Abstract
Previously, we have shown that interleukin (IL)-8 induces the rapid (15 to 30 minutes) mobilization of hematopoietic progenitor cells (HPC) in mice. Because integrins are essential for adhesion and transendothelial migration of HPC, we studied the involvement of the β2-integrin leukocyte function-associated antigen-1 (LFA-1) in IL-8–induced mobilization. After a single injection of blocking anti–LFA-1 antibodies, no mobilization of colony-forming cells was observed. In addition, when mice were pretreated with anti–LFA-1 or saline and subsequently injected with 30 μg of IL-8, mobilization of HPC was completely blocked. We showed that this was not due to anti–LFA-1 antibodies affecting colony formation, as addition of anti–LFA-1 antibodies to colony cultures in semisolid medium had no inhibitory activity. Also, anti-intercellular adhesion molecule (ICAM)-1 antibodies, directed to the main ligand of LFA-1 significantly inhibited the IL-8–induced mobilization. Furthermore, IL-1–induced mobilization was significantly inhibited by anti–LFA-1 antibodies. Because LFA-1 is reported to be expressed on more differentiated HPC, it was considered that the IL-8–induced mobilization of more primitive HPC would not be blocked by anti–LFA-1 antibodies. Transplantation of blood-derived mononuclear cells (MNC) from IL-8–mobilized animals pretreated with anti–LFA-1 antibodies protected only 25% of lethally irradiated recipient mice, whereas the radioprotection rate of control mice transplanted with MNC derived from IL-8-mobilized animals was 86% (P < .01). Anti-LFA–1 antibodies did not interfere with stem cell homing, as transplantation of IL-8-mobilized blood MNC, incubated in vitro with these antibodies resulted in 100% radioprotection. We conclude that anti–LFA-1 antibodies completely prevent the rapid mobilization of colony-forming cells and of cells with radioprotective capacity induced by IL-8. These results indicate a major role for the β2-integrin LFA-1 in the IL-8–induced mobilization of hematopoietic stem cells.
TRANSPLANTATION WITH mobilized progenitor cells is increasingly being applied in the treatment of a variety of hematologic disorders. The use of blood-derived stem cells is associated with a more rapid restoration of the marrow function in comparison with autologous bone marrow transplantation.1-3This advantage is obtained only when the numbers of circulating stem cells are greatly expanded using mobilization regimens. Although stem cell mobilization is a property of most hematopoietic growth factors (granulocyte colony-stimulating factor [G-CSF],4-6granulocyte-macrophage colony-stimulating factor [GM-CSF],1 interleukin [IL]-3,7,8 stem cell factor [SCF],9 flt-3 ligand10), relatively prolonged treatment is required to induce mobilization. Only a few cytokines, including IL-1,11 IL-8,12,13 and macrophage inflammatory protein (MIP-1α),14induce rapid mobilization after a single injection. We have recently shown that IL-8 induces rapid mobilization of cells with lymphomyeloid repopulating ability in mice12 and of progenitor cells in monkeys.13
Adhesion molecules have been implicated to play a role in retaining progenitor cells in the bone marrow microenvironment.15-21Among these, integrins are involved in the cellular interactions between hematopoietic progenitor cells, stromal cells, and components of the extracellular matrix. From the two major families, the β1-integrins, very late antigen (VLA)-4 (CD49d/CD29) and VLA-5 (CD49e/CD29), as well as the β2-integrins leukocyte function-associated antigen-1 (LFA-1) (CD11a/CD18) and Mac-1 (CD11b/CD18) have been reported to be expressed on progenitor cells.16,20,22-27 In addition, ligands for these integrins are abundantly expressed on cells of the bone marrow microenvironment.17,19,28 For instance, fibronectin, a component of the extracellular matrix, is a ligand for VLA-4, and vascular cell adhesion molecule-1 (VCAM-1), as well as intercellular adhesion molecule (ICAM)-1, which are ligands for VLA-4 and LFA-1, respectively, are both expressed on activated endothelial and bone marrow stromal cells.16,17,19,28,29 Adhesion blocking experiments have indicated that the VLA-4/VCAM-1, VLA-5/fibronectin, and β2-integrins/ICAM-1 pathways play a role in the attachment of CD34+ cells to stromal cells.16 The prominent role of VLA-4 in retaining progenitor cells in the bone marrow is shown by Papayannopoulou and Nakamoto,30 who demonstrated that anti–VLA-4 antibodies, but not anti-β2 antibodies, induce mobilization of HPC in primates. Likewise, adhesion molecules have been implicated to play a role in the induction of stem cell mobilization.
In the present study, we have used antibodies against β1- or β2-integrins to study the role of these molecules in IL-8–induced stem cell mobilization. We found that anti–LFA-1 antibodies, but not anti–VLA-4 antibodies, completely prevented the rapid mobilization of colony-forming cells, as well as cells with radioprotective capacity, without interfering with stem cell homing. These results indicate a major role for the β2-integrin LFA-1 in the IL-8–induced mobilization of stem cells.
MATERIALS AND METHODS
Mice.
Balb/c mice, with an age ranging between 8 and 12 weeks and weight between 20 and 25 g, were purchased from Broekman BV, Someren, The Netherlands. Donor animals were fed commercial rodent chow and acidified water ad libitum. Recipient animals were maintained in a pathogen-free environment and fed water containing ciprofloxacin 1 mg/mL (Bayer Nederland BV, Mijdrecht, The Netherlands), polymyxin-B 70 μg/mL and saccharose 2 g/100 mL.
Experimental design.
Mobilization of HPC was induced by a single intraperitoneal (IP) injection of 30 μg of IL-8. After 20 minutes, the mice were killed by CO2 asphyxiation and peripheral blood was obtained by intracardiac puncture. In blocking experiments, mice were pretreated with an IP injection of anti–LFA-1 blocking antibodies or saline. The following day, 30 μg of IL-8 was administered IP, and blood was obtained after 20 minutes. In transplantation experiments, recipient mice were placed in a polymethylmetaacetate (PMMA) box and given total body irradiation (8.5 Gy, Philips SL 75-5/6 mV linear accelerator, Philips Medical Systems, Best, The Netherlands), divided in two parts in posterior-anterior and anterior-posterior position, at a dose rate of 4 Gy/min. In survival experiments, a fixed number of 5 × 105 blood-derived mononuclear cells (MNC) obtained from IL-8–mobilized donor animals pretreated with anti–LFA-1 antibodies or saline, was injected in the tail vein of lethally irradiated recipients. The experimental protocol was approved by the institutional ethical committee on animal experiments.
Cytokines.
Recombinant human IL-8 was purified from Escherichia coli(E coli) expressing a synthetic gene31 and provided by the Novartis Forschungsinstitut, Vienna, Austria. IL-8 has no colony-stimulating activity as reported previously.32 The concentration of endotoxin was less than 0.05 EU/mL as determined by the Limulus amoebocyte lysate assay. Human rIL-1α was kindly provided by Hoffmann-La Roche (Nutley, NJ) and does not contain detectable levels of endotoxin (<60 pg/mL). For in vivo experiments, all agents were diluted to the desired concentration in endotoxin-free phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and administered as an IP injection.
Monoclonal antibodies and detection of free circulating antibody.
Rat antimurine monoclonal antibodies directed against different adhesion molecules were used: H154.163 (anti-CD11a, LFA-1, IgG2a, kindly provided by Dr M. Pierres, Centre d'Immunologie, Marseille, France),33 R1-2 (anti-CD49d, VLA-4, IgG2b, kindly provided by Dr B. Holzmann, Department of Pathology, Stanford University, Stanford, CA),35 and YN1/1.7 (anti- CD54, ICAM-1, IgG2b).34 To detect circulating free antibody, plasma was obtained from mice at various time intervals after a single IP injection of 100 μg anti–LFA-1. A volume of 50 μL plasma was incubated for 30 minutes at 4°C with 2 × 105 peripheral blood leukocytes of untreated mice. After washing and labeling with phycoerythrin-conjugated goat antirat-IgG (GaRa-PE) (Caltag, San Francisco, CA), fluorescence intensity was analyzed by fluorescence-activated cell sorting (FACS) (Becton Dickinson, Mountain View, CA).
Preparation of cell suspensions.
Mice were killed by CO2 asphyxiation. Blood was obtained by intracardiac puncture and cell counts were performed on a Sysmex F800 (TOA Medical Electronics Co, LTD, Kobe, Japan). Manual neutrophil counts were performed after May Grünwald-Giemsa staining. Blood-derived MNC suspensions were obtained by Ficoll separation as described.11
Progenitor cell assays.
Colony-forming unit–granulocyte macrophage (CFU-GM) were cultured as described previously.11 Briefly, peripheral blood MNC were cultured in 3.5-cm dishes containing 5 × 105 cells per mL in semisolid medium in the presence of murine GM-CSF (1.25 ng/mL). After 6 days of culture in a fully humidified atmosphere of 37°C containing 5% CO2, the number of colonies (defined as aggregates of > 20 cells) were scored using an inverted microscope.
Statistical analysis.
Differences were evaluated using the Student's t-test. In survival analysis, differences were evaluated using the Mantel-Haenszel test for linear association. P values of < .05 were considered statistically significant.
RESULTS
Effect of pretreatment with anti–LFA-1 antibodies on the mobilization of progenitor cells by IL-8.
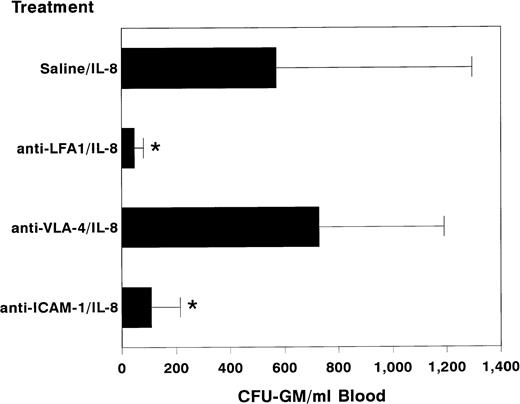
Balb/c mice were injected IP with a single dose of 100 μg of anti–LFA-1 antibodies or saline. At various time intervals ranging from 30 minutes to 24 hours, the numbers of circulating progenitor cells were assessed. Anti–LFA-1 antibodies alone did not induce the mobilization of progenitor cells. Free circulating antibodies could be detected up to 72 hours after a single injection (data not shown). Subsequently, mice pretreated with a single dose of anti–LFA-1 antibodies (100 μg) were treated after 24 hours with a single dose of 30 μg IL-8 IP. After 20 minutes, the mice were killed and peripheral blood was obtained by cardiac puncture for progenitor cell assays. Pretreatment with anti–LFA-1 antibodies completely prevented the IL-8–induced mobilization of circulating progenitor cells (anti-LFA-1 + IL-8 46 ± 34, n = 21 v saline + IL-8 570 ± 724 CFU-GM/mL blood, n = 18; mean ± standard deviation [SD], P < .01, Fig1). This effect appeared to be dose dependent, complete blocking being obtained after injecting a dose of 100 μg (Fig 2). Addition of anti–LFA-1 antibodies to colony cultures in semisolid medium of IL-8–mobilized blood or steady-state bone marrow had no inhibitory activity, indicating that the lack of mobilization after pretreatment with anti–LFA-1 was not due to interference of the antibody with colony growth in vitro (data not shown). Also, blocking antibodies to ICAM-1, the main ligand of LFA-1, significantly inhibited the IL-8–induced mobilization of colony-forming cells, although inhibition was not complete (108 ± 107 CFU-GM/mL blood, n = 8; mean ± SD, P < .01, Fig 1). In contrast, anti–VLA-4 antibodies did not block the IL-8–induced mobilization of HPC (728 ± 462 CFU-GM/mL blood, n = 10; mean ± SD, Fig 1), nor did they induce themselves the peripheralization of progenitor cells. After a single injection of 300 μg anti–VLA-4, no significant increase in the number of circulating progenitor cells was observed (anti–VLA-4 19 ± 13 v saline 20 ± 17 CFU-GM/mL blood, n = 3). In addition, administration of anti–VLA-4 (300 μg) for 3 consecutive days did not induce mobilization of HPC (data not shown).
Effect of pretreatment with anti–LFA-1 on the mobilization of progenitor cells by IL-8. Mice were pretreated with a single IP injection of 100 μg of anti–LFA-1 (H154.163, n = 20), 300 μg of anti–VLA-4 (R1/2, n = 10), 300 μg of anti–ICAM-11 (YN1/1.7, n = 8) or saline (n = 20). The following day 30 μg of IL-8 was administered as a single IP injection 20 minutes before harvesting peripheral blood. Results are expressed as mean ± SD. *,P < .01 as compared with saline pretreated controls.
Effect of pretreatment with anti–LFA-1 on the mobilization of progenitor cells by IL-8. Mice were pretreated with a single IP injection of 100 μg of anti–LFA-1 (H154.163, n = 20), 300 μg of anti–VLA-4 (R1/2, n = 10), 300 μg of anti–ICAM-11 (YN1/1.7, n = 8) or saline (n = 20). The following day 30 μg of IL-8 was administered as a single IP injection 20 minutes before harvesting peripheral blood. Results are expressed as mean ± SD. *,P < .01 as compared with saline pretreated controls.
Prevention of IL-8–induced mobilization of progenitor cells by anti–LFA-1 is dose dependent. Mice were pretreated with a single injection of increasing doses of anti–LFA-1 (10, 30, and 100 μg) or saline IP. The following day 30 μg of IL-8 was administered IP 20 minutes before harvesting peripheral blood. Results are expressed as mean (n = 20 for saline and 100 μg anti–LFA-1, and n = 2 for 10 and 30 μg anti–LFA-1).
Prevention of IL-8–induced mobilization of progenitor cells by anti–LFA-1 is dose dependent. Mice were pretreated with a single injection of increasing doses of anti–LFA-1 (10, 30, and 100 μg) or saline IP. The following day 30 μg of IL-8 was administered IP 20 minutes before harvesting peripheral blood. Results are expressed as mean (n = 20 for saline and 100 μg anti–LFA-1, and n = 2 for 10 and 30 μg anti–LFA-1).
Effect of anti–LFA-1 antibodies on blood cell counts.
Mice pretreated with anti–LFA-1 antibodies showed a consistent thrombocytopenia 24 hours after injection, whereas mice treated with saline or control antibodies did not (312 ± 60, n = 21 v739 ± 109, n = 18; mean ± SD, P < .001, Table 1). The decrease in platelets started 2 hours after injection of the antibody, was maximal at 8 hours, and lasted several days. Concomitant with the decline of free circulating antibodies, the platelet count returned to baseline values. No significant effect on the numbers of white blood cells, red blood cells, or total number of granulocytes was observed after 24 hours. The instant neutropenia and subsequent neutrophilia seen after IL-8 injection was unaltered in mice pretreated with blocking anti–LFA-1 antibodies before IL-8 injection (data not shown).
Effect of Anti–LAF-1-Antibodies on Blood Cell Counts
| . | Treatment-150 . | ||||||
|---|---|---|---|---|---|---|---|
| PBS/IL-8 . | Anti–LAF-1/PBS . | Anti–LAF-1/IL-8 . | Anti–VLA-4/IL-8 . | Anti–ICAM-1/IL-8 . | PBS/IL-1 . | Anti–LAF-1/IL-1 . | |
| RBCs (×1012/L) | 8.2 ± 0.7 | 8.9 ± 0.6 | 8.7 ± 0.8 | 8.1 ± 0.3 | 7.7 ± 0.8 | 7.2 ± 0.2 | 7.2 ± 0.3 |
| WBCs (×109/L) | 7.9 ± 1.5 | 9.8 ± 1.7 | 6.7 ± 1.9 | 8.3 ± 1.9 | 7.4 ± 1.7 | 7.2 ± 1.2 | 5.1 ± 0.8 |
| Neutrophils (%) | 16.6 ± 5.5 | 13.8 ± 4.6 | 17.7 ± 9.7 | ND | 11 ± 7.5 | 53 ± 3.5 | 49 ± 8.0 |
| Plts (×109/L) | 739 ± 109 | 290 ± 36-151 | 312 ± 60-151 | 634 ± 100 | 682 ± 87 | 599 ± 47 | 235 ± 44-151 |
| . | Treatment-150 . | ||||||
|---|---|---|---|---|---|---|---|
| PBS/IL-8 . | Anti–LAF-1/PBS . | Anti–LAF-1/IL-8 . | Anti–VLA-4/IL-8 . | Anti–ICAM-1/IL-8 . | PBS/IL-1 . | Anti–LAF-1/IL-1 . | |
| RBCs (×1012/L) | 8.2 ± 0.7 | 8.9 ± 0.6 | 8.7 ± 0.8 | 8.1 ± 0.3 | 7.7 ± 0.8 | 7.2 ± 0.2 | 7.2 ± 0.3 |
| WBCs (×109/L) | 7.9 ± 1.5 | 9.8 ± 1.7 | 6.7 ± 1.9 | 8.3 ± 1.9 | 7.4 ± 1.7 | 7.2 ± 1.2 | 5.1 ± 0.8 |
| Neutrophils (%) | 16.6 ± 5.5 | 13.8 ± 4.6 | 17.7 ± 9.7 | ND | 11 ± 7.5 | 53 ± 3.5 | 49 ± 8.0 |
| Plts (×109/L) | 739 ± 109 | 290 ± 36-151 | 312 ± 60-151 | 634 ± 100 | 682 ± 87 | 599 ± 47 | 235 ± 44-151 |
Abbreviation: ND, not done.
Mice were treated with a single IP injection of 100 μg anti–LFA-1 (H154.163), 300 μg anti–VLA-4 (R1-2), 300 μg anti–ICAM-1 (YN1/1.7) or saline (PBS) at day −1. At day 0, 30 μg of IL-8, 1 μg of IL-1 or PBS was administered IP. Blood was obtained by cardiac puncture at 20 minutes after IL-8 and at 5 hours after IL-1 injection. Results are expressed as mean ± SD (n = 20 for saline/IL-8 and anti–LFA-1/IL-8, n = 4 for anti–LFA-1/saline, n = 10 for anti–VLA-4/IL-8, n = 8 for anti–ICAM-1/IL-8, and n = 6 for saline/IL-1 and anti–LFA-1/IL-1).
P < .001.
Effect of anti–LFA-1 antibodies on IL-1–induced mobilization of progenitor cells.
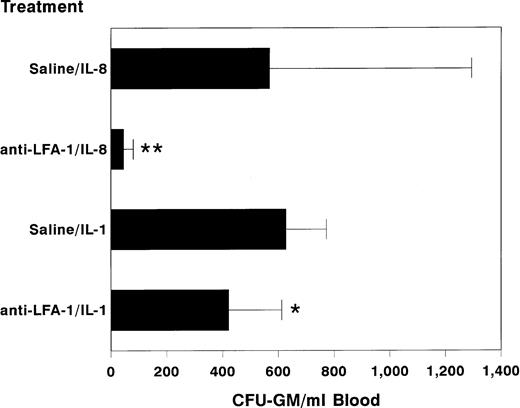
Because IL-1 is a potent inducer of IL-836 and because it also induces the mobilization of hematopoietic stem cells after a single injection,11 we studied the involvement of LFA-1 in IL-1–induced progenitor cell mobilization. In these experiments, mice were pretreated with anti–LFA-1 antibodies (100 μg) at 24 hours before a single IP injection of IL-1 (1 μg). Circulating HPC numbers were assessed at 5 hours after injection of IL-1. In comparison with IL-1–mobilized control animals, mice treated with IL-1 after pretreatment with anti–LFA-1 had significantly lower numbers of circulating HPC (anti–LAF-1 + IL-1 423 ± 191 v saline + IL-1 628 ± 146 CFU-GM/mL blood; n = 6 per treatment group, mean ± SD, P < .05, Fig 3), showing the involvement of LFA-1 in IL-1–induced progenitor cell mobilization.
Effect of pretreatment with anti–LFA-1 on IL-1–induced mobilization of progenitor cells. Mice were pretreated with 100 μg of anti–LFA-1 or saline. The following day, 1 μg of IL-1 (n = 6 per treatment group) or 30 μg of IL-8 (n = 20 per treatment group) was administered as a single IP injection, 4 to 6 hours or 20 minutes before harvesting peripheral blood, respectively. Results are expressed as mean ± SD. **, P < .01 and *, P < .05 as compared with saline pretreated controls.
Effect of pretreatment with anti–LFA-1 on IL-1–induced mobilization of progenitor cells. Mice were pretreated with 100 μg of anti–LFA-1 or saline. The following day, 1 μg of IL-1 (n = 6 per treatment group) or 30 μg of IL-8 (n = 20 per treatment group) was administered as a single IP injection, 4 to 6 hours or 20 minutes before harvesting peripheral blood, respectively. Results are expressed as mean ± SD. **, P < .01 and *, P < .05 as compared with saline pretreated controls.
Effect of anti–LFA-1 antibodies on the mobilization of hematopoietic progenitor cells with radioprotective capacity.
The possibility was considered that pretreatment with anti–LFA-1 antibodies, while blocking mobilization of HPC, did not preclude the IL-8–induced mobilization of more primitive HPC. To test this hypothesis, we studied the radioprotective capacity of the mobilized MNC fraction. Recipient mice were lethally irradiated (8.5 Gy) and subsequently transplanted with 5 ×105 MNC, derived from the peripheral blood of pretreated donor mice. The survival of recipients transplanted with (5 × 105) MNC derived from IL-8–mobilized animals pretreated with 100 μg anti–LFA-1 was only 25%, not different from control recipients injected with saline only (19%). In contrast, transplantation of (5 × 105) MNC from IL-8–mobilized animals pretreated with saline protected 86% of the recipient mice (n = 20 per group in two experiments, P < .01, Fig 4). These results show that anti–LFA-1 blocking antibodies prevent the IL-8–induced mobilization of progenitor cells with radioprotective capacity. In control experiments, animals transplanted with 5 × 105 IL-8–mobilized MNC incubated in vitro with anti–LFA-1 blocking antibodies before injection into lethally irradiated recipient animals (100 μg per 5 × 106 cells for 30 minutes at 4°C) showed a 100% survival. Moreover, pretreatment of recipient mice with 100 μg anti–LFA-1 antibodies, followed by transplantation with 5 × 105 IL-8–mobilized MNC resulted in a survival rate of 90%, indicating that these antibodies did not interfere with homing (n = 10 per group, Fig 4).
Survival of lethally irradiated recipients at 33 days after transplantation. Recipient mice were transplanted with 5 × 105 blood-derived MNC from IL-8–mobilized animals (30 μg IP) pretreated with anti–LFA-1 (100 μg IP) (anti–LFA-1 + IL-8) or saline (saline + IL-8) at day -1. To exclude the possibility that anti–LFA-1 antibodies would interfere with homing of HPC, IL-8–mobilized blood was incubated with anti–LFA-1 (IL-8/anti–LFA-1) in one control experiment. Furthermore, recipient mice were pretreated with anti–LFA-1 (IL-8 + anti–LFA-1) as another control. Survival data are expressed as absolute percentages of two experiments with 10 mice transplanted per group in each experiment.
Survival of lethally irradiated recipients at 33 days after transplantation. Recipient mice were transplanted with 5 × 105 blood-derived MNC from IL-8–mobilized animals (30 μg IP) pretreated with anti–LFA-1 (100 μg IP) (anti–LFA-1 + IL-8) or saline (saline + IL-8) at day -1. To exclude the possibility that anti–LFA-1 antibodies would interfere with homing of HPC, IL-8–mobilized blood was incubated with anti–LFA-1 (IL-8/anti–LFA-1) in one control experiment. Furthermore, recipient mice were pretreated with anti–LFA-1 (IL-8 + anti–LFA-1) as another control. Survival data are expressed as absolute percentages of two experiments with 10 mice transplanted per group in each experiment.
DISCUSSION
Since the observation that cytokines are capable of inducing stem cell mobilization, adhesion molecules have been suggested to play a role in the process of mobilization.15,21,22,25,37 However, direct evidence that these molecules are essential for mobilization has been lacking. In this report, evidence is presented that the functional expression of the β2-integrin LFA-1 is required for the IL-8–induced mobilization of hematopoietic progenitor cells and shows the direct involvement of the β2-integrin LFA-1 in cytokine-induced mobilization. A single injection of anti–LFA-1 antibodies completely prevented IL-8–induced stem cell mobilization. This was not due to the effect of anti–LFA-1 antibodies on colony formation, as addition of these antibodies to colony cultures in semisolid medium had no inhibitory activity. As LFA-1 is reported to be expressed on more differentiated HPC,22,23 it was considered that the IL-8–induced mobilization of more primitive HPC would not be blocked by anti–LFA-1 antibodies. This appeared not to be the case because the radioprotective capacity of IL-8–induced MNC derived from anti–LFA-1 pretreated donor mice was not different from control recipients injected with saline only. Moreover, transplantation of IL-8–mobilized MNC in recipients pretreated with anti–LFA-1 antibodies or transplantation with in vitro-incubated IL-8–mobilized MNC engrafted normally, indicating that these antibodies did not interfere with stem cell homing. These results are in accordance with the reported improved engraftment in immunodeficient children after treatment with anti–LAF-1antibodies as part of their conditioning regimen for allogeneic bone marrow transplantation.38 39
Anti–LAF-1 antibodies have been reported to inhibit the migration of lymphocytes through IL-1–stimulated human umbilical vein endothelial cells.40 Möhle et al41 have used blocking anti–LFA-1 antibodies to inhibit migration of CD34+ cells in a transmigration assay using a bone marrow endothelial cell-derived cell line. In the transmigrated cell population, primitive CD34+ CD38− progenitor cells were virtually absent, while in comparison with the starting cell population, the transmigrated cells had a significantly higher plating efficiency. These data indicate differences in the ability to migrate between primitive and lineage committed progenitor cells. Indeed, LFA-1 was found to be preferentially expressed on more mature HPC, while immature progenitor cells seemed to lack expression.22,23 It is therefore possible that the effect of anti–LFA-1 antibodies on IL-8–induced mobilization is mediated by the inhibition of the transmigration of progenitor cells through the bone marrow endothelium. In accordance with this hypothesis, also anti–ICAM-1 antibodies, directed to the main ligand of LFA-1 and expressed on endothelial cells,41-44 significantly inhibited IL-8–induced mobilization of HPC. The lack of expression of LFA-1 on primitive HPC, however, would argue against such a mechanism and suggests the involvement of an accessory cell expressing both LFA-1 and receptors for IL-8.
The inhibitory effect of anti–LFA-1 on the mobilization of progenitor cells does not only affect IL-8–induced mobilization, but also mobilization induced by IL-1. In previous studies in our laboratory, IL-1 has been found to induce HPC mobilization after an interval of 4 to 6 hours.11 Because IL-1 is a potent inducer of other cytokines,36,45 the possibility was considered that an intermediate cytokine, induced by IL-1, was responsible for this effect. Because the production of IL-8 by endothelial cells is induced by IL-1,36 the observations in this study are compatible with the hypothesis that IL-1–induced mobilization is at least in part mediated by the induction of IL-8.
The interaction between progenitor cells and bone marrow stromal cells with β1-integrins appears to be essential for adhesion of hematopoietic progenitor cells. Leavesley et al46 reported that VLA-4, but not LFA-1, contributes to the adhesive phenotype of steady-state bone marrow-derived CD34+ cells. In vitro experiments have shown that blocking anti–VLA-4 antibodies completely abrogate lymphopoiesis and severely reduce myelopoiesis in long-term bone marrow cultures.47Furthermore, in vivo, incubation of bone marrow cells with anti–β1-integrin antibodies before transplantation into lethally irradiated mice inhibited the formation of CFU-S derived spleen colonies, as well as the reconstitution of medullary hematopoiesis.18 These data indicate the prominent role of β1-integrins in the retention and homing of progenitor cells in the bone marrow microenvironment. In our experiments, we have used anti–VLA- 4 antibodies to study the effect on IL-8–induced mobilization. The antibodies did not influence mobilization or induce mobilization when administered alone. These data are in contrast with those reported by Papayannopoulou who found that VLA-4 antibodies were able to induce the peripheralization of colony-forming progenitor cells in primates and mice.30 48 These discrepancies may be due to the use of other antibodies, recognizing different epitopes of the VLA-4 molecule.
Mice treated with anti–LFA-1 antibodies exhibited distinct thrombocytopenia. Studies from Tavassoli and Makoto49,50indicated that megakaryocyte adhesion to endothelial cells is an important physiologic process for platelet formation. Subsequently, Avraham et al51 showed that LFA-1 is expressed on megakaryocytes and that anti-CD18 antibodies inhibit the binding of megakaryocytes to cytokine-stimulated endothelial cells. These observations suggest a direct interaction between the binding of the anti–LFA-1 antibody and platelet production. Because the thrombocytopenia was already observed at 2 hours after injection, a direct interaction with platelets is also likely. At present, the sequence of events underlying this thrombocytopenia remains unclear.
In conclusion, we have found that anti–LFA-1 antibodies completely prevent the IL-8–induced mobilization of progenitor cells with colony-forming or radioprotective capacity. We showed that these antibodies did not interfere with homing of IL-8–induced mobilized progenitor cells after transplantation. Our data clearly show the important role of the β2-integrin, LFA-1, in IL-8– and IL-1–induced mobilization of HPC. Whether LFA-1 expression on hematopoietic progenitor cells or on accessory cells is responsible for this activity remains to be determined. Recent experiments from our group indicate that the majority of the colony-forming cells in murine steady-state bone marrow are LFA-1 negative.52 These observations do not imply a direct inhibition of the anti–LFA-1 antibodies on the egression of HPC through the bone marrow endothelium and indicate the involvement of an intermediate cell population expressing receptors for IL-8, as well as LFA-1. We are currently studying the hypothesis that neutrophils may fulfill such a role. In accordance with this hypothesis Liu et al53 have found severely impaired IL-8–induced mobilization in G-CSF–receptor-deficient mice. Furthermore, activation of neutrophils by IL-8 induces the release of the metalloproteinase gelatinase-B (MMP-9), involved in the degradation of extracellular matrix molecules.54 Preliminary experiments in monkeys have indicated the rapid induction of MMP-9 by IL-8 and the blocking of IL-8–induced mobilization of HPC by antibodies against MMP-9.55 Taken together, these data are in line with a central role for neutrophils and metalloproteinases in the induction of IL-8–induced stem cell mobilization.
ACKNOWLEDGMENT
The authors thank Arie Boon of the Department of Radiotherapy for his technical assistance in irradiating the animals and Peter de Jong of the facilities for laboratory animals for his excellent animal care.
Supported by the Dutch Cancer Society (NKB) (Amsterdam, The Netherlands) Grant No. RUL 95-1091.
Address reprint requests to Johannes F.M. Pruijt, MD, Department of Hematology, Leiden University Medical Center, Bldg 1, C2-R, PO Box 9600, 2300 RC Leiden, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal