difficult to examine the chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC on this rare thymocyte population. Instead, we isolated TN (CD3−CD4−CD8−) thymocytes that were depleted of committed T cells and non-T cells. We tracked chemotaxis of 4 thymocyte populations defined by expression of CD44 and CD25.25 The phenotypic flow of TN thymocyte differentiation is CD25−CD44+ → CD25+CD44+ → CD25+CD44− → CD25−CD44−. SDF-1 attracted all 4 TN thymocyte populations (Fig 5), which was predictable from the data in the earlier figures showing strong chemotactic activity of SDF-1 on DN thymocytes. CKβ-11/MIP-3β/ELC had no significant chemotactic activity for CD25+CD44+, CD25+CD44−, and CD25−CD44− TN thymocyte subsets. It did demonstrate some chemotactic activity for the CD25−CD44+ thymocyte population over background level, but this was not a statistically significant effect.
Chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for TN thymocyte subsets, CD25−CD44+(A), CD25+CD44+ (B), CD25+CD44− (C), and CD25−CD44− (D) subsets. Isolated TN thymocytes were added to the upper chambers, and the 2 chemokines were added to the lower chambers as indicated in the figure. Data are expressed as the mean percentage of migration (±differences of duplicated experiments) for each subset. *Significant differences (P < .05) from background migration.
Chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for TN thymocyte subsets, CD25−CD44+(A), CD25+CD44+ (B), CD25+CD44− (C), and CD25−CD44− (D) subsets. Isolated TN thymocytes were added to the upper chambers, and the 2 chemokines were added to the lower chambers as indicated in the figure. Data are expressed as the mean percentage of migration (±differences of duplicated experiments) for each subset. *Significant differences (P < .05) from background migration.
Effects of selection processes on chemotactic responsiveness of DP thymocytes to SDF-1 and CKβ-11/MIP-3β/ELC.
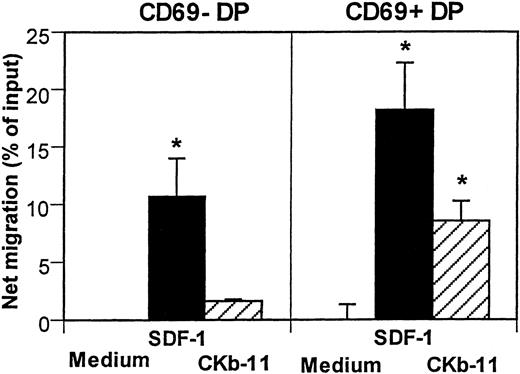
The specificity and affinity of T-cell antigen receptors (TCR) on DP thymocytes determine the fate of T-cell development.26-29 The negative selection process eliminates potentially harmful DP T cells that are reactive with self-antigens, whereas positive selection saves DP T cells with TCR that recognizes self-MHC. Expression of CD69 is induced after initiation of the selection on DP thymocytes.30 31 We examined the chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC to test whether chemotactic responsiveness of developing thymocytes is regulated by the selection process. The 2 chemokines attracted CD69+ DP cells better than CD69− DP cells (Fig 6). SDF-1 showed stronger chemotactic activity for both CD69− DP and CD69+ DP thymocytes than CKβ-11/MIP-3β/ELC. CKβ-11/MIP-3β/ELC attracted mainly CD69+ DP thymocytes, but not much CD69− DP thymocytes, suggesting it has a preference for positively selected DP thymocytes. CD69+ DP cells accounts for 12% of total DP thymocytes (data not shown). After chemotaxis, the CD69+ DP cells were enriched to account for 24% of total DP thymocytes in response to SDF-1 and 43% in response to CKβ-11/MIP-3β/ELC, suggesting that the thymic selection process modulates the chemotactic responsiveness of DP thymocytes to the chemokines (data not shown).
Chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for DP thymocyte subsets defined by CD69 expression. Total thymocytes were added to the upper chambers and optimal concentrations of SDF-1 (300 ng/mL) and CKβ-11/MIP-3β/ELC (500 ng/mL) were added to the lower chambers. Input and migrated cells were stained with antibodies to CD4, CD8, and CD69. CD4+CD8+CD69+ cells and CD4+CD8+CD69− cells were counted by FACscan for 30 seconds. Data are shown as the net mean percentage of migration after background subtraction for each subset (±SD of triplicated experiments). *Significant migration (P< .05) from background migration.
Chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for DP thymocyte subsets defined by CD69 expression. Total thymocytes were added to the upper chambers and optimal concentrations of SDF-1 (300 ng/mL) and CKβ-11/MIP-3β/ELC (500 ng/mL) were added to the lower chambers. Input and migrated cells were stained with antibodies to CD4, CD8, and CD69. CD4+CD8+CD69+ cells and CD4+CD8+CD69− cells were counted by FACscan for 30 seconds. Data are shown as the net mean percentage of migration after background subtraction for each subset (±SD of triplicated experiments). *Significant migration (P< .05) from background migration.
Mature splenic CD4+ SP T cells are more responsive to CKβ-11/MIP-3β/ELC and SDF-1 than their thymic counterparts.
It is known that SDF-1 and CKβ-11/MIP-3β/ELC are potent chemoattractants for human peripheral blood lymphocytes and T cells.12 18 However, SDF-1 was a weaker, whereas CKβ-11/MIP-3β/ELC was a stronger chemoattractant for murine thymic SP T cells in this study. It is possible that the chemotactic responsiveness of SP T cells to SDF-1 would be upregulated after migration to peripheral blood or secondary lymphoid tissues. To test this possibility, we compared the chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC on mouse thymic and spleen CD4+ and CD8+ SP T cells. Spleen CD4+ T cells were more responsive than their thymic counterparts to SDF-1 and CKβ-11/MIP-3β/ELC (Fig 7A). In mice, SDF-1 was less potent than CKβ-11/MIP-3β/ELC in chemotaxis of thymic and spleen CD4+ T cells (Fig 7A). CD8+ T cells from mouse spleen and thymus were equally responsive to CKβ-11/MIP-3β/ELC (Fig 7B). Although SDF-1 appeared to show higher chemotactic activity for mouse spleen CD8+ SP than their thymic counterparts, this was not a statistically significant difference (Fig 7B).
Comparison of chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for splenic T cells with that for thymic T cells. Murine CD4+ (A) and CD8+ (B) SP T cells from thymus and spleen were assayed for their chemotactic responsiveness to SDF-1 and CKβ-11/MIP-3β/ELC. Input and migrated cells were stained with fluorescent antibodies to human or mouse CD4 and CD8, acquired for 30 seconds, and analyzed by FACscan. Cell migration to lower chambers is expressed as the mean percentage (±differences of duplicated experiments) of input subset cell number added in the upper chamber at the start time of chemotaxis. Background migration was subtracted from the mean migration to show net migration. Significant differences were observed between the following 2 peak points: A and B, A and C, A and D, B and C, B and D, and C and D (for [A]); and A and D and B and D (for [B]).
Comparison of chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for splenic T cells with that for thymic T cells. Murine CD4+ (A) and CD8+ (B) SP T cells from thymus and spleen were assayed for their chemotactic responsiveness to SDF-1 and CKβ-11/MIP-3β/ELC. Input and migrated cells were stained with fluorescent antibodies to human or mouse CD4 and CD8, acquired for 30 seconds, and analyzed by FACscan. Cell migration to lower chambers is expressed as the mean percentage (±differences of duplicated experiments) of input subset cell number added in the upper chamber at the start time of chemotaxis. Background migration was subtracted from the mean migration to show net migration. Significant differences were observed between the following 2 peak points: A and B, A and C, A and D, B and C, B and D, and C and D (for [A]); and A and D and B and D (for [B]).
Differential sensitivity of SDF-1– and CK-11/MIP-3β/ELC-dependent chemotaxis to pertussis toxin.
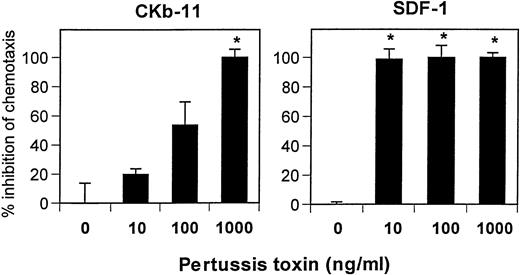
Pertussis toxin specifically inhibits only Gi/Go proteins among other G-proteins such as Gq, Gs, or G12/G13 proteins.32 Both SDF-1– and CKβ-11/MIP-3β/ELC–dependent chemotaxis were sensitive to pertussis toxin (Fig 8). However, SDF-1–dependent chemotaxis was more sensitive to pertussis toxin than that of CKβ-11/MIP-3β/ELC. Pertussis toxin at 10 ng/mL was effective in inhibiting SDF-1–dependent chemotaxis, whereas higher concentrations (∼1,000 ng/mL) were required for complete inhibition of CKβ-11/MIP-3β/ELC–dependent chemotaxis (Fig 8). This result supports that the 2 chemokines use different receptors (CXCR4 receptor for SDF-1 and CCR7 receptor for CKβ-11/MIP-3β/ELC). It also suggests that these 2 receptors have different sensitivities to pertussis toxin, possibly by using different G-proteins for signaling.
Chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC on thymocytes is sensitive to pertussis toxin. Low-density total thymocytes were preincubated with pertussis toxin at indicated concentrations for 1 hour and used for chemotaxis to SDF-1 (300 ng/mL) and CKβ-11/MIP-3β/ELC (500 ng/mL). Data are expressed as the mean percentage of inhibition (±differences of duplicated experiments). Complete inhibition (100%) means that chemotaxis to chemokines is inhibited to background or lower than background levels, and no inhibition (0%) represents maximum chemotaxis to chemokines observed without pertussis toxin treatments. Partial inhibition is between 0% and 100%. *Significant inhibition (P < .05) from controls (no pertussis toxin treatment).
Chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC on thymocytes is sensitive to pertussis toxin. Low-density total thymocytes were preincubated with pertussis toxin at indicated concentrations for 1 hour and used for chemotaxis to SDF-1 (300 ng/mL) and CKβ-11/MIP-3β/ELC (500 ng/mL). Data are expressed as the mean percentage of inhibition (±differences of duplicated experiments). Complete inhibition (100%) means that chemotaxis to chemokines is inhibited to background or lower than background levels, and no inhibition (0%) represents maximum chemotaxis to chemokines observed without pertussis toxin treatments. Partial inhibition is between 0% and 100%. *Significant inhibition (P < .05) from controls (no pertussis toxin treatment).
DISCUSSION
In this study, we examined the activity of 2 thymus-expressed chemokines, SDF-1, a CXC chemokine, and CKβ-11/MIP-3β/ELC, a CC chemokine, for chemoattraction of total and subsets of thymocytes. The thymus is organized into subcompartments supporting the proliferation and differentiation of thymocytes at different stages of maturation. Thymocyte migration between compartments correlates with the differentiation stage of thymocytes. Thymic chemoattractants may be involved in thymocyte migration within the thymus and also in thymocyte differentiation. One way to understand migration of thymocytes would be to investigate selective chemoattraction of thymocyte subsets by multiple thymic chemoattractants. This model would require multiple thymic chemoattractants that have specificity for each or a group of thymocyte subsets. Alternatively, thymic anatomy is designed such that thymocytes migrate in a unidirectional manner according to their differentiation stages by simple overflow of thymocytes from one microenvironment to another by chemoattraction to a nonselective chemoattractant(s). These 2 models are not necessarily incompatible. The preferences of the 2 chemokines, SDF-1 and CKβ-11/MIP-3β/ELC, for selective chemoattraction of thymocyte subsets demonstrated in this study provide evidence for the first model.
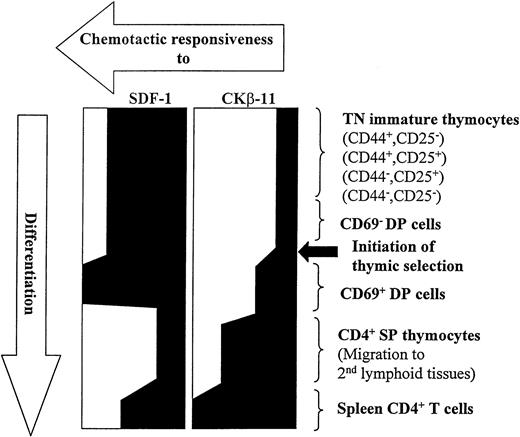
In the present study, we consistently observed the modulation of chemotactic responsiveness of thymocyte subsets to SDF-1 and CKβ-11/MIP-3β/ELC during their development (Fig 9). SDF-1 maintains its chemotactic potency on thymocytes from the early stages of TN thymocytes (CD25−CD44+, CD25+CD44+, CD25+CD44−, and CD25−CD44−) to intermediary DP thymocytes (before and after initiation of thymic selection). After DP thymocytes become SP CD4+ T cells, SDF-1 shows reduced chemotactic activity for SP thymocytes. In contrast, CKβ-11/MIP-3β/ELC shows minimal chemotactic activity for the TN and CD69− DN thymocyte subsets and begins to enhance chemotactic activity for CD69+ DP thymocytes. CKβ-11/MIP-3β/ELC demonstrates potent chemotactic activity for mature SP thymocytes. These data allow us to speculate that SDF-1 may be a major chemoattractant regulating migration of immature DN and DP thymocytes at premedullar stage (cortex) migration, whereas CKβ-11/MIP-3β/ELC regulates migration of mature thymocytes from the medullar compartment into peripheral blood. There was no significant desensitization or inhibition of chemotaxis to the major chemoattractant by the minor chemoattractant, suggesting that, in the event of microenvironmental coexpression, the preferential chemotaxis by the major chemoattractant may not be significantly affected by coexisting minor chemoattractants. Unfortunately, the spatial expression of SDF-1 and CKβ-11/MIP-3β/ELC proteins in different murine thymic microenvironments is not yet known. Thymic SP T cells enter the peripheral blood or lymphatic system and migrate to a secondary lymphoid system such as the spleen. Both SDF-1 and CKβ-11/MIP-3β/ELC demonstrate enhanced chemotactic activity for these spleen T cells. These results suggest that chemokines in different lymphoid organs (thymus v spleen) may have different roles to help achieve differential organ function. On the other hand, cells in different developmental stages or organs appear to actively modulate their responsiveness to chemokines to achieve the same goals.
Modulation of chemotactic responsiveness to SDF-1 and CKβ-11/MIP-3β/ELC during T-cell development from early TN stage to mature CD4+ T cells in secondary lymphoid tissues.
Modulation of chemotactic responsiveness to SDF-1 and CKβ-11/MIP-3β/ELC during T-cell development from early TN stage to mature CD4+ T cells in secondary lymphoid tissues.
The thymus is a site of massive cell death or apoptosis as a result of thymic selection processes.29 These selection processes occur with thymocytes at the DP stage. If chemokines are important in these processes, they can facilitate apoptosis by recruiting macrophages and apoptotic thymocytes together in specialized thymic compartments. The majority of DP thymocytes cannot survive the selection processes, and only a small fraction of DP cells are allowed to contribute to the total T-cell repertoire.27 It is also possible that thymic chemoattractants may be involved in the rescue of selected DP thymocytes by inducing the migration of selected thymocytes away from phagocytic macrophages. SDF-1 and CKβ-11/MIP-3β/ELC may be appropriate chemokines for such functions because they attract CD69+ DP thymocytes.
CXCR4, the receptor for SDF-1, is expressed on human peripheral blood lymphocytes, neutrophils, T cells, and blood monocytes.33CCR7, the receptor for CKβ-11/MIP-3β/ELC, is expressed in activated peripheral blood lymphocytes, Epstein-Barr virus-positive B-cell lines, and most lymphoid tissues.34,35 We have demonstrated herein that the mRNA for these receptors is also expressed in most subsets of thymocytes. Recently, it was reported that CXCR4 is expressed at a higher level on immature DP than mature CD4+ SP human thymocytes, suggesting a possible role of the CXCR4 coreceptor for HIV infection of immature thymocytes.36 This differential CXCR4 expression level among human thymocyte subsets is similar to what we have seen on mouse thymocytes. Taken together, the murine and human data imply that there are potential roles for thymic chemokine receptors and their ligands in thymocyte migration and differentiation. Expression of CXCR4 mRNA appears higher in DP, DN, and CD8+SP thymocytes than in CD4+ SP thymocytes, whereas CCR7 mRNA expression appears higher in SP cells than in immature DP and DN thymocytes. Overall, the receptor message levels correlate with the chemotactic activity and selectivity of SDF-1 and CKβ-11/MIP-3β/ELC for thymocyte subsets.
Recently, several chemokines have been reported to have chemotactic activity for thymocytes. Thymus-expressed chemokine (TECK) and lymphotactin have been reported to attract thymocytes, although the precise chemotactic specificity of each chemokine is not known.37 Interestingly, TECK shows chemotactic activity for thymic dendritic cells, suggesting that this chemokine might regulate thymic dendritic cell migration within or to thymus.37 It is known that peripheral blood T cells recirculate to the thymus.38,39 These peripheral blood T cells, especially CD4+ T cells, negatively regulate CD4+ T-cell generation from DP thymocytes through unknown mechanisms to maintain homeostasis of T lymphopoiesis.40 Thymic chemoattractants such as SDF-1 and CKβ-11/MIP-3β/ELC may have the potential to regulate thymopoiesis by recruiting appropriate amounts of peripheral blood T cells into the thymus. The discovery and characterization of thymic chemoattractants would potentially provide further information on intrathymic thymocyte migration or migration of thymocytes and accessory cells into/out of thymus, information that would further our understanding of T lymphopoiesis.
ACKNOWLEDGMENT
The authors thank Edward Appelbaum, Kyoung Johanson, Donna Cusimono, Joyce Mao, Stephanie Van Horn, Laura Grayson, Gil Scott, and Dean McNulty (SmithKline Beecham) for the production and analytical characterization of CKβ-11. We also thank Rebecca Miller and Cindy Booth (Indiana University) for assistance in the preparation of this manuscript.
Supported by US Public Health Service Grants No. R01 HL 56416 and R01 HL 54037 and by a project in P01 HL 53586 from the National Institutes of Health to H.E.B.
Address reprint requests to Hal E. Broxmeyer, PhD, Department of Microbiology/Immunology and the Walther Oncology Center, Indiana University School of Medicine, Bldg R4, Room 302, 1044 W Walnut St, Indianapolis, IN 46202.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. Migration behavior of total thymocytes (A) and thymocyte subsets, CD4+ SP (B), DP (C), DN (D), and CD8+SP (E) subsets, in chemotactic environments formed by SDF-1 and CKβ-11/MIP-3β/ELC. The 2 chemokines were added to upper or lower chambers as indicated in the Fig (+ and − denote the presence and absence of indicated chemokines, respectively) at optimal concentrations (500 ng/mL for CKβ-11/MIP-3β/ELC and 300 ng/mL for SDF-1). Thymocytes depleted of non-T cells were used as input cells for chemotaxis. Cell migration to lower chambers is expressed as the mean percentage of migration (±differences of duplicated experiments) of input thymocyte subsets added in the upper chamber at the start time of chemotaxis. Significant differences (P < .05) were observed between the following 2 bars: 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 2 and 8, 3 and 8, 8 and 10, and 9 and 11 (for [A]); 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 3 and 8, 8 and 9, and 10 and 11 (for [B]); 1 and 2, 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [C]); 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [D]); and 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 3 and 8, 8 and 9, 8 and 10, and 10 and 11 (for [E]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4434/4/m_blod41245004ax.jpeg?Expires=1769108496&Signature=Gtfhq6Ls1pbEO24e831olGI5wJFVC9lWaIMgSjuCtlZ6PtfUiUL1xhF8G6UEL2mT1hPpSmY7GaNqkOQj~7HaL6G6dME00EweEhplyF1bplSDMOuRCMFEUfO8AYY~8vXq2JLt7lPU6ndwDuCrbmlN22jJQ5ugC8D6MLUd5UDlcifIjES4A7Z1b-y6JiWdHx34XWq3~J-xjqiKPjBejHOjEfALW2CdoSysxaExUqSdk991parW1TdExwObGSGWUrhATMaRdwhvbJjHvfkPyP3~fSpHyLG1hMyE8YLchNFlxxiMQpHdfhoflGDM4l5M~N3BN2jTKPZuMArfXsAy7~uWVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Migration behavior of total thymocytes (A) and thymocyte subsets, CD4+ SP (B), DP (C), DN (D), and CD8+SP (E) subsets, in chemotactic environments formed by SDF-1 and CKβ-11/MIP-3β/ELC. The 2 chemokines were added to upper or lower chambers as indicated in the Fig (+ and − denote the presence and absence of indicated chemokines, respectively) at optimal concentrations (500 ng/mL for CKβ-11/MIP-3β/ELC and 300 ng/mL for SDF-1). Thymocytes depleted of non-T cells were used as input cells for chemotaxis. Cell migration to lower chambers is expressed as the mean percentage of migration (±differences of duplicated experiments) of input thymocyte subsets added in the upper chamber at the start time of chemotaxis. Significant differences (P < .05) were observed between the following 2 bars: 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 2 and 8, 3 and 8, 8 and 10, and 9 and 11 (for [A]); 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 3 and 8, 8 and 9, and 10 and 11 (for [B]); 1 and 2, 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [C]); 1 and 3, 3 and 5, 5 and 6, 2 and 8, and 9 and 11 (for [D]); and 1 and 2, 1 and 3, 2 and 4, 3 and 5, 4 and 7, 5 and 6, 3 and 8, 8 and 9, 8 and 10, and 10 and 11 (for [E]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4434/4/m_blod41245004bx.jpeg?Expires=1769108496&Signature=eML4sCCEJ~2rFQsiRB8ObESLUb5n4LZX4zJRzoL6q9AlGxQNWNKflwp2X1ogMqsvJVo-AAfeWY~O1nVQ3LvB~Xs8FHMhS5bTN0Mo3V8EMes9gLwrpwa4E-8RpqcN5XgxDvJrCTcHMcTdedLO05bFW8lp6F25B7NjeSps0iI3XamX8ZLB8TnKqP8K23Y2DJ3JYF28i1vJuQXHVtoGioQkAUqb~l~vJwO4ldyJ~G1EgYCkRhmGQ9Lml3EPtjktsO-5YJpX1WH6bQ~BnZOcyfQZ45dJBMAUBYa7zZuqGviOEYq-wjdm0LFwgrjN3wDFDU2wDro9440l8kQrC9JCiyKt3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Comparison of chemotactic activity of SDF-1 and CKβ-11/MIP-3β/ELC for splenic T cells with that for thymic T cells. Murine CD4+ (A) and CD8+ (B) SP T cells from thymus and spleen were assayed for their chemotactic responsiveness to SDF-1 and CKβ-11/MIP-3β/ELC. Input and migrated cells were stained with fluorescent antibodies to human or mouse CD4 and CD8, acquired for 30 seconds, and analyzed by FACscan. Cell migration to lower chambers is expressed as the mean percentage (±differences of duplicated experiments) of input subset cell number added in the upper chamber at the start time of chemotaxis. Background migration was subtracted from the mean migration to show net migration. Significant differences were observed between the following 2 peak points: A and B, A and C, A and D, B and C, B and D, and C and D (for [A]); and A and D and B and D (for [B]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4434/4/m_blod41245007x.jpeg?Expires=1769108496&Signature=FpomSZYtCY77Zji92VEcsvBIY6reSwjj1J~2eiTZh-yOw0Fgzbo4RjzsHRz1Mxa9VY6DcCOV5XbJU9Oye2ZzBAbCv071In5zJ1tF5Ub7XSl4jQvwZDRhRhrWUosNmXdD9~v5SoX46UmmO8FSsbeVEoYF4zLFs8DCfUANDsuAx4OHaD5Rq-Eo6ZZN6lY-wBQR2EaUkfNIG67aDP485f8wmKgtHt5uOS1UB6d8Z0gFUTUm3CXhAZXJoNmEaYUqearY5oPMkxaMLO0hlbNQUHRZDk~P1N~CKo~KendYBj4Kyci3enfxC2ITxVQF3n7xvgwqkFxXWKPJQ2YVbWvlCdk6Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal