Abstract
In the acute promyelocytic leukemia (APL) cell line NB4, as well as in APL patients' cells, arsenic trioxide (As2O3) leads to incomplete cell maturation, induction of apoptosis, as well as to the degradation of the oncogenic PML/RARα fusion protein. We have isolated an arsenic-resistant NB4 subline (NB4-AsR), which fails to undergo apoptosis, but maintains the partial differentiation response to this drug. When grown in the presence of As2O3, NB4-AsRcells degrade PML/RARα, slightly differentiate, and become more sensitive to serum deprivation-induced apoptosis. Similarly, in RA-resistant NB4-R1 cells, RA induced a significant PML/RARα degradation and yet failed to induce cell maturation. Thus, As2O3- or retinoic acid (RA)-induced PML/RARα degradation may be a prerequisite, but is not sufficient for the full differentiative/apoptotic response to these drugs. Strikingly, RA-triggered differentiation and apoptosis were greatly accelerated in As2O3-treated NB4-AsR cells. The synergism between these two agents in this setting could provide an experimental basis for combined or sequential RA/As2O3 therapies.
ACUTE PROMYELOCYTIC leukemia (APL) is a rare disease representing 10% to 15% of all the acute myelogeneous leukemias of adults. APL is characterized by developmental arrest of granulopoiesis at the promyelocytic stage and is generally associated with a specific t(15;17) translocation, which fuses the PML and retinoic acid receptor α (RARα) genes to yield a PML/RARα fusion protein.1 RARα belongs to the steroid receptor superfamily and activates transcription in response to all-trans retinoic acid (RA).2 PML is a zinc finger protein containing a coiled-coil domain, which has growth and transformation suppressor activity.3-5 Immunofluorescence analysis indicates that PML is localized on discrete subnuclear structures called PML nuclear bodies (NBs) whose function remains unknown.6-9 In APL cells, both the PML/RARα fusion protein and the normal RARα and PML proteins are expressed.10 Recent results, obtained in vivo with PML/RARα transgenic mice, show that this chimeric protein is sufficient to impair neutrophilic differentiation and initiate development of leukemia.11-13 PML/RARα bears most of the functional domains of both PML and RARα and consequently may interfere with the normal function of these proteins. However, it remains to be elucidated how leukemogenesis relates to altered RARα and/or PML function(s). PML/RARα (as well as a dominant negative RARα mutant) interferes with nuclear receptor function and myeloid differentiation.14,15 In addition, PML/RARα displaces PML and several other NB antigens from NBs to other ill-defined nuclear sites, presumably through the formation of PML:PML/RARα heterodimers.6-9 Such loss of specific PML localization could antagonize its growth suppressive properties and contribute to deregulated cell growth. Finally, the PML/RARα fusion may exhibit new functional properties, as suggested by the observation that PML/RARα homodimers recognize new DNA target sites.16 17
Paradoxically, the APL cells are exquisitely sensitive to the differentiating action of pharmacologic concentrations (10−6 mol/L) of RA, leading to high rates of temporary clinical remissions.18,19 In fact, in APL cells or in the APL cell line, NB4, RA administration causes the degradation of the fusion protein20 associated with the reaggregation of PML and other NB-antigens to yield their normal pattern of nuclear speckled localization.8 Arsenic trioxide (As2O3) has also proven to be an effective drug in the treatment of APL patients, inducing remission even in RA-resistant APL cases.21-23 As2O3triggers apoptosis at micromolar concentrations, and this is associated with a strong downregulation of the bcl-2 protein,21 while at lower concentrations, some differentiation can be observed.23 In addition, we have shown that, in NB4 cells, As2O3 targets PML and PML/RARα onto NBs and induces the degradation of both proteins.24 Thus, As2O3 and RA target the PML and RAR moities of the fusion protein, respectively, and both induce its degradation, providing the first example of oncogene-targeted therapy.
In the present work, the isolation and the characterization of an As2O3-resistant NB4-derived population, NB4-AsR, is reported. These cells, when grown in the presence of As2O3, are more differentiated than parental NB4 cells, constantly degrade PML/RARα, and display a normal speckled NBs pattern. NB4-AsR cells treated with the combination of As2O3 and RA show accelerated differentiation and/or a dramatic induction of apoptosis. By showing that RA and As2O3 synergize for induction of differentiation and apoptosis and that cells resistant to one agent are sensitive to the other, our results provide a rational for combined therapies.
MATERIALS AND METHODS
Cell culture conditions and reagents.
The NB4 APL cell line and the NB4-derived lines (NB4-R1 and NB4-R2 ) were kindly provided by M. Lanotte.25-27 All cell lines were grown in RPMI-1640 supplemented with 10% fetal calf serum (FCS) (GIBCO-BRL, Gaithersburg, MD) under the same conditions as previously reported for the isolation and maintenance of the NB4 cell line.25 All-trans RA and As2O3 were purchased from Sigma-Aldrich (St Louis, MO).The 10−2mol/L RA stock solution was prepared by dissolving the compound in dimethyl sulfoxide (DMSO). A 100 mmol/L stock solution of As2O3 was obtained by dissolving As2O3 in 1 N NaOH and dilution in H2O.
Selection of NB4-AsR cells.
An As2O3-resistant NB4 subline was derived from the NB4 cell line by growth in medium supplemented with 10−7 mol/L As2O3. While most of the cell population died, rare As2O3-resistant cells became evident in about 4 months of selection in 10−7 mol/L As2O3. The few living cells were recovered following the method described by Andersen and Junker.28 To restore the required minimal cell density, the surviving cells (1/105 cells) were replated at 30 cells/well in a 96-well microtiter plate and selection continued. After 2 months in the presence of 10−6 mol/L As2O3, a pool of resistant cells was isolated, which was used in the subsequent experiments.
Characterization of cell differentiation.
Morphology and granulocytic differentiation was evaluated by light microscopy of May-Grünwald-Giemsa–stained cytospin preparation. Differentiation of NB4 and NB4-AsR cells was also assessed by the ability of the cells to produce superoxide. This was measured by the degree of reduction of nitroblue-tetrazolium (NBT, Sigma-Aldrich) achieved by the cells over a 30-minute period at 37°C in the presence of phorbol myristate acetate (PMA, Sigma-Aldrich).29
Measurement of leukocyte alkaline phosphatase (LAP) activity.
A total of 106 cells were harvested, washed once with phosphate-buffered saline (PBS), and pelleted. The cell pellet was resuspended in homogenization buffer30 and disrupted by vigorous pipetting. The homogenate was used for the LAP assay, which was performed using p-nitrophenol phosphate (Sigma-Aldrich) as a substrate. LAP activity was normalized for the protein content in the sample. Protein concentration was measured according to the Bradford method using bovine serum albumin (BSA) fraction V (Sigma) as a standard and a commercially available kit (BioRad, Hercules CA). One unit of LAP activity was defined as the amount of enzyme able to transform 1 nmol of substrate in 1 minute at 37°C. Enzyme assays were performed in conditions of linearity relative to the substrate and to the concentration of proteins.
Measurement of plastic adherence.
After incubation with medium alone or with medium supplemented with the different compounds, NB4 and NB4-AsR cells still growing in suspension were collected and counted, the plastic wells were then washed twice with PBS, and adhering cells were detached with trypsin-EDTA and counted. Adherence was estimated as the percentage of cells in each well adhering to the plastic (ie, adhering cell number/total (adhering + nonadhering) cell number × 100%).
Analysis of cytodifferentiation markers and interferon (IFN) titration.
The myeloid surface markers CD33, CD11b, CD11c, and CD14 were determined by flow cytometry analysis using appropriate antibodies purchased from DAKO, Glostrup, Denmark (CD11b, CD11c) or Coulter Corp, Miami, FL (CD33 and CD14). The percentage of positive cells (%) and the mean associated fluorescence were quantitated using a FACScan analyzer (LYSIS II software, Becton Dickinson, Mountain View, CA). IFN was titrated on Madin Darby bovine kidney (MDBK) cells as described.31
Apoptosis and cell cycle distribution.
DNA degradation was estimated by labelling DNA strand breaks with terminal deoxynucleotidyl transferase (TdT). The tailing reaction named TUNEL was performed according to the manufacturer's instructions (Boehringer, Mannheim GmbH, Germany) except that the paraformaldehyde fixation was replaced by a 4% formaldehyde fixation. Cells were analyzed by fluorescence microscopy and by flow cytometry using a FACScan. All data were collected, stored, and analyzed by LYSIS II software (Becton Dickinson). Cells were also analyzed for their DNA content and cell cycle distribution (CELLFIT cell cycle analysis, Becton Dickinson) by flow cytometry analysis of propidium iodine (PI)-stained nuclei.32
Immunofluorescence and Western blot analysis.
Immunofluorescence was performed as previously described.6PML/RARα and PML were revealed by rabbit polyclonal antibodies6 followed by a fluorescein isothiocyanate (FITC)-labeled antirabbit polyclonal antibody (Biosys, Compiegne, France). RARα was revealed by monoclonal antibodies (kindly provided by B. Allegretto, Ligand Co, La Jolla, CA) followed by a Texas Red antimouse polyclonal antibody (Amersham, Little Chalfont, Buckinghadshire, UK). For Western blot analysis, cells were washed and resuspended in PBS, lysed in 1 X hot Läemmli sample buffer, and boiled for 5 minutes. About 30 μg of protein was analyzed on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and transferred to nitrocellulose by semidry blotting. Membranes were blocked with 10% skimmed milk in Tris buffered saline (TBS)-0.1% Tween for 1 hour and incubated overnight with the following specific rabbit polyclonal antibodies; anti-RARα (RPαF') (kindly provided by P. Chambon, Strasbourg, France), anti-STAT1α, anti-P21WAF1/CIP1 (Santa Cruz Biotechnology, Santa Cruz, CA), as well as with antihuman Bcl-2 mouse monoclonal antibody (DAKO Corp, Carpinteria, CA). These initial incubations were followed by incubation with antirabbit IgG or antimouse IgG horse radish peroxidase-conjugated antibodies (Biosys). Antibody complexes were detected by chemiluminescence using the ECL kit (Amersham Aylesbury). To estimate the apparent molecular mass of polypeptides, kaleidoscope prestained standards from BioRad were used (BioRad Laboratories, Richmond, CA).
RESULTS
Arsenic triggers differentiation, but not apoptosis in the NB4-AsR subline.
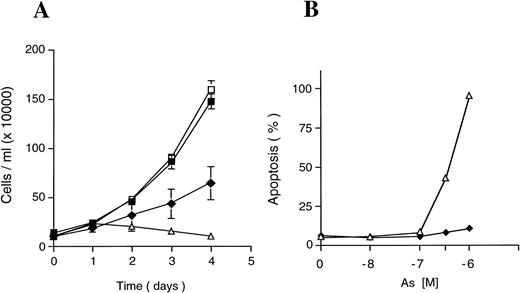
Arsenic-resistant cells were selected from NB4 cells treated with 10−6 mol/L As2O3 over a 4-month period (see Materials and Methods). In normal medium or in low doses (10−7 mol/L) of arsenic, NB4, and NB4-AsR cells have similar growth rates (Fig 1A and see Fig 7A) . At higher doses (10−6 mol/L) of As2O3, a sharp difference in growth was observed between parental and resistant cells: the later grow, although at a reduced rate, while parental cells die (Fig 1A). Indeed, a sharp reduction in As2O3-induced apoptosis was demonstrated by TUNEL in resistant compared with parental cells (Fig 1B). Resistant cells withdrawn from arsenic for over a month still achieve a significant resistance to apoptosis on de novo arsenic exposure (not shown). Thus, NB4-AsR cells remain sensitive to growth retardation of arsenic (a property also seen in HL60 or U937 cells), but fail to apoptose at high concentrations (Fig 1B). Consistent with this result, two proteins, p21 and Bcl-2, which are modulated during As2O3 triggered NB4 apoptosis, failed to respond to As2O3 in resistant cells (see Fig8). As2O3 content of NB4 and NB4-AsR cells was very similar (respectively 0.025 ± 0.003 μmol/L/107 cells v 0.021 ± 0.004 μmol/L/107 cells when grown for 24 hours in 10−7 mol/L As2O3and 0.105 ± 0.008 μmol/L/107 cells v0.097 ± 0.007μmol/L/107 cells when grown for 24 hours in 10−6 mol/L As2O3). This suggests that the intracellular As2O3 content of resistant cells was unaffected, in contrast to, for instance, As2O3-resistant bacteria, which actively exclude arsenicals.33
Effect of As2O3 on NB4 and NB4-AsR cell growth. (A) Exponentially growing cells were seeded at 105 cells/mL and incubated for 4 days without As2O3 (NB4 (□), NB4-AsR cells (▪), or with 10−6 mol/L As2O3(NB4-AsR (⧫), NB4 (▵). Each value represents the mean ± standard deviation (SD) of three independent determinations. (B) Apoptotic effect of As2O3on NB4 or NB4-AsR cells. NB4 (▵) and NB4-AsR(⧫) were treated for 5 days with the concentrations indicated in the plot. The percentage of apoptosis (%) was determined by a TUNEL assay.
Effect of As2O3 on NB4 and NB4-AsR cell growth. (A) Exponentially growing cells were seeded at 105 cells/mL and incubated for 4 days without As2O3 (NB4 (□), NB4-AsR cells (▪), or with 10−6 mol/L As2O3(NB4-AsR (⧫), NB4 (▵). Each value represents the mean ± standard deviation (SD) of three independent determinations. (B) Apoptotic effect of As2O3on NB4 or NB4-AsR cells. NB4 (▵) and NB4-AsR(⧫) were treated for 5 days with the concentrations indicated in the plot. The percentage of apoptosis (%) was determined by a TUNEL assay.
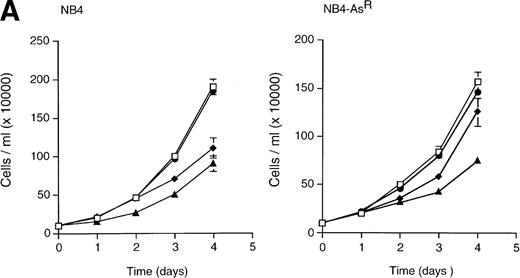
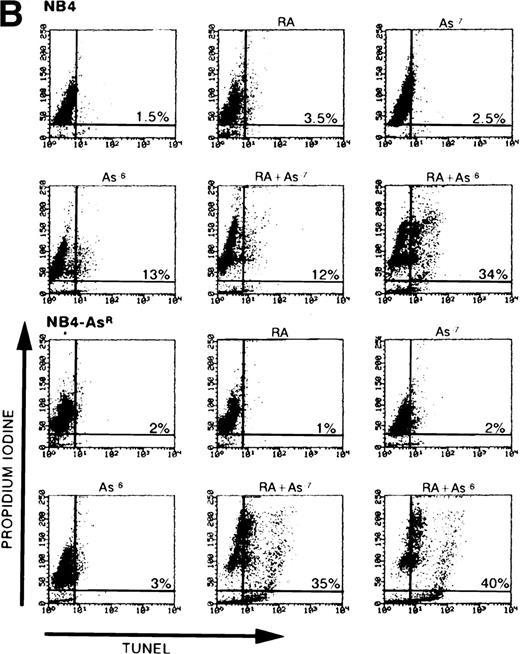
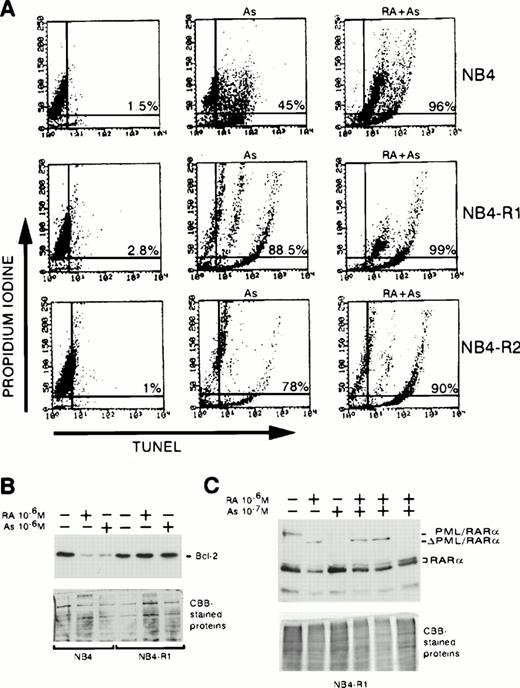
(A) Effect of As2O3and/or RA on NB4 and NB4-AsR cell growth. NB4 and NB4-AsR were seeded at 105 cells/mL and incubated with 10−7 mol/L As2O3(•), with 10−6 mol/L RA (⧫), with the combination of 10−6 mol/L RA and 10−7 mol/L As2O3 (▴) or without As2O3 (□). A representative experiment among three separate assays is shown. Each value represents the mean ± SD of three determinations. (B) Effect of As2O3and/or RA on NB4 or NB4-AsR cell apoptosis. NB4 and NB4-AsR were treated for 3 days as indicated in the pictures. The percentage of apoptosis (%) was determined by a TUNEL assay (abscissa) and PI staining (ordinate), and analyzed by flow cytometry.
(A) Effect of As2O3and/or RA on NB4 and NB4-AsR cell growth. NB4 and NB4-AsR were seeded at 105 cells/mL and incubated with 10−7 mol/L As2O3(•), with 10−6 mol/L RA (⧫), with the combination of 10−6 mol/L RA and 10−7 mol/L As2O3 (▴) or without As2O3 (□). A representative experiment among three separate assays is shown. Each value represents the mean ± SD of three determinations. (B) Effect of As2O3and/or RA on NB4 or NB4-AsR cell apoptosis. NB4 and NB4-AsR were treated for 3 days as indicated in the pictures. The percentage of apoptosis (%) was determined by a TUNEL assay (abscissa) and PI staining (ordinate), and analyzed by flow cytometry.
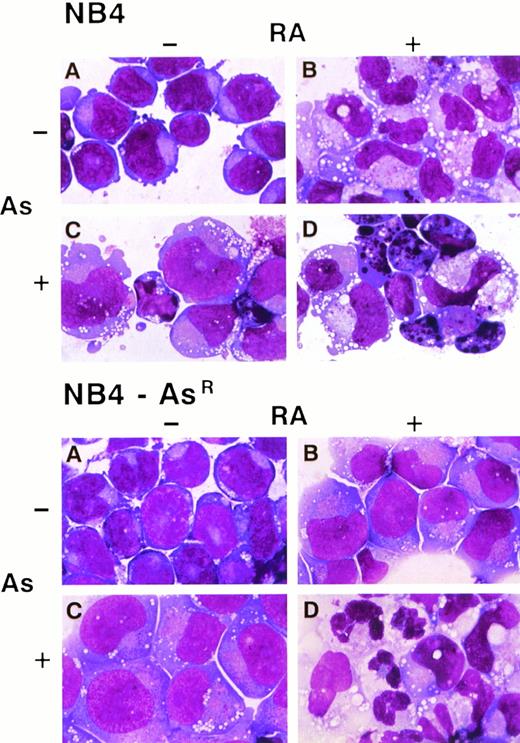
Morphologic features of NB4 and NB4-AsR cell lines on RA, As2O3 or the combined treatment. NB4 and NB4-AsR cells were treated for 5 days, respectively, without (A), with 10−6 mol/L RA (B), 10−6 mol/L As2O3 (C) or with the combination of 10−6 mol/L RA plus 10−6mol/L As2O3 (D).
Morphologic features of NB4 and NB4-AsR cell lines on RA, As2O3 or the combined treatment. NB4 and NB4-AsR cells were treated for 5 days, respectively, without (A), with 10−6 mol/L RA (B), 10−6 mol/L As2O3 (C) or with the combination of 10−6 mol/L RA plus 10−6mol/L As2O3 (D).
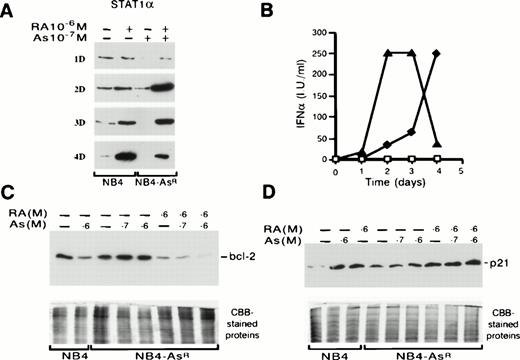
Western blot analysis of Bcl-2, STAT1α, p21 expressions, and IFNα production in NB4 and NB4-AsR cells (A) NB4-AsR, maintained in 10−7 mol/L As2O3, and NB4 cells were treated with medium alone or 10−6 mol/L RA. The time-course of synthesis of STAT1α protein is depicted. (B) Time-course of IFNα secretion. NB4 cells were treated with medium alone (□) or 10−6 mol/L RA (⧫). NB4-AsR cells were treated with 10−7 mol/L As2O3 (completely coinciding with □) or 10−7 mol/L As2O3 plus 10−6 mol/L RA (▴). (C and D) NB4 or NB4-AsR cells were grown for 36 hours with the different treatments indicated in the figure.
Western blot analysis of Bcl-2, STAT1α, p21 expressions, and IFNα production in NB4 and NB4-AsR cells (A) NB4-AsR, maintained in 10−7 mol/L As2O3, and NB4 cells were treated with medium alone or 10−6 mol/L RA. The time-course of synthesis of STAT1α protein is depicted. (B) Time-course of IFNα secretion. NB4 cells were treated with medium alone (□) or 10−6 mol/L RA (⧫). NB4-AsR cells were treated with 10−7 mol/L As2O3 (completely coinciding with □) or 10−7 mol/L As2O3 plus 10−6 mol/L RA (▴). (C and D) NB4 or NB4-AsR cells were grown for 36 hours with the different treatments indicated in the figure.
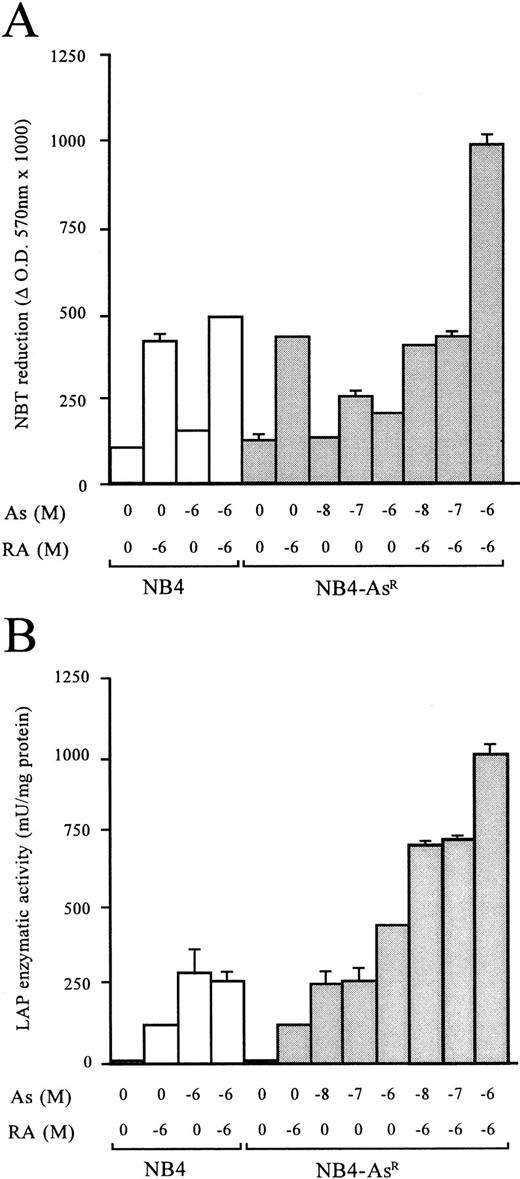
Morphologic analysis of May-Grünwald-Giemsa stained cells showed that untreated NB4-AsR cells were identical to their parental cells. However, when grown for 3 days in 10−6 mol/L As2O3-supplemented medium, the resistant cells became significantly larger than untreated NB4 cells. Moreover, a higher fraction of cells presented an excentric nucleus. The nucleus/cytoplasm ratio, azurophic granules, and basophily were reduced, and in the cytoplasm, neutrophilic granules and vacuoles were increased (Fig 2). Nevertheless, despite these changes, the resistant cells maintained immature features, as previously noted in NB4 cells treated with low doses of As2O3 (10−7mol/L).23 An increased rate of spontaneous adherence to plastic was also noted (data not shown). To gain further insight into the phenotypic changes of the NB4-AsR cell line, surface markers specific for granulocytic or monocytic differentiation were analyzed (Table 1). NB4 cells have a high expression of CD33, absence of CD14 (a marker of the monocytic lineage), and a low expression of CD11b (a marker of the granulocytic lineage, like CD11c). When NB4 cells were grown 3 days in 10−8 mol/L or 10−7 mol/L As2O3, CD33 expression was weakly downregulated, while CD11b, CD11c, and CD14 expression increased.23 NB4-AsR cells grown without As2O3 have a more differentiated immunophenotype than the parental cell lines. These cells grown in 10−8 mol/L or 10−7 mol/L As2O3 showed a sharp increase in CD11b and CD11c expression, while CD33 and CD14 expression remained almost unchanged (Table 1). Because maturation along both the granulocytic and monocytic lineages is associated with the ability of myeloid cells to produce superoxide, NB4-AsR cells were examined for this ability with a NBT reduction assay. An increase of superoxide production was found in the As2O3-treated resistant or parental cells, which was much weaker than the one induced by RA (Fig 3). LAP (a marker of terminal granulocytic differentiation) was absent from NB4 and NB4-AsR grown in the absence of As2O3 and only modestly induced by RA.34 However, a sharp induction was caused by arsenic treatment at 3 days in both cell lines (Fig 3). Importantly, NB4-AsR cells respond to RA like the parental clone in their surface antigen expression, NBT reduction, LAP activity, and morphologic differentiation, showing that resistance to As2O3-triggered apoptosis did not impair RA response. In conclusion, NB4-AsR are apoptosis-resistant cells that remain sentitive to As2O3- or RA-triggered differentiation.
(A) Effects of As2O3, RA, or their combination on the NBT-reducing activity of NB4 and NB4-AsR cells. Aliquots of 5 × 105 cells were treated for 3 days as indicated under the plot. (B) Effects of As2O3, RA, or the combination of the two compounds on LAP enzymatic activity. Aliquots of 106 cells were treated with RA, As2O3, or the combination of 10−6 mol/L RA plus As2O3 for 3 days. Each value represents the mean ± SD of three independent measurements.
(A) Effects of As2O3, RA, or their combination on the NBT-reducing activity of NB4 and NB4-AsR cells. Aliquots of 5 × 105 cells were treated for 3 days as indicated under the plot. (B) Effects of As2O3, RA, or the combination of the two compounds on LAP enzymatic activity. Aliquots of 106 cells were treated with RA, As2O3, or the combination of 10−6 mol/L RA plus As2O3 for 3 days. Each value represents the mean ± SD of three independent measurements.
Expression of Myeloid Cell Surface Markers on NB4 and in NB4-AsR Cells (% positive cells)
| . | Medium . | RA 10−6 mol/L . | As 10−8 mol/L . | As 10−7 mol/L . | RA + As 10−8 mol/L . | RA + As 10−7 mol/L . |
|---|---|---|---|---|---|---|
| NB4 | ||||||
| CD33 | 86.5 | 60 | 80 | 60 | 30 | 38 |
| CD11b | 13.8 | 76 | 36 | 73 | 68 | 65 |
| CD11c | 0 | 80 | 53 | 80 | 80 | 81.5 |
| CD14 | 0 | 34 | 40 | 49 | 0 | 0 |
| NB4-AsR | ||||||
| CD33 | 93 | 60 | 85 | 65 | 0 | 0 |
| CD11b | 25 | 76 | 92 | 74 | 69 | 70 |
| CD11c | 25 | 80 | 95 | 86 | 74 | 70 |
| CD14 | 56 | 60 | 50 | 38 | 0 | 0 |
| . | Medium . | RA 10−6 mol/L . | As 10−8 mol/L . | As 10−7 mol/L . | RA + As 10−8 mol/L . | RA + As 10−7 mol/L . |
|---|---|---|---|---|---|---|
| NB4 | ||||||
| CD33 | 86.5 | 60 | 80 | 60 | 30 | 38 |
| CD11b | 13.8 | 76 | 36 | 73 | 68 | 65 |
| CD11c | 0 | 80 | 53 | 80 | 80 | 81.5 |
| CD14 | 0 | 34 | 40 | 49 | 0 | 0 |
| NB4-AsR | ||||||
| CD33 | 93 | 60 | 85 | 65 | 0 | 0 |
| CD11b | 25 | 76 | 92 | 74 | 69 | 70 |
| CD11c | 25 | 80 | 95 | 86 | 74 | 70 |
| CD14 | 56 | 60 | 50 | 38 | 0 | 0 |
Effect of As2O3, RA or the combination of the two compounds on the expression of the myeloid surface markers CD33, CD11b, CD11c, and CD14. NB4 cells and NB4-AsR cells (105/mL) were incubated for 3 days with the different compounds. The number of positive cells for the expression of the various markers, as well as the mean associated fluorescence, were quantified by flow cytometry. One presentative experiment among 3 different assays is reported.
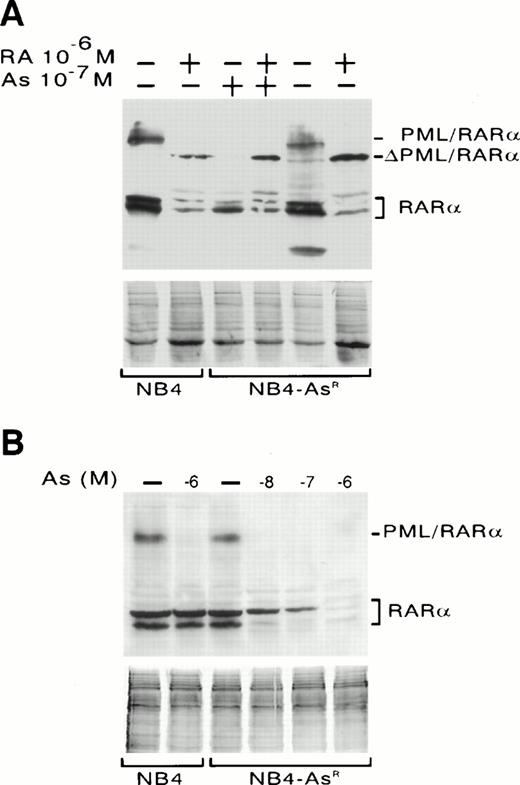
Western blot analysis of PML/RARα and RARα expression in NB4 and NB4-AsR cells. (A) NB4 cells were treated for 1 day with medium alone or 10−6 mol/L RA. NB4-AsR cells were grown continuously in 10−7mol/L As2O3, then washed with medium and treated for 1 day with 10−7 mol/L As2O3, 10−6 mol/L RA plus 10−7 mol/L As2O3 or grown for 2 weeks without As2O3 and treated for 1 day with medium alone or 10−6 mol/L RA. ▵PML/RARα indicates a cleavage product of PML/RARα. (B) NB4 cells were treated for 1 day with medium alone or 10−6 mol/L As2O3. NB4-AsR cells were treated for 1 week with medium alone, As2O3 at 10−6 mol/L, 10−7 mol/L, 10−8mol/L. CBB waived gels are shown below to ensure equal protein loading.
Western blot analysis of PML/RARα and RARα expression in NB4 and NB4-AsR cells. (A) NB4 cells were treated for 1 day with medium alone or 10−6 mol/L RA. NB4-AsR cells were grown continuously in 10−7mol/L As2O3, then washed with medium and treated for 1 day with 10−7 mol/L As2O3, 10−6 mol/L RA plus 10−7 mol/L As2O3 or grown for 2 weeks without As2O3 and treated for 1 day with medium alone or 10−6 mol/L RA. ▵PML/RARα indicates a cleavage product of PML/RARα. (B) NB4 cells were treated for 1 day with medium alone or 10−6 mol/L As2O3. NB4-AsR cells were treated for 1 week with medium alone, As2O3 at 10−6 mol/L, 10−7 mol/L, 10−8mol/L. CBB waived gels are shown below to ensure equal protein loading.
NB4-AsR–resistant cells degrade PML/RARα on As2O3 exposure.
Western blot analysis with anti-RARα antibodies was performed on NB4 cells treated for 1 day with As2O3(Fig 4B) or RA (Fig 4A). As2O3 caused a near total degradation of PML/RARα,24 while 10−6 mol/L RA led to the degradation of PML/RARα and the generation of a cleavage intermediate, ΔPML/RARα (Fig 4A). Essentially identical results were found for NB4-AsR cells, showing that As2O3-triggered degradation of the fusion protein is not sufficient to trigger apoptosis (Fig 4A). RA also induced the degradation of RARα proteins after an overnight treatment in both parental and arsenic-resistant cells (Fig 4A). Interestingly, NB4-AsR cells exposed for a week to different concentrations of As2O3 presented a dose-dependent RARα degradation (Fig 4B). Arsenic-triggered apoptosis precluded analysis of long-term effects of As2O3 on RARα protein expression in parental NB4 cells. In both cell lines, PML/RARα and RARα transcripts (assessed by Northern blot analysis) were almost unchanged on 1 day treatment with As2O3 or RA, showing that the decrease in the corresponding proteins was due to protein catabolism (data not shown).
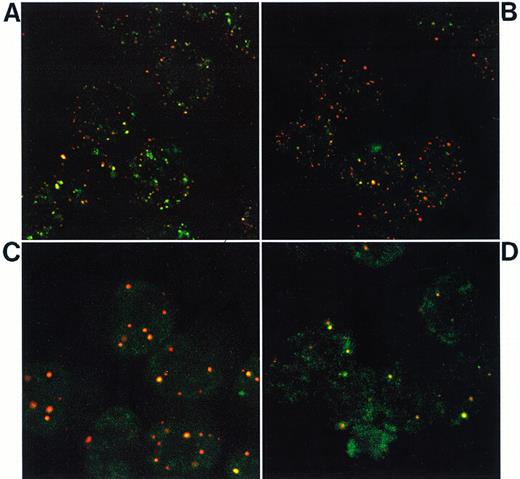
Confocal laser microscopy analysis of NB4 and NB4-AsR cells double-labeled with anti-PML antibodies (revealed with a Texas Red-labeled conjugate) and anti-RARα antibodies (revealed by a FITC-labeled conjugate). (A) NB4; (B) NB4-AsR; (C) NB4-AsR treated for 1 day with 10−6 mol/L RA; or (D) NB4-AsR treated for 1 day with 10−6 mol/L As2O3.
Confocal laser microscopy analysis of NB4 and NB4-AsR cells double-labeled with anti-PML antibodies (revealed with a Texas Red-labeled conjugate) and anti-RARα antibodies (revealed by a FITC-labeled conjugate). (A) NB4; (B) NB4-AsR; (C) NB4-AsR treated for 1 day with 10−6 mol/L RA; or (D) NB4-AsR treated for 1 day with 10−6 mol/L As2O3.
NB4-AsR cells grown 24 hours or more in As2O3, lacked the microspeckled RARα staining, but presented a strong PML staining on nuclear speckles (NBs) (Fig 5D). Contrary to RA-treated NB4 cells, in which approximatively 10 NBs were found in each nucleus, NB4-AsR cells maintained in As2O3, had a lower number of NBs that appeared smaller than RA-induced NBs in NB4 (data not shown) or in NB4-AsR cells (Fig 5C). This suggests that there was a further degradation of PML and NBs aggregation in As2O3-treated NB4-AsR cells, consistent with our previous work.24 NB4-AsR cells withdrawn from As2O3 for a month recovered a normal synthesis of PML/RARα and RARα proteins (Fig 4A and B) and hence a microspeckled PML and RARα staining like in NB4 cells (Fig 5A and B), but rechallenge with arsenic led again to PML/RARα degradation and relocalization of NBs. These observations suggest that As2O3 resistance was not due to a genetic defect affecting PML/RARα, PML, or RARα expression and could rather be ascribed to other As2O3-induced events.
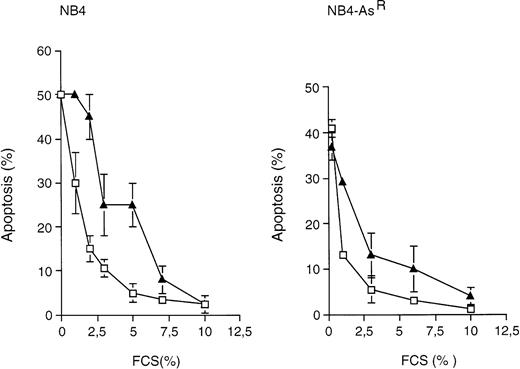
Effects of FCS depletion on NB4 and NB4-AsRapoptosis. NB4 (left graph) and NB4-AsR cells (right graph) were grown in the absence (□) or in the presence (▴) of 10−7 mol/L As2O3 for 3 days. Medium was supplied with the concentrations of FCS indicated in the plot. Apoptosis (%) was determined, after a TUNEL assay, by flow cytometry. Each value represents the mean ± SD of three independent measurements.
Effects of FCS depletion on NB4 and NB4-AsRapoptosis. NB4 (left graph) and NB4-AsR cells (right graph) were grown in the absence (□) or in the presence (▴) of 10−7 mol/L As2O3 for 3 days. Medium was supplied with the concentrations of FCS indicated in the plot. Apoptosis (%) was determined, after a TUNEL assay, by flow cytometry. Each value represents the mean ± SD of three independent measurements.
Arsenic facilitates induction of apoptosis in NB4-AsRcells.
The property of NB4-AsR cells to express or degrade PML/RARα protein in the presence or in the absence of As2O3 and the proposed links between PML/RARα expression and apoptosis14 35 led us to study the role of the fusion protein on NB4 cell survival. We submitted NB4 and NB4-AsR cells to serum deprivation in the presence or absence of 10−7 mol/L As2O3 (which led to the complete degradation of PML/RARα). Arsenic sensitized NB4 and NB4-AsRcells to both cell death (measured by Trypan blue exclusion) and apoptosis (assessed by TUNEL) triggered by serum deprivation (Fig 6). Although we cannot rule out a direct action of arsenic on apoptotic pathways, these results are consistent with the idea that PML/RARα degradation favors apoptosis triggered by serum deprivation.
RA and arsenic synergize to induce differentiation and apoptosis.
The effects of As2O3, RA, or both agents on the differentiation and apoptosis of NB4 cells were examined. When parental or arsenic-resistant NB4 cells were treated with 10−7mol/L As2O3 and 10−6 mol/L RA in combination, a sharp decrease in cell growth was observed (Fig 7A) and these drugs synergized for induction of apoptosis (assessed by TUNEL and PI labeling) (Figs 2 and 7B). Such massive induction of apoptosis makes morphologic assessment of differentiation difficult (Fig 2); nevertheless, immunophenotypic analysis showed a weak synergy between As2O3 and RA for CD33 and CD14 expressions (Table 1). Under similar conditions, at both 3 (not shown) and 5 days (Fig 2), dual treated NB4-AsR cells presented highly differentiated features (an increased number of cells presented lobed or multiple nuclei) and more neutrophilic granules and vacuoles than RA-treated cells, as well as a smaller cell size and a less basophilic cytoplasm (Fig 2). Increased plastic adhesion was also noted (data not shown). The NBT reduction assay, LAP activity, and surface marker analysis support the idea of enhanced differentiation with the combination of RA and As2O3. Therefore, these agents appear to synergize for the induction of apoptosis and/or differentiation, primarily in arsenic-resistant, but also in NB4 cells.
The expression of several key genes involved in differentiative and/or apoptotic pathways and known to be directly or indirectly responsive to RA, was then studied. Bcl-2 protein was expressed at the same levels in parental or resistant NB4 cells. In NB4 cells, the protein was downregulated by 10−6 mol/L As2O3 or 10−6 mol/L RA (see Fig 9).21,36 Arsenic did not modulate this protein in NB4-AsR cells, while RA treatment did (data not shown). However, increasing the As2O3 concentration in the presence of RA did lead to a further decrease in bcl-2 protein (Fig 8C), which was not due to cell death, as STAT1α expression sharply increased (see below). RA induces the STAT1α protein and also stimulates IFNα secretion.31,37In NB4 or in NB4-AsR, 10−7 mol/L As2O3 did not modify STAT1α protein levels (data not shown), while 10−6 mol/L RA induced expression of this protein (Fig 8A and not shown). RA and As2O3 combination sharply shortened the response time in NB4-AsR cells (2 days) compared with NB4 cells (4 days) for both STAT1α synthesis (Fig 8A) and IFNα secretion (Fig 8B). p21WAF1/CIP1 is a primary target gene of both RA38 and STAT1α.39 p21 was strongly upregulated by either As2O3 or RA in NB4 cells (Fig 8D). NB4-AsR cells have an elevated baseline of p21 expression and 10−6 mol/L As2O3 induced a moderate increase of p21, while RA alone or with As2O3 induced high levels of p21 expression. Taken together, these findings show that RA and As2O3 strongly cooperate in their activation of effectors of differentiation, growth control, and apoptosis.
(A) Effect of As2O3and/or RA on NB4, NB4-R1, and NB4-R2 cell apoptosis. NB4, NB4-R1, and NB4-R2 were treated for 3 days with 10−6mol/L As2O3 or 10−6 mol/L RA, as indicated. Apoptosis was determined, after a TUNEL assay and PI-staining, by flow cytometry, and the % of apoptotic cells is reported in the plots. The abscissa of the plot represents TUNEL (apoptosis) and the ordinate represents PI (DNA quantity). (B) Western blot analysis of Bcl-2 in NB4 and NB4-R1 cells. Cells were grown for 48 hours in the presence of the indicated treatments. (C) Effect of As2O3 and/or RA on PML/RARα and RARα expression in NB4-R1 cells. The cells were treated for 2 days with either medium, 10−6 mol/L RA, 10−7mol/L As2O3, or the combination of 10−6 mol/L RA plus 10−7 mol/L As2O3. The two right lanes represent sequential treatments: 10−6 mol/L RA for 1 day followed by 10−7 mol/L As2O3 for the next day or 10−7 mol/L As2O3 for 1 day followed by 10−6 mol/L RA for the next day (far right lane).
(A) Effect of As2O3and/or RA on NB4, NB4-R1, and NB4-R2 cell apoptosis. NB4, NB4-R1, and NB4-R2 were treated for 3 days with 10−6mol/L As2O3 or 10−6 mol/L RA, as indicated. Apoptosis was determined, after a TUNEL assay and PI-staining, by flow cytometry, and the % of apoptotic cells is reported in the plots. The abscissa of the plot represents TUNEL (apoptosis) and the ordinate represents PI (DNA quantity). (B) Western blot analysis of Bcl-2 in NB4 and NB4-R1 cells. Cells were grown for 48 hours in the presence of the indicated treatments. (C) Effect of As2O3 and/or RA on PML/RARα and RARα expression in NB4-R1 cells. The cells were treated for 2 days with either medium, 10−6 mol/L RA, 10−7mol/L As2O3, or the combination of 10−6 mol/L RA plus 10−7 mol/L As2O3. The two right lanes represent sequential treatments: 10−6 mol/L RA for 1 day followed by 10−7 mol/L As2O3 for the next day or 10−7 mol/L As2O3 for 1 day followed by 10−6 mol/L RA for the next day (far right lane).
RA-resistant cell lines are sensitive to As2O3 apoptotic effects.
NB4-R1 and NB4-R2 are two stable RA-resistant cell lines.27The As2O3-induced apoptosis in NB4-R1 and NB4-R2 cell lines (with or without RA) appeared faster compared with parental cells. The whole cell population died after a 4-day incubation with As2O3, while at the same time, only 50% of the parental NB4 cells were apoptotic (Fig 9A). This observation is consistent with the clinical finding that RA-resistant patients are As2O3-sensitive and that another RA-resistant subclone is As2O3 sensitive.23,40The NB4-R1 and NB4-R2 apoptosis pattern was reproducibly different from parental NB4 cells and may present two apoptotic populations. Surprisingly, RA and/or As2O3-treated NB4-R1 cells presented high levels of Bcl-2 protein (Fig 9B and not shown), again suggesting that Bcl-2 protein does not play a key role in NB4 apoptosis.36 Western blot analysis confirmed that untreated NB4-R1 cells expressed PML/RARα and RARα proteins (Fig9C), while NB4-R2 no longer expressed the PML/RARα protein.41 In NB4-R1, as in NB4 cells, a 2-day RA treatment caused the degradation of PML/RARα, a partial degradation of RARα proteins, and the stabilization of ΔPML/RARα (Fig 9C). Moreover, sequential treatment with As2O3 and RA showed that the ΔPML/RARα cleavage product, once generated by RA exposure, was resistant to the degradative action of As2O3 both in NB4 (not shown) and NB4-R1 cells (Fig 9C). This suggests that an initial proteolytic step triggered by RA renders ΔPML/RARα insensitive to As2O3. Overnight As2O3 treatment again caused the complete degradation of PML/RARα, but not RARα, in NB4-R1 cells (Fig 9C). By showing that As2O3 and RA induce PML/RARα degradation in NB4 sublines resistant to their respective actions, our data demonstrate that PML/RARα degradation may be required, but is not sufficient for the induction of differentiation or apoptosis.
DISCUSSION
In this report, an arsenic-resistant NB4 population, NB4-AsR, was characterized and used to show that arsenic and RA cooperate to induce differentiation and/or apoptosis. Resistance of NB4-AsR cells to apoptosis was not due to a decreased As2O3 uptake in presence of the drug or to altered PML/RARα As2O3-induced degradation. Rather, resistance to the apoptotic effects of As2O3 is linked to alterations in some unknown effectors. Evidence for this process comes from the failure of As2O3 to regulate expression of bcl-2 or p21 proteins in As2O3-resistant NB4 cells. However, NB4-AsR cells remain sensitive to some of the effects of As2O3, such as induction of partial differentiation or growth retardation and thus provide a model system in which the two effects of As2O3(differentiation and apoptosis) are dissociated. The NB4-AsR cell line shows an As2O3concentration-dependent degradation of PML/RARα and thus provides a powerful means by which to study the effects of PML/RARα (such as NB antigens dispersion8 and/or control of apoptosis14,35) in the context of a myeloid cell. In NB4 cells, the degradation of PML/RARα would be expected to restore myeloid maturation by relieving the differentiation block. We previously argued that during As2O3 treatment, differentiation may not be apparent because of the rapid onset of apoptosis.24 Indeed, in NB4 cells grown in low As2O3 concentrations, as well as in NB4-AsR treated with high As2O3concentrations, some differentiation is observed. Although we cannot exclude that As2O3 might activate specific genes involved in myeloid differentiation (such as LAP), it could be suggested that PML/RARα degradation is responsible for the partial myeloid differentiation. Consistent with this hypothesis, in NB4-R1 cells, RA-induced PML/RARα decrease is also associated with changes in surface markers but not terminal maturation.27Nevertheless, our data both in As2O3- and in RA-resistant cells clearly show that induction of PML/RARα degradation may be a first step, but is not sufficient for the full response to these two agents.
Besides PML/RARα degradation, long-term, but not short-term, As2O3 exposure induced a pronounced and dose-dependent downregulation of RARα protein. We have previously shown that As2O3 leads to modifications in RARα phosphorylation, which could induce modifications in the half-life of the RARα protein.24 In addition, a major, lactacystin (a proteasome-inhibitor)–sensitive RA-induced RARα degradation was consistently observed in all cell lines examined to date (data not shown). Together with the recent observation that RA also induces the catabolism of the PML/RARα20,42 and PLZF/RARα43 fusion proteins, this observation strongly suggests that RA induces the degradation of RARα-containing proteins. The less pronounced downregulation of RARα proteins compared with PML/RARα may be due to the concomitant RA induction of RAR genes.2 Future studies should delineate the mechanisms responsible for the catabolism of RARα induced by RA, as well as the links between RARα degradation and the RA target gene activation.
When NB4-AsR cells are primed with As2O3, RA induces apoptosis. This apoptosis could reflect the terminal differentiation of the myeloid cells, as strongly suggested from the sharp synergy between As2O3 and RA for morphologic differentiation. Alternatively, as some retinoids have been shown to induce apoptosis independently from differentiation, RA may directly trigger apoptosis in As2O3-primed cells.44-46Reciprocally, arsenicals may induce apoptosis, and RA-mediated PML/RARα catabolism (which sensitize the cells to cell death), could favor As2O3-triggered apoptosis and thus account for the RA/As2O3 synergy in NB4 cells. Bcl-2 protein is downregulated during RA-induced NB4 differentiation, while in NB4-R1 cells, RXR-specific agonists induce apoptosis despite high levels of bcl-2 protein.36 Moreover, the protein levels of bcl-2 are unchanged during As2O3-induced apoptosis of NB4-R1 cells (Fig9B) and an NB4-R1 cell-line that overexpresses bcl-2 protein47 presented the same kinetics of arsenic-induced apoptosis as parental cells (data not shown). Altogether, these results imply that Bcl-2 modulation per se is not significant for apoptosis of NB4 cells. STAT1α signaling pathway could induce apoptosis through the induction of interleukin converting enzyme (ICE) genes48 or other unknown ways. However, although STAT1α induction precedes RA-triggered apoptosis, it is not upregulated during arsenic-induced apoptosis, questioning its role in apoptosis. A candidate gene, which could participate in both As2O3 and RA-induced apoptosis, is p21, which blocks the cell cycle at both the G1/S and G2/M transitions during the apoptosis process after DNA damage49 and whose upregulation by either As2O3 or RA always precedes induction of apoptosis.
Several nonexclusive hypotheses can be put forward to account for the arsenic/RA effects on differentiation. Either, arsenic alone leads to a significant differentiation not dependent on RARα, which would lead to additive effects between As2O3 and RA. This can be a property of arsenic per se due, for example, to modifications in phosphorylation of transcription factors such as AP150 or to DNA hypomethylation, which in turn, facilitates the expression of some genes.51 In this respect, it is worth noting that As2O3 sharply induced LAP activity (Fig 3B). Arsenic could also control differentiation through PML/RARα degradation and hence modulation of putative PML/RARα-regulated genes involved in myeloid differentiation, which are not controlled by RA.16 Alternatively, As2O3 could affect the RA response, for instance through changes in phosphorylation of RARα or other proteins involved in RA transduction pathways, leading to cooperative effects. Although arsenic did not alter the RA response in transient transfection assays, our demonstration that long-term As2O3 exposure leads to RARα degradation could relate to its activation.24 Finally, As2O3-induced PML/RARα degradation might be expected to facilitate the RA response through the normal receptor by liberating DNA binding sites and/or RARα cofactors. However, that the STAT1α gene induction by RA was identical in NB4 cells pretreated or not with 10−7 mol/L As2O3 (not shown) does not favor this hypothesis. Our results are compatible with all three possibilities: there are additive effects on differentiation, facilitated responses for STAT1α, NBT reduction, and synergistic effects on LAP activity and apoptosis. That As2O3- or RA-resistant cells not only show no cross-resistance but, indeed, an enhanced sensitivity to the other agent, has important implications for APL, supporting the idea that the combination of RA and As2O3 treatments will be of clinical benefit.
ACKNOWLEDGMENT
We thank Prof. P. Chambon for the generous gifts of anti-RARα antibodies. We thank E. Garattini and all members of the de Thé's laboratory for critical reading of the manuscript. We also thank M.T. Daniel for critical comments on “Giemsa stained” NB4-AsR cells and M. Schmid for his help in the confocal microscopy.
Supported by grants from the “Association pour la Recherche sur le Cancer,” “Ligue contre le Cancer” (Nationale, Comité de Paris and Hauts-de-Seine), and “Fondation St Louis,” Paris, France. M.G. was supported by La “Ligue Contre le Cancer” and partially supported by the “FIRC” (Fondazione Italiana per la Ricerca sul Cancro, Milano, Italy).
Address reprint requests to Hugues de Thé, MD, PhD, CNRS, UPR 9051, Hôpital St Louis, Paris, 1 Avenue Vellefaux, 75475 Paris Cedex 10, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal