Abstract
Human hematopoietic stem cells are pluripotent, ie, capable of producing both lymphoid and myeloid progeny, and are therefore used for transplantation and gene therapy. An in vitro culture system was developed to study the multi-lineage developmental potential of a candidate human hematopoietic stem cell population, CD34+CD38− cells. CD34+CD38− cells cocultivated on the murine stromal line S17 generated predominantly CD19+ B-cell progenitors. Transfer of cells from S17 stroma to myeloid-specific conditions (“switch culture”) showed that a fraction of the immunophenotypically uncommitted CD19− cells generated on S17 stroma had myeloid potential (defined by expression of CD33 and generation of colony-forming unit-cells). Using the switch culture system, single CD34+CD38− cells were assessed for their lymphoid and myeloid potential. Nineteen of 50 (38%) clones generated from single CD34+CD38− cells possessed both B-lymphoid and myeloid potential. 94.7% of the CD34+CD38− cells with lympho-myeloid potential were late-proliferating (clonal appearance after 30 days), demonstrating that pluripotentiality is detected significantly more often in quiescent progenitors than in cytokine-responsive cells (P = .00002). The S17/switch culture system permits the in vitro assessment of the pluripotentiality of single human hematopoietic cells.
HEMATOPOIETIC STEM CELLS (HSC) have the unique capacity to provide long-term and complete restoration of myelopoiesis and lymphopoiesis after marrow ablation. The clinical fields of hematopoietic cell transplantation and gene therapy have stimulated intense interest in ex vivo manipulation of HSC. However, conclusions about the effects of ex vivo manipulations have been based on in vitro assays of committed progenitors. These in vitro assays may be misleading when used as surrogate markers for true pluripotent HSC.1 An assay able to detect single cells possessing both myeloid and lymphoid potential (pluripotentiality) is required to understand how approaches aimed at HSC proliferation may affect HSC function.
In vivo assays of human hematopoiesis have been developed in which human hematopoietic cells engraft in immunodeficient mice.2-4 These models have demonstrated the existence of human pluripotent cells either by limiting dilution analysis or by clonal integration of a retroviral marker gene.5,6 Until recently, in vitro systems of human hematopoiesis have been limited to lineage-specific cultures.7-12 Our goal in the present studies was to determine whether lineage-specific culture systems for myeloid and lymphoid cells could be modified to show in vitro the presence of pluripotent human hematopoietic cells. In this report we describe the development of a switch culture system based on cocultivation on the murine stromal line S17 in which single cord blood CD34+CD38− cells with pluripotentiality can be identified. Furthermore, the lympho-myeloid cells so detected are found almost exclusively in late-proliferating CD34+CD38− cells, demonstrating that more rapidly proliferating, cytokine-responsive progenitors lack multilineage potential.
MATERIALS AND METHODS
Isolation of CD34+CD38− cells.
Umbilical cord blood samples were collected by the Labor and Delivery Staff at Kaiser Permanente Hospital Sunset (Los Angeles, CA) following clamping of the cord as previously described9 and according to guidelines reviewed by the Committee on Clinical Investigations at Childrens Hospital Los Angeles. Mononuclear cells were prepared using Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density centrifugation within 24 hours of collection. Enrichment for CD34+ cells was performed as per manufacturer's guidelines using the MiniMACS system (Miltenyi Biotec, Auburn, CA). CD34+ enriched cells were washed in phosphate-buffered solution (PBS; Irvine Scientific, Santa Ana, CA) and incubated for 30 minutes at 4°C in fluorescein isothiocyanate (FITC)-CD34 (HPCA2; Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) and phycoerythrin (PE)-CD38 (leu 17; BDIS). CD34+CD38− cells were then isolated using the gating previously described9 on a FACSVantage flow cytometer (BDIS) with an argon laser tuned at 488 nm using Lysys II software (BDIS). CD34+CD38− cells, defined by the R2 gate in Fig 1, comprised 3.53% ± 0.68% of the CD34+ cells.
CD34+CD38− cord blood cells are immunophenotypically uncommitted to lymphoid or myeloid lineages. Three-color analysis of CD34+ enriched cord blood cells from region R1 (based on forward and side scatter (not shown9). (B) Region R2 defining CD34+CD38− cells (ie, high CD34 expression with CD38 expression less than half the maximal fluorescence of the isotype control). R2 comprises 3.53% ± 0.68% (mean ± SEM) of all CD34+ cells (defined by upper and lower right quadrants). (C, D, and E) The lack of expression of lineage-specific antigens on cells from R2. (A and F) Respective isotype controls.
CD34+CD38− cord blood cells are immunophenotypically uncommitted to lymphoid or myeloid lineages. Three-color analysis of CD34+ enriched cord blood cells from region R1 (based on forward and side scatter (not shown9). (B) Region R2 defining CD34+CD38− cells (ie, high CD34 expression with CD38 expression less than half the maximal fluorescence of the isotype control). R2 comprises 3.53% ± 0.68% (mean ± SEM) of all CD34+ cells (defined by upper and lower right quadrants). (C, D, and E) The lack of expression of lineage-specific antigens on cells from R2. (A and F) Respective isotype controls.
An aliquot of CD34+ MiniMACS enriched cells was removed for analysis of lineage-specific antigen expression using three-color fluorescence-activated cell sorter (FACS) analysis. For these studies CD34+ enriched cells were incubated with FITC-CD34, APC-CD38 (leu 17; BDIS), and one or more of the following antibodies conjugated to PE: CD19 (leu 12; BDIS), CD10 (Calla; Coulter, Hialeah, FL), CD33 (My 9; Coulter), CD11b (leu 15; BDIS), and CD14 (My 4; Coulter). Analysis was performed on the FACSVantage using argon and HeNe lasers and CellQuest software (BDIS).
Stromal cultures.
S17 stroma is a murine bone marrow (BM) stromal line and was generously provided by Dr Kenneth Dorshkind (University of California at Los Angeles).13 S17 stroma was expanded in RPMI 1640 (Irvine Scientific), 5% fetal calf serum (FCS), 50 μmol/L 2-mercaptoethanol (2-ME; Sigma, St Louis, MO), penicillin/streptamycin (P/S; Gemini Bio Products, Calabasas, CA) and glutamine (Gemini Bio Products). S17 stroma was not irradiated before plating of human hematopoietic cells and was maintained in a confluent state during long-term culture by contact inhibition. Primary human stroma (HS) was prepared from mononuclear BM cells as previously described.9 10 HS was then trypsinized, irradiated at 20 Gy, and replated at a concentration of 7 × 103 cells/well in 96-well plates (Becton Dickinson Labware, Lincoln Park, NJ).
Primary cultures.
Primary cultures consisted of cocultivation of CD34+CD38− cells on S17 in “lymphoid medium” (RPMI, 5% FCS, 2-ME, P/S, glutamine). In some experiments (see Fig 6) primary cultures were established on either S17 or HS with either lymphoid medium or “myeloid medium-noHC/GF” (ie, myeloid medium [see below] without hydrocortisone or added growth factors).
B-cell potential is lost during primary culture on HS. CD34+CD38− cord blood cells were cultured for 1 week in each of the four primary culture conditions shown and then switched to BSC on S17 stroma to measure maintenance of B-cell potential. At days 34 and 60 after switching, cells were obtained from BSC, counted, and analyzed by FACS for CD19 expression. Bars shown are means (±SEM) compiled from three independent experiments. LM, lymphoid medium; MM, myeloid medium-noHC/GF (see Materials and Methods). (▪), HS/LM; (□), HS/MM; (▧), S17/LM; (▧), S17/MM.
B-cell potential is lost during primary culture on HS. CD34+CD38− cord blood cells were cultured for 1 week in each of the four primary culture conditions shown and then switched to BSC on S17 stroma to measure maintenance of B-cell potential. At days 34 and 60 after switching, cells were obtained from BSC, counted, and analyzed by FACS for CD19 expression. Bars shown are means (±SEM) compiled from three independent experiments. LM, lymphoid medium; MM, myeloid medium-noHC/GF (see Materials and Methods). (▪), HS/LM; (□), HS/MM; (▧), S17/LM; (▧), S17/MM.
Immunophenotypic analysis of cultured cells.
Cells from primary cultures were incubated first with 1% human IVIG (Cutter, Berkely, CA) to block nonspecific binding, and then with PE-CD33 and FITC-CD19. In view of the nonspecific background staining which occurs in cultured cells, it was necessary to exclude surface antigen expression occurring at low frequency (<5%) from the analyses. Thus, the presence of myeloid cells in primary culture was defined as CD33 expression in greater than 5% of cells. B-lymphoid differentiation was defined as CD19 expression in greater than 20% of cells.
Lineage-specific (switch) cultures.
Hematopoietic cells were switched from primary culture into either B-cell–specific culture (“BSC,” ie, S17 and lymphoid medium as described above) or myeloid-specific culture (“MSC,” ie, HS and myeloid medium = Iscoves Modified Dulbecco's Medium [IMDM; GIBCO-BRL, Bethesda, MD], 30% FCS, 1% bovine serum albumin [BSA; Sigma], 2-ME, P/S, glutamine, 10−6 mol/L hydrocortisone [Sigma] and the myeloid growth factors interleukin-3 [IL-3; 10 ng/mL], IL-6 [50 U/mL], and Steel factor [SF, 50 ng/mL]).9 10 Hydrocortisone was included in MSC to prevent B-cell proliferation; cytokines were added to induce myeloid differentiation. The presence of myeloid progenitors in MSC was assayed by replating nonadherent cells into semisolid medium (myeloid medium with the addition of 1.3% methylcellulose, 50 ng/mL granulocyte-macrophage colony-stimulating factor [GM-CSF] and 2 U/mL erythropoietin) and enumeration of colony-forming unit-cells (CFU-C) after 14 days.
Cloning efficiency of CD34+CD38−cells grown on S17 stroma.
Cloning efficiency was determined by plating CD34+CD38− cells by the Automated Cell Deposition Unit (ACDU) of the FACSVantage onto established S17 stroma either in limiting dilution or as single cells into each well of 96-well plates. Accuracy of ACDU sorts was checked by sorting single chicken red blood cells (Axell; Accurate Chemical and Scientific Corp, Westbury, NY) and FITC-conjugated beads (Calibrite beads; BDIS) into each well of 96-well flat-bottom microtiter plates (n = 6). These checks showed that an average 6% of wells contained no visible cells. No instances of more than one cell per well were seen. In the limiting dilution experiments, between 22 and 144 wells containing each of the following cell numbers were plated: 1, 3, 10, 20, 30, 40, 80 cells. Timing of clonal appearance was estimated only in the experiments using single-cell deposition. Wells were inspected every week and the presence of new clones recorded to note the timing of first appearance. Because B cells are small and grow as a dispersed population rather than as easily visible cobblestone areas, only clones containing more than 500 cells could be visualized with certainty and were thus scored as positive. Clones containing more than 1,000 cells were procured for cell enumeration after Trypan blue staining. Those clones that were visible but too small to enumerate were recorded as containing 500 to 1,000 cells.
Clones were obtained for CD19 staining, FACS sorting, and switch culture when they contained at least 3,000 cells. This occurred within 1 week of first identification and was constant for both late and early appearing clones large enough for analysis. In single-cell studies, myeloid potential within the CD19− cells of each clone was defined as a combination of (1) proliferation of cells after switching CD19− cells to MSC, and (2) CD33 expression in greater than 5% of cells and/or CFU-C production after switching to MSC.
Statistical analysis.
Significant differences between data groups were determined by T-test using Fisher's Exact and Aspin-Welch methods. Cloning efficiency using limiting dilution analysis (LDA) data was analyzed by Poisson statistics and the weighted mean method.
RESULTS
Our goal in these studies was to determine whether single human hematopoietic cells with both lymphoid and myeloid potential (pluripotentiality) could be detected in vitro.
CD34+CD38− cells lack expression of lineage-specific antigens.
We sought to initiate cultures with a CD34+ subpopulation which lacked immunophenotypic evidence of lineage commitment. Expression of lineage-specific antigens on CD34+ subsets (defined by CD38 expression) from cord blood was studied using three-color FACS analysis. The CD34+CD38−immunophenotype defines a highly primitive, largely quiescent population of hematopoietic progenitors.9,10 14-16 Only cells defined as CD34+CD38− using the stringent R2 gate (ie, high CD34 expression with CD38 expression less than half the maximum fluorescence of the isotype control, Fig 1) uniformly lacked expression of the B-cell antigens (CD19 and CD10) and the myeloid antigens (CD33, CD14, and CD11b). Therefore, we used the R2 gate to define CD34+CD38− cells for all studies to maximally enrich for uncommitted cord blood cells.
Cloning efficiency of B-lymphoid progenitors from single cord blood CD34+CD38− cells.
Cocultivation of human CD34+CD38− cells on S17 stroma has recently been shown to generate cultures containing predominantly B-lymphoid progenitors; 80% to 95% of cultured cells express CD19, CD10, and CD45, do not express CD34 or CD20, and are predominantly germline at the Ig locus.12 To ascertain the feasibility of adapting the S17 system to analysis of single cells, the cloning efficiency of B-lymphoid progenitors from cord blood CD34+CD38− cells was determined. CD34+CD38− cells were isolated by FACS and plated onto S17 stroma in limiting dilution. Day 40 cloning efficiency of cord blood CD34+CD38− cells was 2.4% ± 0.4% (mean ± SEM, n = 3). In experiments with single-cell plating by ACDU, cloning efficiency of CD34+CD38− cord blood cells was 3.7% ± 1.3% (n = 3). Combining these data, the frequency of CD34+CD38− cells capable of proliferating on S17 stroma by day 40 was 3.02 ± 0.67% (n = 6).
Immunophenotype of clones generated from single CD34+CD38− cells on S17 stroma.
Of 224 clones generated from single CD34+CD38− cells on S17 stroma (n = 4), 88 clones contained sufficient cells to be analyzed for expression of the B-cell–specific antigen CD19 by FACS. The unambiguous presence of B-cell progenitors (defined as >20% CD33−CD19+ cells) was found in 85 of 88 (96.6%) clones (Fig 2).
CD19 expression in clones from single CD34+CD38− cells in S17 culture. Bars indicate mean ± SEM from four individual experiments (total 88 clones).
CD19 expression in clones from single CD34+CD38− cells in S17 culture. Bars indicate mean ± SEM from four individual experiments (total 88 clones).
Although most clones contained predominantly CD19+ cells, CD19− cells were also present, and comprised 5% to 80% of the cells. Immunophenotypic evidence of myeloid differentiation (defined as CD33 expression in >5% of cells) was not detectable in any clones. Analysis of additional clones showed no expression of another myeloid antigen, CD11b.
Onset of clonal proliferation and clone size.
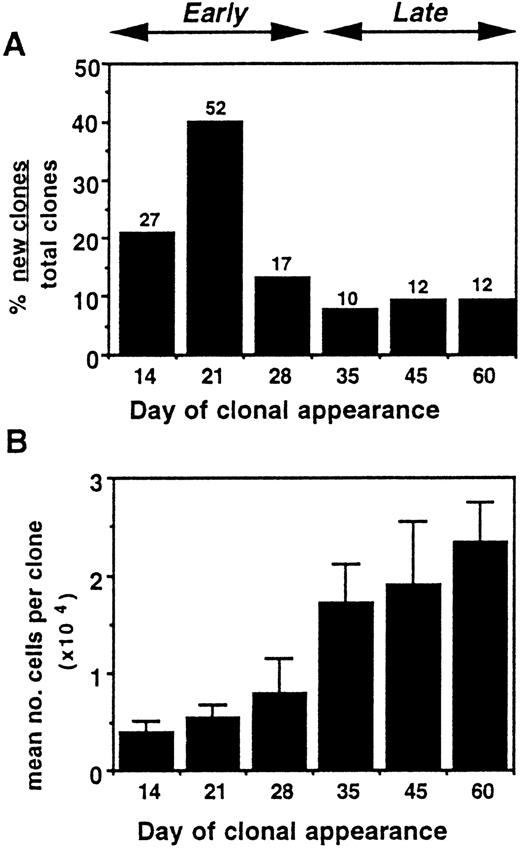
The timing of clonal proliferation was determined by plating single cells in individual wells and noting the time at which clones first became visible during 100 days of culture. The time of appearance of the clones generated from single CD34+CD38−cells was variable, ranging from as early as day 13 to as late as day 76. Most clones appeared before day 30 (from “early proliferating” cells). However, “late-proliferating cells” (those which formed clones after day 30) comprised 1.6% (34 of 2,112) of all CD34+CD38− cells plated and 26.2% (34 of 130) of all clones (Fig 3A). The relationship between timing of clonal proliferation and generative capacity (clonal size) was also determined. Late-proliferating cells produced clones containing a significantly greater number of progeny (1.77 ± 0.33 × 104 cells, mean ± SEM) than did early proliferating cells (0.54 ± 0.11 × 104 cells) (P < .01) (Fig 3B).
Proliferation from single CD34+CD38− cord blood cells. (A) Timing of clonal appearance. Above the bars are shown the number of clones analyzed at each time point. (B) Relationship of timing of clonal appearance to generative capacity. Bars denote mean number of cells/clone ± SEM. Data for (A) and (B) are compiled from a total 130 clones initiated from 2,112 single CD34+CD38− cells.
Proliferation from single CD34+CD38− cord blood cells. (A) Timing of clonal appearance. Above the bars are shown the number of clones analyzed at each time point. (B) Relationship of timing of clonal appearance to generative capacity. Bars denote mean number of cells/clone ± SEM. Data for (A) and (B) are compiled from a total 130 clones initiated from 2,112 single CD34+CD38− cells.
CD19− cells from primary S17 cultures have lymphoid and myeloid potential.
Having established that CD34+CD38− cells are preferentially directed to B-lymphoid differentiation on S17 stroma without immunophenotypic evidence of myeloid commitment, we next determined if the functional capacity to differentiate into the myeloid lineage was preserved on S17. A switch culture system in which cells from primary B-lymphoid cultures were replated into MSC was used to reveal the presence or absence of myeloid progenitors in primary S17 cultures. To determine the lineage potential of the minority of cells not yet committed to B-lymphoid differentiation, CD19−cells were separated by FACS from the more numerous CD19+cells from day 14 to day 60 of primary S17 culture (n = 3). CD19− and CD19+ populations were then split and each replated into BSC and MSC (Fig 4).
CD19− cells replated into BSC proliferated with a 20- to 50-fold increase in cell number (n = 3). CD19 antigen was expressed on 44% to 88% of the cells generated from CD19− cells in BSC. CD19− cells isolated after at least 5 weeks of primary S17 culture retained the ability to differentiate into CD19+ B-lymphoid progenitors in BSC.
CD19− cells also differentiated into myeloid cells when cultured in MSC. After an initial 3 weeks on S17 stroma, isolated CD19− cells replated into MSC expanded from 113- to 1,600-fold and cell proliferation persisted for over 6 weeks (n = 3); CD33 expression was present in over 50% of cells. To confirm the lineage commitment of proliferating cells functionally, nonadherent cells from MSC were replated into methylcellulose culture to detect myeloid progenitors. 0.67% ± 0.24% (mean ± SEM) of CD19− cells gave rise to CFU-C and these CFU-C could be detected in CD19− populations isolated after at least 60 days in S17 culture. Long-term culture-initiating cells (LTC-IC)8 were detected in CD19− populations after an initial 35 days of S17 culture. Thus, the CD19−fraction of S17 primary cultures generates two populations of progenitors: B-lymphoid progenitors that express CD19 after secondary culture with S17 and myeloid progenitors revealed only when they are induced to proliferate and differentiate in myeloid-specific conditions.
CD19+ cells are committed to B-cell differentiation.
When CD19+ cells from primary S17 cultures were isolated by FACS and replated in BSC, they underwent no further proliferation but were maintained viably for at least 4 weeks. After switching to MSC (which contains hydrocortisone), CD19+ cells showed no expansion and disappeared completely within 5 days. Thus, CD19+ cells in S17 culture were irreversibly committed to B-cell differentiation and did not display myeloid potential under the conditions described here.
Single cord blood CD34+CD38−cells have both myeloid and lymphoid potential.
The S17 switch culture system was next applied to single CD34+CD38− cells to conclusively prove their pluripotentiality. Clones expanded from single CD34+CD38− cord blood cells were obtained from S17 and incubated with CD33 and CD19; CD19− cells were isolated from each clone and switched into MSC.
Of 2,112 single CD34+CD38− cells plated in individual wells, a total of 50 clones contained sufficient cells for FACS analysis and switch culture. Of these 50 clones, 31 showed only B-lymphoid potential, ie, they contained greater than 20% CD19+ cells, but showed no myeloid potential upon switching of the CD19− cells to MSC. CD19− cells comprised 35.9% ± 3.5% of the total cells in the 50 clones analyzed. Nineteen clones (38% of all clones analyzed) showed both B-lymphoid (CD19 expression) and myeloid potential (in MSC), representing 0.9% of all CD34+CD38− cord blood cells plated. In the 19 bipotent clones, the CD19−cells comprised 51.2% ± 5.3% of the total cells in each clone. Of the 50 clones analyzed, 22 appeared before day 30, but only one clone (4.5%) which appeared at day 20 demonstrated both B-lymphoid and myeloid potential (Fig 5). Of the 28 clones from late-proliferating cells, 18 clones (64.3%) showed both B-lymphoid and myeloid potential. Thus, pluripotent cells were found almost exclusively in the subpopulation of CD34+CD38− cells which proliferate late in culture (P = .00002, early v late pluripotent cells).
Relationship of B-lymphoid/myeloid potential with timing of proliferation. Shown on the vertical axis is the percent clones with both B-lymphoid and myeloid potential over the total clones analyzed at each time point. Raw numbers of bipotent clones over total clones analyzed are shown above each bar. Data are compiled from a total of 2,112 CD34+CD38− cells plated as single cells per well onto primary S17 culture and analyzed by FACS and switch culture.
Relationship of B-lymphoid/myeloid potential with timing of proliferation. Shown on the vertical axis is the percent clones with both B-lymphoid and myeloid potential over the total clones analyzed at each time point. Raw numbers of bipotent clones over total clones analyzed are shown above each bar. Data are compiled from a total of 2,112 CD34+CD38− cells plated as single cells per well onto primary S17 culture and analyzed by FACS and switch culture.
B-cell potential is rapidly lost during cocultivation on primary HS.
Cocultivation of human cells on primary HS has become the standard in vitro assay of human myelopoiesis.7 17 Therefore, we determined whether the switch culture system could be adapted to use HS instead of S17 stroma during the primary culture of CD34+CD38− cells. To determine if B-cell progenitors can be maintained on primary HS, cells were cultured initially in bulk on HS and then switched to BSC to assay B-cell potential. CD34+CD38− cells cultured in bulk for 1 week on HS could generate B-cell progenitors after switching to BSC, although at reduced numbers compared with cells cultured on S17 stroma continuously (n = 3, Fig 6). The superiority of S17 over HS in maintaining B-cell progenitors was seen irrespective of whether primary cultures contained lymphoid medium or a modification of myeloid medium (ie, myeloid medium without hydrocortisone or growth factors). Thus, the critical component required for maintenance of B-lymphoid potential is the presence of S17 stroma.
Cultures were also initiated with single CD34+CD38− cord blood cells plated in primary culture onto HS (and lymphoid medium). Resultant clones were switched to BSC and analyzed by FACS for evidence of lymphoid development. From a total 607 CD34+CD38− cells, 47 clones developed and 7 were large enough for FACS analysis. B cell progenitors (CD19+CD33− cells) were absent in all clones studied. All clones showed myeloid differentiation (CD33+ > 10%). Thus, although S17 and HS are both able to maintain primitive myeloid progenitors for prolonged periods, B-cell progenitors are rapidly lost during culture on HS.
DISCUSSION
In this report we have developed a switch culture assay to demonstrate the presence of human progenitors with both lymphoid and myeloid potential. Single CD34+CD38− cells from cord blood are plated initially onto the murine stromal line S17 to allow clonal proliferation. In the primary S17 cultures proliferation gives rise to two populations: cells irreversibly committed to B-lymphoid differentiation (CD19+ cells) and a more primitive CD19− population, which is yet to express either myeloid or lymphoid antigens but has the potential to differentiate into either or both lineages. The existence of hematopoietic progenitors with the capacity for B-lymphoid and myeloid differentiation but which lack the potential for T-lymphoid development cannot be excluded by these studies. Application of a T-lymphocyte culture system to the single-cell switch culture assay described here will delineate further the differentiation pathways of uncommitted hematopoietic stem cells.
The presence of S17 in primary culture was crucial for the switch culture system when applied to single cells. Use of primary human BM stroma in otherwise identical culture conditions did not permit clonal expansion into B-lymphoid cells. The reason for this lineage restriction is unclear, but presumably elements within HS either prevent lymphoid commitment from pluripotent cells and/or inhibit the expansion of lymphoid progeny to detectable numbers.
The S17 stromal line was initially identified by its ability to support both murine myelopoiesis and B lymphopoiesis,13 and has been shown to be superior to primary murine BM stroma and numerous other murine stromal lines in its ability to support murine long-term repopulating cells in vitro.18,19 Studies of human hematopoiesis have recently reported that S17 stroma permits B-lymphoid differentiation from cord blood CD34+ and CD34+CD38− cells into cultures containing 80% to 95% CD19+ cells.11 12 This current report shows that S17 stroma also maintains primitive human hematopoietic progenitor cells and that the myeloid potential of these cells can be revealed by switching to conditions allowing myeloid differentiation.
The ability of other murine stromal lines to support human myelopoiesis and B lymphopoiesis has been reported recently.20,21 The Sys1 murine BM stromal line allowed the proliferation of human cord blood CD34hi/lin− cells into myeloid and B-lymphoid lineages.20 The MS5 BM stromal line supported LTC-IC from human BM and has been recently used to identify B-lymphoid/myeloid progenitors from cord blood CD34+CD38low cells (defined as 25% of CD34+ cells with the lowest CD38 expression).21 22 Both of these studies identified the presence of B-lymphoid and myeloid cells by FACS analysis without functional confirmation of lineage commitment and used limiting dilution analysis for assessment of single-cell behavior.
In the present report with S17 stroma, the high percentage of cells expressing CD19 in each clone made determination of the presence of B-lymphoid cells unambiguous. CD19+ cells were irreversibly committed to B-lymphoid lineage; they had lost CD34 expression, died rapidly in myeloid culture, but were sustained in B-cell culture. However, expression of myeloid antigens (CD33 and CD11b) was never found in more than 5% of cells from each clone. Detection of lineage-specific antigens below this level is difficult in cultured cells because of the nonspecific staining commonly seen. Thus, we chose to base the definition of myeloid potential on functional analysis, ie, proliferation in myeloid culture and production of clonogenic myeloid progenitors. Because the S17 culture system allows B-lymphoid differentiation from single CD34+CD38− cells with concomitant maintenance of functionally primitive myeloid progenitors with great proliferative capacity, myeloid potential could be confirmed functionally and unequivocally after serial replating of CD19− cells into myeloid stromal and methylcellulose cultures. Furthermore, the generative capacity of the B-cell and myeloid progenitors upon replating of the CD19− cells allow further study of each population after further cell expansion.
In attempting to define pluripotent cells, it is important that assays are initiated with as homogeneous and uncommitted population as possible. When total CD34+ cord blood cells are cocultivated on S17 stroma, definite CD33 expression is seen early in bulk cultures11 and in clones from early proliferating cells plated in limiting dilution (unpublished data, April 1997). These CD33+ cells (which do not produce CFU-C upon replating) are presumably derived from committed myeloid progenitors sufficiently differentiated at the time of plating to express CD33. Thus, there is a potential for contamination by committed myeloid progenitors when clones are obtained by limiting dilution using heterogeneous starting populations. The stringent definition of CD34+CD38− cells described by the R2 region (which excludes all but the 3% to 4% of CD34+ cells with the highest CD34 and lowest CD38 expression) was therefore used in these studies.
The frequency of B lympho-myeloid progenitors detected using the S17 culture system (0.9% of CD34+CD38− cells) is lower than that reported recently by Berardi et al,21 who found that 7% of CD34+CD38low cells plated by limiting dilution onto MS5 stroma gave rise to cultures with simultaneous CD19 and CD11b expression.The higher frequency may be due to differences in the population of cells used to initiate the cultures, the use of limiting dilution analysis, or functional differences in the supporting stromal lines MS5 and S17. It is also possible that both this study and our own underestimate the true frequency of pluripotent cells as the sensitivity of such analyses depends on sufficient clonal growth. In vivo studies of human B lympho-myeloid cells in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice have found that 0.16% of CD34+CD38− cord blood cells are SCID-repopulating cells that can generate both B-lymphoid and myeloid cells.6 The comparatively low frequency of bipotent cells in these in vivo studies may be secondary to low seeding efficiency in the NOD/SCID mouse. A seeding efficiency of 10% to 20%, similar to that of CFU-S in murine transplant models,23 would result in an estimate of the frequency of pluripotent HSC similar to that presented in this report.
Several in vivo murine transplant studies have led to the notion of a hierarchy of stem cells/progenitors based on quiescence. Long-term repopulating cells can be distinguished from more mature progenitors such as CFU-S by their resistance to cell-cycle–dependent agents.24-27 In vitro murine studies using the cobblestone area forming cell (CAFC) assay showed that cell populations that proliferated late in culture (day 28 CAFC) were those which contributed most to long-term repopulation.28,29 Our previous studies using human myeloid long-term cultures have shown the functional heterogeneity of the CD34+CD38− population in cord blood and in BM.9,10 Although most CD34+CD38− cells which proliferate in myeloid conditions do so during the first 30 days of culture, a small proportion (termed the extended LTC-IC) form clones late in culture, ie, after 30 to 80 days. CD34+CD38− cells that proliferate late in myeloid culture have a greater generative capacity than early proliferating cells.10 These and other in vitro studies using human hematopoietic cells support the concept that quiescence or “cytokine-nonresponsiveness” is associated with the most primitive human hematopoietic progenitors.30-32 The same relationship between late proliferation and greater generative capacity has been noted in this report using CD34+CD38− cells grown on S17 stroma in B-lymphoid conditions. The characteristic of pluripotentiality, fundamental to HSC, was found almost exclusively in the late-proliferating subpopulation of CD34+CD38− cells, providing further evidence that the most primitive human hematopoietic cells are also the most quiescent.
The switch culture system described in this report allows the assay of cells with both lymphoid and myeloid potential, a characteristic considered a unique hallmark of HSC. The ability to expand HSC ex vivo and to transfer therapeutic genes into HSC can now be studied directly rather than relying on the surrogate assays of committed progenitors.
ACKNOWLEDGMENT
We are grateful to the staff of the Labor and Delivery ward, Kaiser Permanente Hospital Sunset (Los Angeles, CA) for their continuing essential contributions in the collection of cord blood samples; to Earl Leonard for biostatistical analysis; and to Drs Robertson Parkman and Donald B. Kohn for helpful advice on the manuscript.
Supported in part by National Institutes of Health Grant Nos. HL54850 and CA-59318. G.M.C. is supported by a Translational Research Grant from the Leukemia Society of America.
Address reprint requests to Gay M. Crooks, MD, Division of Research Immunology/BMT, Childrens Hospital Los Angeles, MS #62, 4650 Sunset Blvd, Los Angeles, CA 90027.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal