Abstract
Iron overload is a major life-threatening complication of thalassemia major and other iron-loading anemias treated by regular blood transfusions. Although the clinical manifestations of iron overload may be prevented by desferrioxamine, the only iron-chelating drug in routine clinical use, this treatment requires subcutaneous infusion of desferrioxamine for 12 hours each day. New orally effective iron chelators are urgently needed, and pyridoxal isonicotinoyl hydrazone (PIH), which was first recognized as an effective iron chelator in vitro and subsequently in vivo, shows promise for the treatment of iron overload. More recently, over 40 analogs of PIH were synthesized, and some of them proved to be very potent in mobilizing 59Fe in vitro from 59Fe-labeled cells. In this study, we show that PIH analogs such as pyridoxal benzoyl hydrazone, pyridoxal p-methoxybenzoyl hydrazone (PMBH), pyridoxal m-fluorobenzoyl hydrazone (PFBH), and pyridoxal-2-thiophenecarboxyl hydrazone, compounds previously shown to mobilize iron from cells in vitro, are also effective in vivo. All of these chelators significantly enhanced biliary excretion of iron (measured by atomic absorption spectrophotometry) following their intraperitoneal (IP) and/or oral administration to rats. The most effective was PFBH, which increased iron concentration in the bile about 150-fold, as compared with basal biliary iron concentration, within 1 hour following a single IP dose of 0.2 mmol/kg body weight. In contrast, desferrioxamine increased the biliary iron concentration only 20-fold to 30-fold under the same conditions. Moreover, while control rats excreted ≈ 0.8 μg Fe in 2 hours, treatment with PFBH, PMBH, and desferrioxamine resulted in cumulative excretions of 87, 59, and 22 μg Fe, respectively, in the same period of time. Interestingly, PMBH was also quite effective following gastric administration, resulting in a 6-hour cumulative value of 34 μg Fe. These compounds are nontoxic and are inexpensive and easy to make. Their further evaluation as candidate drugs for the treatment of iron overload is warranted.
DESFERRIOXAMINE (DFO) is the only iron chelator in routine clinical use for the treatment of secondary iron overload, but this drug is very expensive and may cause toxic side effects. Moreover, desferrioxamine treatment involves a slow subcutaneous delivery over a 12-hour period each day, resulting in lack of compliance with the therapy.1-3 Although the new orally active chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1, deferiprone) increases urinary Fe excretion,4,5 clinical trials have shown a number of serious complications with L1 therapy, including agranulocytosis, arthritis, and gastrointestinal side effects.6-11 Moreover, recent long-term clinical trials showed that there is either no overall change in iron stores12 or that mean body iron increases13 in the course of L1-treatment. In fact, tissue iron can reach concentrations associated with iron-induced complications in 95% of patients.13 Hence, there is an urgent need to develop alternative iron chelators that are economical, orally effective, and highly efficient.
Pyridoxal isonicotinoyl hydrazone (PIH), produced by the Schiff base condensation of pyridoxal and isonicotinic acid hydrazide (INH), was initially identified as an effective iron chelator in vitro.14,15 Subsequently, PIH was found to be effective in vivo when given parenterally to mice15 and orally to rats.16-19 The high activity of PIH both in vitro and in vivo14-26 has prompted a clinical trial, where PIH showed no evidence of toxicity and produced significant iron excretion.27
We have recently synthesized 44 analogues of PIH, which retained the key iron-binding groups, and tested their effect on hepatocyte iron uptake from transferrin, as well as on iron mobilization from intracellular sites of hepatocytes.28 This study identified several PIH analogues, which are relatively simple and inexpensive to synthesize, that are highly effective in blocking 59Fe uptake by hepatocytes and/or mobilizing hepatocyte59Fe. Another study29 confirmed these results and also documented that the compounds do not cause toxic effects when added in effective concentrations to isolated rat hepatocytes incubated in vitro. In the present study, we examined the capacity of these newly identified iron chelators to promote biliary iron excretion following their administration to rats.
MATERIALS AND METHODS
Animals.
Female Wistar rats (220 ± 20 g) fed on a standard pellet diet were used. The rats were starved 24 hours before the experiment during which they had free access to deionized water.
Chelators.
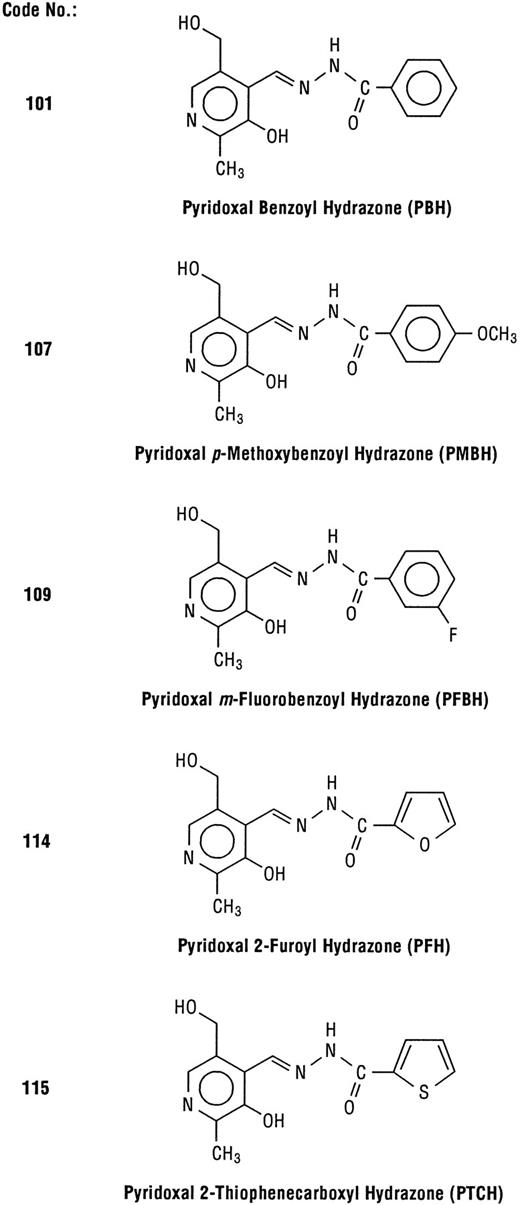
The chelators were synthesized by Schiff base condensation between appropriate aldehydes and acid hydrazides, using standard procedures.30 The number codes (Fig 1) for the hydrazones were identical as in our previous reports.24,28 The compounds were characterized with respect to their carbon-hydrogen analysis, melting points, and infra-red and nuclear magnetic resonance spectra.30 The chelators were administered either intraperitoneally (IP) or by gastric gavage at the dose of 0.2 mmol/kg body weight, roughly corresponding to concentrations shown to be effective in in vitro systems.28 29 Solutions of chelators were prepared within 30 minutes or less before administration, as follows: the appropriate amount of chelator was dissolved in a minimum volume (0.3 to 0.5 mL) of 1 mol/L HCl, then adjusted to pH 6 to 7 by addition of Krebs-Henseleit buffer (pH 7.4). The temperature of the solution was kept within the range of 35°C to 37°C. Injected volumes were approximately 1 mL per rat, depending on the body weight.
Structures of PIH analogs examined in this study. The number codes are identical to those in previous reports.24 28
Experimental procedure.
The bile duct was cannulated using fine tubing (PE-20, Clay-Adams, internal diameter 0.28 mm), which was then subcutaneously conveyed to the dorsal area, exposed extracorporeally, and connected to a small test tube fitted to a jacket that was fixed on the back of the rat. The bile was collected in 12 × 75 mm plastic tubes, which were replaced as needed to allow assessment of the rate of bile flow and the iron content in the bile.31 The animals were kept in metabolic cages and bile samples were collected every hour for 2 hours before and 8 hours after administration of the chelator. Immediately after the collection, the weight of the bile sample was determined, and the samples were kept in a refrigerator until analyzed. The urine was collected for 18 hours before and after the injection of chelator using the method described earlier.31 32 At the end of the experiment, the rats were killed by exsanguination from the heart, and the brains were carefully removed and weighed.
Iron determinations.
All analyses were performed on an AAS-30 Varian spectrophotometer (Varian Techtron Pty, Limited, Mulgrave, Victoria, Australia) equipped with Zeeman's background correction for flameless and deuterium correction for flame technique. The concentration of iron in the bile was determined directly using flameless technique. The content of iron in the urine was determined after extraction of filtration paper with 30 mL of 0.1 mol/L Na-EDTA (flame technique).
RESULTS AND DISCUSSION
Our recent study showed that several hydrazones of pyridoxal,viz those derived from hydrazides of benzoic (#101), p-methoxybenzoic (#107), m-fluorobenzoic (#109), and 2-thio-phenecarboxylic (#115) acids, as well as pyridoxal 2-furoyl hydrazone (#114) are highly effective in mobilizing59Fe from 59Fe-labeled hepatocytes in vitro.28 Before starting in vivo experiments, toxicities of these compounds were tested using isolated hepatocytes in vitro.29 This model was helpful in determining nontoxic and effective concentrations of the chelators.29
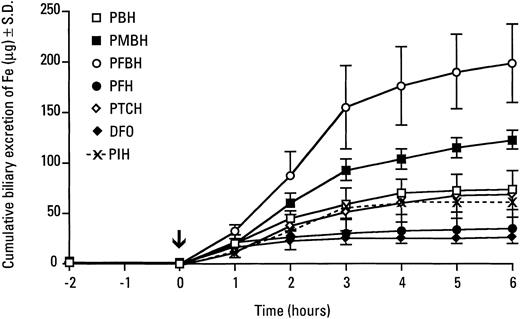
In the present study, the bile ducts of normal female rats were cannulated and the bile was collected for 2 hours before administration of the chelators (either IP or by gastric gavage); bile collection was continued for an additional 7 to 8 hours after drug administration. Each experimental group comprised eight animals, which before chelator administration, served as their own controls. Before chelator treatment, biliary iron concentration was ≤ 1 μg/mL, and dramatic increases were noted within 1 hour after administration of the hydrazones (Table 1). The highest biliary iron concentration was in rats treated with pyridoxalm-fluorobenzoyl hydrazone (PFBH, #109), which increased the concentration of iron in the bile more than 100-fold within 1 hour. Rats treated with this chelator showed a peak of biliary iron excretion between 1 to 3 hours, but very high iron levels in the bile were still detectable at 8 hours after the drug administration (Table 1). The remaining compounds (#101, #107, #114, and #115) were less effective than PFBH in promoting biliary iron excretion, both in terms of increasing the biliary iron levels and the duration of their effect (Table 1). Nevertheless, all of the compounds dramatically increased the cumulative excretion of iron in the bile (Fig 2). As compared with untreated rats, which excreted about 1 μg iron in 2 hours, at 6 hours, the cumulative iron excretion was 69, 74, 122, and 194 μg after a single dose of pyridoxal 2-thiophenecarboxyl hydrazone, pyridoxal benzoyl hydrazone, pyridoxal p-methoxybenzoyl hydrazone and pyridoxalm-fluorobenzoyl hydrazone, respectively (Fig 2). PMBH and, in particular, PFBH were significantly more effective than the parent compound, PIH, which gave a 6-hour cumulative value of 59 μg Fe (Fig2). Pyridoxal 2-furoyl hydrazone was somewhat less effective, leading to the excretion of 34 μg iron in the bile in 6 hours. The efficacy of m-fluorobenzoyl hydrazone in stimulating biliary iron excretion may be related to the high lipophilicity of this compound, either as a free ligand or in a complex with iron.22 With the exception of compound #114 (pyridoxal 2-furoyl hydrazone), all acyl hydrazones tested were significantly more effective than desferrioxamine (Fig 2). Bile appears to be the primary route of iron excretion after the administration of these compounds, as none of the hydrazones tested significantly increased urinary iron excretion (Table 2).
Concentration of Iron in Bile (μg/mL) After IP Administration of the Chelators
| Chelator Time (h) . | PBH (101) . | PMBH (107) . | PFBH (109) . | PFH (114) . | PTCH (115) . | DFO . |
|---|---|---|---|---|---|---|
| −2 | 0.39 ± 0.14 | 0.79 ± 0.21 | 0.49 ± 0.10 | 1.06 ± 0.38 | 0.37 ± 0.1 | 0.66 ± 0.18 |
| −1 | 0.35 ± 0.08 | 0.76 ± 0.21 | 0.70 ± 0.10 | 0.89 ± 0.38 | 0.67 ± 0.27 | 0.71 ± 0.13 |
| +1 | 30.10 ± 5.70 | 36.1 ± 13.8 | 88.50 ± 25.80 | 28.77 ± 8.48 | 39.50 ± 7.40 | 21.23 ± 2.34 |
| +2 | 39.30 ± 9.10 | 64.20 ± 10.30 | 46.90 ± 20.20 | 15.30 ± 2.84 | 81.30 ± 29.40 | 12.48 ± 7.52 |
| +3 | 32.00 ± 6.70 | 62.40 ± 7.50 | 85.60 ± 33.30 | 7.91 ± 3.29 | 58.80 ± 20.70 | 4.78 ± 4.53 |
| +4 | 20.10 ± 6.50 | 55.10 ± 9.30 | 1.60 ± 23.80 | 5.05 ± 2.89 | 34.80 ± 12.90 | 1.05 ± 0.48 |
| +5 | 10.60 ± 3.80 | 30.50 ± 8.10 | 86.60 ± 31.40 | 3.45 ± 2.05 | 26.70 ± 10.20 | 0.94 ± 0.59 |
| +6 | 3.60 ± 1.90 | 19.70 ± 5.30 | 25.20 ± 13.50 | 2.30 ± 1.49 | 13.80 ± 6.50 | 0.93 ± 0.50 |
| +7 | 1.80 ± 0.70 | 8.20 ± 3.30 | 19.80 ± 2.60 | 1.81 ± 0.64 | 2.80 ± 1.40 | — |
| 8 | 0.67 ± 0.22 | 3.20 ± 1.1 | 1.27 ± 0.53 | 1.60 ± 0.70 | — |
| Chelator Time (h) . | PBH (101) . | PMBH (107) . | PFBH (109) . | PFH (114) . | PTCH (115) . | DFO . |
|---|---|---|---|---|---|---|
| −2 | 0.39 ± 0.14 | 0.79 ± 0.21 | 0.49 ± 0.10 | 1.06 ± 0.38 | 0.37 ± 0.1 | 0.66 ± 0.18 |
| −1 | 0.35 ± 0.08 | 0.76 ± 0.21 | 0.70 ± 0.10 | 0.89 ± 0.38 | 0.67 ± 0.27 | 0.71 ± 0.13 |
| +1 | 30.10 ± 5.70 | 36.1 ± 13.8 | 88.50 ± 25.80 | 28.77 ± 8.48 | 39.50 ± 7.40 | 21.23 ± 2.34 |
| +2 | 39.30 ± 9.10 | 64.20 ± 10.30 | 46.90 ± 20.20 | 15.30 ± 2.84 | 81.30 ± 29.40 | 12.48 ± 7.52 |
| +3 | 32.00 ± 6.70 | 62.40 ± 7.50 | 85.60 ± 33.30 | 7.91 ± 3.29 | 58.80 ± 20.70 | 4.78 ± 4.53 |
| +4 | 20.10 ± 6.50 | 55.10 ± 9.30 | 1.60 ± 23.80 | 5.05 ± 2.89 | 34.80 ± 12.90 | 1.05 ± 0.48 |
| +5 | 10.60 ± 3.80 | 30.50 ± 8.10 | 86.60 ± 31.40 | 3.45 ± 2.05 | 26.70 ± 10.20 | 0.94 ± 0.59 |
| +6 | 3.60 ± 1.90 | 19.70 ± 5.30 | 25.20 ± 13.50 | 2.30 ± 1.49 | 13.80 ± 6.50 | 0.93 ± 0.50 |
| +7 | 1.80 ± 0.70 | 8.20 ± 3.30 | 19.80 ± 2.60 | 1.81 ± 0.64 | 2.80 ± 1.40 | — |
| 8 | 0.67 ± 0.22 | 3.20 ± 1.1 | 1.27 ± 0.53 | 1.60 ± 0.70 | — |
Cumulative excretion of iron in the bile of rats injected IP with pyridoxal-derived hydrazones. The bile ducts of normal rats were cannulated and the bile was collected for 2 hours, after which the hydrazones were injected IP (0.2 mmol/kg body weight) and then bile collection continued as indicated.
Cumulative excretion of iron in the bile of rats injected IP with pyridoxal-derived hydrazones. The bile ducts of normal rats were cannulated and the bile was collected for 2 hours, after which the hydrazones were injected IP (0.2 mmol/kg body weight) and then bile collection continued as indicated.
Urinary Excretion of Fe After IP Administration of the Chelators
| Chelator . | No. . | Fe (μg/18 h) . |
|---|---|---|
| Control | 29.88 ± 9.42 | |
| PBH | 101 | 25.26 ± 3.79 |
| PMHB | 107 | 65.40 ± 29.89 |
| PFBH | 109 | 46.40 ± 10.33 |
| PFH | 114 | 30.23 ± 10.91 |
| PTCH | 115 | 44.90 ± 15.34 |
| DFO | 30.04 ± 14.31 |
| Chelator . | No. . | Fe (μg/18 h) . |
|---|---|---|
| Control | 29.88 ± 9.42 | |
| PBH | 101 | 25.26 ± 3.79 |
| PMHB | 107 | 65.40 ± 29.89 |
| PFBH | 109 | 46.40 ± 10.33 |
| PFH | 114 | 30.23 ± 10.91 |
| PTCH | 115 | 44.90 ± 15.34 |
| DFO | 30.04 ± 14.31 |
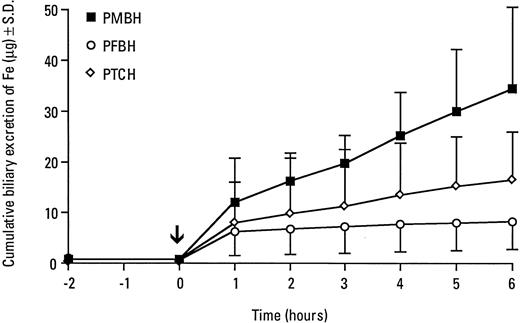
Three of the most efficient hydrazones (PFBH, PMBH, and PTCH) were further tested after administration by gastric gavage. The hydrazones were less effective in increasing biliary iron excretion when administered by gavage (Table 3) compared with IP administration (see Table 1). The most effective chelator after gastric administration was pyridoxal p-methoxybenzoyl hydrazone (PMBH) which, within 1 hour, increased biliary iron concentration more than 10-fold and maintained a high iron content in the bile for the additional 5 hours (Table 3). Consequently, PMBH given intragastrically significantly increased cumulative excretion of iron, resulting in a 6-hour cumulative value of 34 μg Fe (Fig3). As compared with PFBH, PMBH is more hydrophilic,28 a property that would seem to make this compound more soluble than PFBH in gastrointestinal fluids. If so, the availability of PMBH for absorption would be higher than that of PFBH, and this could explain the higher efficacy of PMBH, as compared with PFBH, when this compound was administered by gavage. Gastric administration of PMBH, PFBH, and PTCH did not increase urinary iron excretion (Table 4).
Concentration of Iron in Bile (μg/mL) After Administration of the Chelators by Gastric Gavage
| Chelator Time (h) . | PMBH (107) . | PFBH (109) . | PTCH (115) . |
|---|---|---|---|
| −2 | 1.01 ± 0.08 | 0.61 ± 0.14 | 0.23 ± 0.07 |
| +1 | 11.26 ± 6.06 | 6.72 ± 4.11 | 3.94 ± 2.56 |
| +2 | 6.38 ± 1.94 | 1.20 ± 0.19 | 3.96 ± 2.22 |
| +3 | 4.61 ± 2.86 | 0.85 ± 0.26 | 3.52 ± 2.22 |
| +4 | 7.55 ± 4.98 | 0.92 ± 0.15 | 4.17 ± 4.24 |
| +5 | 5.90 ± 2.82 | 0.82 ± 0.28 | 3.16 ± 2.82 |
| +6 | 6.98 ± 3.39 | 0.73 ± 0.28 | 2.37 ± 2.05 |
| Chelator Time (h) . | PMBH (107) . | PFBH (109) . | PTCH (115) . |
|---|---|---|---|
| −2 | 1.01 ± 0.08 | 0.61 ± 0.14 | 0.23 ± 0.07 |
| +1 | 11.26 ± 6.06 | 6.72 ± 4.11 | 3.94 ± 2.56 |
| +2 | 6.38 ± 1.94 | 1.20 ± 0.19 | 3.96 ± 2.22 |
| +3 | 4.61 ± 2.86 | 0.85 ± 0.26 | 3.52 ± 2.22 |
| +4 | 7.55 ± 4.98 | 0.92 ± 0.15 | 4.17 ± 4.24 |
| +5 | 5.90 ± 2.82 | 0.82 ± 0.28 | 3.16 ± 2.82 |
| +6 | 6.98 ± 3.39 | 0.73 ± 0.28 | 2.37 ± 2.05 |
Cumulative excretion of iron in bile of rats after gastric administration of pyridoxal-derived hydrazones. The bile ducts of normal rats were cannulated and the bile was collected for 2 hours, after which the hydrazones were administered by gavage (0.2 mmol/kg body weight) and then bile collection continued as indicated.
Cumulative excretion of iron in bile of rats after gastric administration of pyridoxal-derived hydrazones. The bile ducts of normal rats were cannulated and the bile was collected for 2 hours, after which the hydrazones were administered by gavage (0.2 mmol/kg body weight) and then bile collection continued as indicated.
Urinary Excretion of Iron After Administration of the Chelators by Gastric Gavage
| Chelator . | No. . | Fe (μg/18 h) . |
|---|---|---|
| Control | 12.50 ± 7.20 | |
| PMBH | 107 | 9.65 ± 3.51 |
| PFBH | 109 | 8.80 ± 4.13 |
| PTCH | 115 | 14.80 ± 3.72 |
| Chelator . | No. . | Fe (μg/18 h) . |
|---|---|---|
| Control | 12.50 ± 7.20 | |
| PMBH | 107 | 9.65 ± 3.51 |
| PFBH | 109 | 8.80 ± 4.13 |
| PTCH | 115 | 14.80 ± 3.72 |
These results, as well as our previous observations,16suggest that the limited oral efficacy of PIH and other acyl hydrazones is caused by lower absorption. Similarly, Brittenham's27evaluation of PIH in patients has yielded lower than expected effect on iron excretion at doses permissible for clinical use. Because PIH was given as a powder in gelatin capsules, it appears likely that the chelator was poorly available for absorption. Hence, a more bioavailable formulation of PIH and its analogs should be designed and evaluated as a means of increasing Fe excretion. One promising strategy may involve the use of chelators encapsulated into biodegradable polymers.33 However, the bioavailability of PIH and analogs may also be limited by hydrolysis of acyl hydrazones in the stomach, as it has been reported that these compounds hydrolyze in strongly acidic solutions.34
A clinically useful iron chelator should have high affinity for iron, but low affinity for all other biologically important cations. It is well known that DFO fullfils this criterion. Importantly, PIH and several of its analogs have very low affinity for Ca(II) and Mg(II) and complexation with these ions only occurs at pH greater than 8.35 Hence, chelation of these biologically important cations should not occur under physiologic conditions. Although the PIH class of chelators can bind Zn(II) at pH 7.4, their affinity for Zn(II) is much less than for Fe(III),35 and complexation of Zn(II) probably does not occur in vivo. In rats, PIH did not increase biliary excretion of zinc and copper.16
One of the aims of this study was to identify a chelator with the highest efficacy to promote biliary iron excretion at a relatively low dose (0.2 mmol/kg). It is possible that at this dose the chelators do not produce maximum effects, and further experiments are being planned to examine whether PMBH and PFBH would be more effective at higher doses administered either IP or orally. DFO is known to promote urinary iron excretion in iron overloaded rats,36 and it should be pointed out that in this study we used rats that were not iron overloaded. The use of noniron overloaded rats can probably explain low urinary iron excretion after DFO administration.
In conclusion, this study showed that pyridoxal hydrazones with benzoyl (#101), p-methoxybenzoyl (#107), m-fluorobenzoyl (#109), and 2-thiophene-carboxyl (#115) substituents show potential as candidate iron chelating drugs. The most promising compounds are pyridoxal m-fluorobenzoyl hydrazone (#109) and pyridoxalp-methoxybenzoyl hydrazone (#107) that deserve further vigorous evaluation for iron chelation therapy.
ACKNOWLEDGMENT
The excellent technical assistance of Jana Tumova and Eva Nagy is gratefully acknowledged. We would like to thank Sandy Fraiberg for her excellent editorial assistance.
Supported by a grant from the Medical Research Council of Canada, Ottawa, Cananda; and Grant No. 0259-3 from the Czech Ministry of Health, Prague, Czech Republic.
Presented at the 37th Annual American Society of Hematology Meeting, Seattle, WA, December 1-5, 1995 and published in abstract form (Blood 86:300a, 1995 [suppl 1]).
Address reprint requests to Premysl Ponka, MD, PhD, Lady Davis Institute for Medical Research, Jewish General Hospital, 3755 Cote Ste-Catherine Rd, Montreal, Quebec, Canada H3T 1E2.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal