Abstract
We previously reported the aberrant growth of granulocyte-macrophage (GM) progenitors induced by a combination of stem cell factor (SCF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in juvenile chronic myelogenous leukemia (JCML). We examined here the effects of thrombopoietin (TPO) on the proliferation and differentiation of hematopoietic progenitors in JCML. In serum-deprived single-cell cultures of normal bone marrow (BM) CD34+CD38high cells, the addition of TPO to the culture containing SCF + GM-CSF resulted in an increase in the number and size of GM colonies. In the JCML cultures, in contrast, the number of SCF + GM-CSF–dependent GM colonies was not increased by the addition of TPO. However, the TPO addition caused an enlargement of GM colonies in cultures from the JCML patients to a significantly greater extent compared with the normal controls. There was no difference in the type of the constituent cells of GM colonies with or without TPO grown by JCML BM cells. A flow cytometric analysis showed that the c-Mpl expression was found on CD13+ myeloid cells generated by CD34+CD38high BM cells from JCML patients, but was at an undetectable level in normal controls. The addition of TPO to the culture containing SCF or SCF + GM-CSF caused a significant increase in the production of GM colony-forming cells by JCML CD34+CD38neg/lowpopulation, indicating the stimulatory effects of TPO on JCML primitive hematopoietic progenitors. Normal BM cells yielded a significant number of megakaryocytes as well as myeloid cells in response to a combination of SCF, GM-CSF, and/or TPO. In contrast, megakaryocytic cells were barely produced by the JCML progenitors. Our results may provide a fundamental insight that the administration of TPO enhances the aberrant growth of GM progenitors rather than the recovery of megakaryocytopoiesis.
JUVENILE CHRONIC myelogenous leukemia (JCML) is a rare myeloproliferative disorder that occurs in infancy and early childhood.1 The International Juvenile Myelomonocytic Leukemia Working Group has proposed the term of juvenile myelomonocytic leukemia.2 In general, the prognosis of patients with JCML is poor. One reason is that intensive chemotherapy is not superior to nonintensive chemotherapy or even to no chemotherapy.2Supportive care such as control for thrombocytopenia is important in most cases.
Thrombopoietin (TPO), c-Mpl ligand, has been cloned by several independent groups3-7 as a growth factor specific for the megakaryocyte-platelet lineage. Like the administration of granulocyte-colony-stimulating factor (G-CSF) for neutropenia, the clinical application of TPO may be a potent strategy for thrombocytopenia in patients with JCML. Methia et al8showed that c-mpl transcript is detected in human CD34+cells as well as megakaryocytes and platelets. Kobayashi et al9 reported that TPO synergizes with stem cell factor (SCF) and/or interleukin-3 (IL-3) in support of the formation of multilineage colonies, granulocyte-macrophage (GM) colonies, and erythroid colonies. In addition, TPO has been indicated to stimulate the proliferation of acute myeloblastic leukemia cells.10Thus, it is necessary to elucidate the effects of TPO on the growth of hematopoietic progenitors in JCML.
Emanuel et al11 proposed that the primary mechanism for the myeloproliferation in JCML is hypersensitivity of the GM progenitors to GM-CSF. In our previous study, JCML GM progenitors showed the favorable response to GM-CSF plus SCF.12 We examined here whether TPO exerted the effects on the proliferation and differentiation of hematopoietic progenitors in JCML using a serum-deprived culture system.
MATERIALS AND METHODS
Patients.
Bone marrow (BM) cells were obtained from five patients who showed the clinical and laboratory characteristics of JCML at the time of diagnosis, ie, hepatosplenomegaly and/or skin manifestations, leukocytosis with monocytosis, erythroblastosis with or without anemia and thrombocytopenia. The clinical data of four of the five patients (cases 1 to 4) were presented in our previous report.12 The laboratory findings of case 5 (a 5-month-old girl) were as follows: white blood cell (WBC) count, 68,600/μL with 12% immature myeloid cells and 13% monocytes; hemoglobin (Hb) level, 10.2 g/dL; erythroblast count, 2/100 WBC; platelet count, 151,000/μL; and fetal hemoglobin (HbF) level, 7.8%. A BM examination showed myeloid hyperplasia and a decreased number of megakaryocytes. No chromosomal abnormalities were observed. The patient's BM specimens yielded high numbers of GM colonies in the absence of hematopoietic growth factors.
When the BM cells were obtained, two of the five patients had been treated with 6-mercaptopurine (6-MP) and the remaining two with either etoposide or a combination of 6-MP and cytosine arabinoside. No treatment had been given to case 5. Four patients treated with the antileukemic drug(s) had hepatosplenomegaly when the samples were obtained. The relevant hematological findings were as follows: WBC counts 4,900 to 14,070/μL; Hb levels 11.0 to 12.2 g/dL; and platelet counts 27,000 to 321,000/μL. The myeloid hyperplasia, elevated HbF level, and spontaneous GM colony formation by BM cells were still observed.
Factors and antibodies.
Human recombinant TPO, SCF, IL-3, and GM-CSF were generously provided by Kirin Brewery Co Ltd (Takasaki, Japan). Human recombinant G-CSF was provided by Chugai Pharmaceutical Co (Tokyo, Japan).
For the flow cytometric analysis, monoclonal antibodies (MoAbs) for CD34 (HPCA-2, fluorescein isothiocyanate, FITC) and CD38 (Leu17, phycoerythrin, PE) were purchased from Becton Dickinson Immunocytometry Systems (Mountain View, CA); MoAbs for CD34 (581, phycoerythrin-cyanin 5, PE-Cy 5), CD13 (SJ1D1, PE), and CD 41b (SZ.22) were from Immunotech S.A. (Marseilles, France). An MoAb against human c-Mpl domain 1 (M113) was obtained from Genzyme Co (Cambridge, MA). In our preliminary experiments, the Western blot analysis showed that M1 MoAb recognized the c-Mpl with the molecular weight of 82 kD in a platelet lysate.
Cell sorting.
BM cells were aspirated in heparinized plastic syringes from the five patients with JCML and from the three healthy volunteers after provision of informed consent. BM mononuclear cells (MNCs) were separated by density centrifugation over Ficoll-Paque (Pharmacia, Piscataway, NJ), washed twice, and suspended in α-medium (Flow Laboratories Inc, Rockville, MD). One milliliter of cell suspension containing 5 × 106 cells in α-medium with a final volume of 10% dimethyl sulfoxide (Sigma Chemical Co, St Louis, MO) and 10% fetal bovine serum (FBS; Hyclone, Logan, UT) was kept in a freezing tube (Sarstedt, Rommelsdorf, Germany). The samples were frozen with liquid nitrogen until the study. The MNCs were rapidly thawed, and then passed through a 200-μm monofilament nylon filter and suspended in α-medium consisting of Ca2+- and Mg2+-free cold phosphate-buffered saline (PBS), 1 mmol/L EDTA 2 Na, and 2.5% FBS. Cells (2 × 106) were incubated with both 20 μL FITC-conjugated anti-CD34 MoAb and 20 μL PE-conjugated anti-CD38 MoAb for 30 minutes at 4°C. As negative controls, cells were stained with FITC- and PE-conjugated mouse IgG1 (DAKO, Glostrup, Denmark). After two washes, CD34+CD38high cells or CD34+CD38neg/low cells were sorted by a FACStar plus flow cytometer (Becton Dickinson), as described previously.12
Serum-deprived single-cell culture.
Single-cell sorting was performed by two-step sorting. BM CD34+CD38high cells were collected in 5-mL tubes, and were resorted into the individual wells of a 96-well U-bottomed tissue culture plate (#3077; Becton Dickinson) containing 100 μL of α-medium supplemented with 1% crystallized deionized bovine serum albumin (BSA; Sigma), 600 μg/mL of fully iron-saturated human transferrin (approximately 98% pure; Sigma), 16 μg/mL of soybean lecithin (Sigma), 9.6 μg/mL of cholesterol (Nakalai Tesque Inc, Kyoto, Japan), and 10 ng/mL of TPO, 10 ng/mL of SCF, 10 ng/mL of GM-CSF, 100 U/mL of IL-3, 10 ng/mL of G-CSF, alone or in combination, using the FACStar plus flow cytometer equipped with an automatic cell deposition unit (Becton Dickinson), as described previously.14 Ninety-nine percent of the wells contained a single cell on the first day of culture. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. On day 14, GM colonies consisting of more than 50 cells were scored in situ on an inverted microscope according to the criteria described previously.15 16
Serum-deprived suspension culture.
To examine the effects of TPO on JCML primitive hematopoietic progenitors, serum-deprived liquid culture was performed in a 96-well U-bottomed tissue-culture plate according to the procedure described by Kobayashi et al.9 One hundred CD34+CD38neg/low cells or 2,000 CD34+CD38high cells were cultured in a well containing 100 μL of α-medium supplemented with 1% BSA, 600 μg/mL of fully iron-saturated transferrin, 16 μg/mL of soybean lecithin, 9.6 μg/mL of cholesterol, and 10 ng/mL of TPO, 10 ng/mL of SCF, 10 ng/mL of GM-CSF, alone or in combination. After 7 days, the cultured cells were washed twice and incubated in methylcellulose culture supplemented with 30% FBS, 1% BSA, 10 ng/mL of SCF, 10 ng/mL of GM-CSF, 100 U/mL of IL-3, and 10 ng/mL of G-CSF for 14 days. GM colonies consisting of more than 50 cells were scored.
Determination of colony size, cytochemical staining, and immunocytochemical staining.
The size of small GM colonies (<500 cells) was determined by direct cell counting in situ under an inverted microscope at a magnification of 150×. Colonies consisting of more than 500 cells were individually lifted with an Eppendorf micropipet and prepared as single-cell suspensions. The colony size was estimated by using a counting chamber.
After determination of the colony size, 20 GM colonies were obtained from each of the patients or normal controls with an Eppendorf micropipet under an inverted microscope and pooled. After one washing with PBS, the cells were spread on glass slides using a Cytospin II (Shandon Southern, Sewickly, PA), and stained with May-Grünwald-Giemsa, α-naphthyl butylate esterase (ANB), or naphthol AS-D chloroacetate esterase (NASDCA), as described previously.17 Differential counts were done on more than 300 cells in all experiments.
To examine megakaryocyte generation in the cultures of CD34+CD38+ BM cells and SCF + GM-CSF, immunocytochemical staining was performed on the Cytospin preparations using a DAKO LSAB Kit (DAKO) as described previously.18Briefly, the samples were fixed with buffered paraformaldehyde-acetone for 30 seconds. After treatment with blocking reagents, the specimens were incubated with anti-CD41b MoAb for 60 minutes at room temperature. Next, they were incubated with biotinylated goat anti-mouse antibody for 30 minutes, followed by alkaline phosphatase–conjugated streptavidin for 10 minutes and substrate-chromogen solution for 15 minutes. The specimens were counterstained with hematoxylin. More than 300 cells were examined.
Fluorescence-activated cell sorter (FACS) analysis.
To analyze surface markers on the cultured cells, 1 × 105cells were incubated with 20 μL anti–c-mpl MoAb for 30 minutes at 4°C. Isotype MoAb was used as a control. The cells were washed three times and stained with FITC-conjugated goat anti-mouse Ig (GAM; Becton Dickinson) for 15 minutes. After the three washings and treatment with mouse serum for 15 minutes, the cells were stained with PE-conjugated anti-CD13 MoAb and/or PE-Cy 5–conjugated CD34 MoAb for 30 minutes. PE– and PE-Cy 5–labeled isotype control antibodies were used as control. After two washings, cells were analyzed with a FACScan flow cytometer using the Lysis 2 software program.
Statistical analysis.
All experiments were performed at least twice and were shown to by reproducible. Colony numbers are expressed as the mean ± SD of triplicate 96-well culture plates containing a single cell per well. The probability of significant differences was determined according to Student's t-test. The statistical analysis of the GM colony size was performed on logarithms of cell numbers of individual colonies.
RESULTS
Effects of TPO on the growth of GM progenitors from JCML CD34+CD38high cells in serum-deprived single-cell culture.
To examine the effects of TPO on the growth of hematopoietic progenitors from the patients with JCML, we used BM CD34+CD38high and CD34+CD38neg/low cells as the target cells. As shown in Fig 1, the percentage of CD34+CD38high cells among the total BM MNCs was 1.5% ± 0.4% (mean ± SD) in the five JCML patients, being similar to the value of the three normal controls (1.4% ± 0.6%). The percentage of CD34+CD38neg/low cells was 0.037% ± 0.02% in JCML patients and 0.042% ± 0.01% in the normal controls. First, we examined whether TPO enhanced the GM colony growth by stimulation with SCF alone, GM-CSF alone, SCF plus GM-CSF, and a combination of SCF, GM-CSF, IL-3, and G-CSF (growth factors, GFs) using BM CD34+CD38high cells, since our previous study showed that GM progenitors in JCML have an intrinsic abnormality in response to hematopoietic factors, in particular, to SCF plus GM-CSF.12 Serum-deprived single-cell cultures were used to reduce the influence of cytokines secreted by the paracrine mechanism. The results are presented in Table1. In the normal control cultures, the addition of TPO to the culture containing SCF + GM-CSF significantly increased the number of GM colonies. However, TPO failed to augment the number of GM colonies under stimulation with SCF alone, GM-CSF alone, or GFs. In the JCML cultures, in contrast, there was no difference in the number of GM colonies in the presence or absence of TPO, when CD34+CD38high cells were plated in SCF alone, GM-CSF alone, SCF + GM-CSF, or GFs.
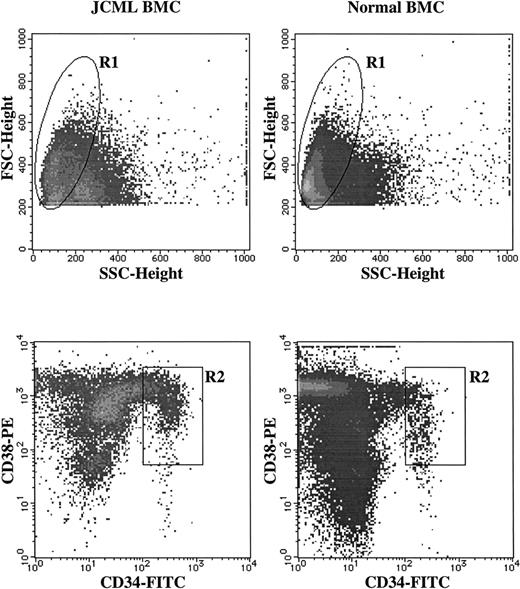
Sorting of CD34+CD38+ cells from JCML and normal BM MNCs. BM MNCs were stained with FITC-conjugated anti-CD34 MoAb and PE-conjugated anti-CD38 MoAb. As negative controls, FITC- and PE-conjugated mouse IgG1 were used. The lymphocyte/blast region (R1) were gated according to their FSC and SSC. The cells in the R2 region were sorted as CD34+CD38highcells.
Sorting of CD34+CD38+ cells from JCML and normal BM MNCs. BM MNCs were stained with FITC-conjugated anti-CD34 MoAb and PE-conjugated anti-CD38 MoAb. As negative controls, FITC- and PE-conjugated mouse IgG1 were used. The lymphocyte/blast region (R1) were gated according to their FSC and SSC. The cells in the R2 region were sorted as CD34+CD38highcells.
Effects of TPO on the Growth of JCML GM Progenitors in Serum-Deprived Single-Cell Cultures
| Cases . | TPO . | None . | SCF . | GM-CSF . | SCF + GM-CSF . | GFs . |
|---|---|---|---|---|---|---|
| JCML | ||||||
| Case 1 | − | 0 | 0 | 0 | 11 ± 2 | 15 ± 2 |
| + | 0 | 1 ± 1 | 0 | 10 ± 1 | 14 ± 1 | |
| Case 2 | − | 0 | 0 | 0 | 12 ± 2 | 11 ± 1 |
| + | 0 | 0 | 0 | 10 ± 2 | 10 ± 1 | |
| Case 3 | − | 0 | 0 | 0 | 10 ± 1 | 10 ± 1 |
| + | 0 | 0 | 0 | 9 ± 1 | 9 ± 1 | |
| Case 4 | − | 0 | 0 | 0 | 11 ± 2 | 14 ± 1 |
| + | 0 | 0 | 0 | 12 ± 1 | 13 ± 1 | |
| Case 5 | − | 0 | 0 | 0 | 12 ± 1 | 11 ± 2 |
| + | 0 | 1 ± 1 | 0 | 10 ± 2 | 12 ± 4 | |
| Normal | ||||||
| Control 1 | − | 0 | 0 | 0 | 1 ± 1 | 11 ± 2 |
| + | 0 | 0 | 0 | 7 ± 2* | 10 ± 0 | |
| Control 2 | − | 0 | 0 | 0 | 2 ± 1 | 11 ± 2 |
| + | 0 | 0 | 0 | 7 ± 2† | 12 ± 2 | |
| Control 3 | − | 0 | 0 | 0 | 2 ± 1 | 13 ± 2 |
| + | 0 | 0 | 0 | 6 ± 1† | 11 ± 2 |
| Cases . | TPO . | None . | SCF . | GM-CSF . | SCF + GM-CSF . | GFs . |
|---|---|---|---|---|---|---|
| JCML | ||||||
| Case 1 | − | 0 | 0 | 0 | 11 ± 2 | 15 ± 2 |
| + | 0 | 1 ± 1 | 0 | 10 ± 1 | 14 ± 1 | |
| Case 2 | − | 0 | 0 | 0 | 12 ± 2 | 11 ± 1 |
| + | 0 | 0 | 0 | 10 ± 2 | 10 ± 1 | |
| Case 3 | − | 0 | 0 | 0 | 10 ± 1 | 10 ± 1 |
| + | 0 | 0 | 0 | 9 ± 1 | 9 ± 1 | |
| Case 4 | − | 0 | 0 | 0 | 11 ± 2 | 14 ± 1 |
| + | 0 | 0 | 0 | 12 ± 1 | 13 ± 1 | |
| Case 5 | − | 0 | 0 | 0 | 12 ± 1 | 11 ± 2 |
| + | 0 | 1 ± 1 | 0 | 10 ± 2 | 12 ± 4 | |
| Normal | ||||||
| Control 1 | − | 0 | 0 | 0 | 1 ± 1 | 11 ± 2 |
| + | 0 | 0 | 0 | 7 ± 2* | 10 ± 0 | |
| Control 2 | − | 0 | 0 | 0 | 2 ± 1 | 11 ± 2 |
| + | 0 | 0 | 0 | 7 ± 2† | 12 ± 2 | |
| Control 3 | − | 0 | 0 | 0 | 2 ± 1 | 13 ± 2 |
| + | 0 | 0 | 0 | 6 ± 1† | 11 ± 2 |
Data are the means ± SD of triplicate 96-well culture plates containing a single cell per well. TPO, 10 ng/mL; SCF, 10 ng/mL; GM-CSF, 10 ng/mL; GFs, combination of SCF (10 ng/mL), GM-CSF (10 ng/mL), IL-3 (100 U/mL), and G-CSF (10 ng/mL).
Significantly different from SCF + GM-CSF (*P < .01, †P < .05).
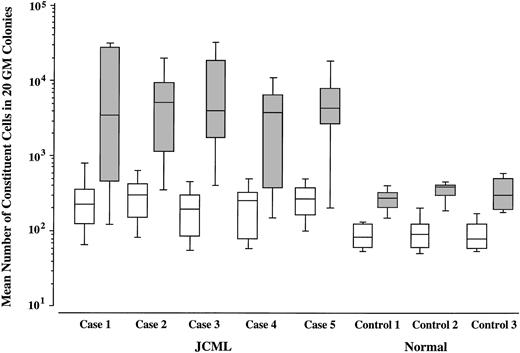
We then compared the size of GM colonies grown by SCF + GM-CSF with that of GM colonies grown by SCF + GM-CSF + TPO in JCML patients and normal controls. As shown in Figs 2 and3, upon stimulation with SCF + GM-CSF, the JCML CD34+CD38high cells generated significantly larger GM colonies compared with the normal CD34+CD38high cells (P < .001), which is consistent with our previous result.12 The addition of TPO resulted in the enlargement of GM colonies by 33.1 ± 13.0 (20.8 to 50.8)-fold in the JCML cultures, and by only 3.5 ± 0.4 (3.1 to 3.8)-fold in the normal control cultures. The difference in the degree of enlargement was significant (P < .001).
In situ appearance and the constituent cells of SCF + GM-CSF–dependent or SCF + GM-CSF + TPO–dependent colonies. (A) and (C) show the colonies with the maximal size grown by stimulation with SCF + GM-CSF or with SCF + GM-CSF + TPO from JCML CD34+CD38high cells, respectively. (B) and (D) show the staining of the constituent cells of 20 pooled colonies grown by SCF + GM-CSF or with SCF + GM-CSF + TPO from JCML CD34+CD38high cells with both ANB (brown) and NASDCA (blue), respectively. (E) and (F) show the staining of the constituent cells of 20 pooled colonies grown by SCF + GM-CSF + TPO from normal or JCML BM CD34+CD38high cells with an MoAb for CD41b using the LSAB Kit, respectively.
In situ appearance and the constituent cells of SCF + GM-CSF–dependent or SCF + GM-CSF + TPO–dependent colonies. (A) and (C) show the colonies with the maximal size grown by stimulation with SCF + GM-CSF or with SCF + GM-CSF + TPO from JCML CD34+CD38high cells, respectively. (B) and (D) show the staining of the constituent cells of 20 pooled colonies grown by SCF + GM-CSF or with SCF + GM-CSF + TPO from JCML CD34+CD38high cells with both ANB (brown) and NASDCA (blue), respectively. (E) and (F) show the staining of the constituent cells of 20 pooled colonies grown by SCF + GM-CSF + TPO from normal or JCML BM CD34+CD38high cells with an MoAb for CD41b using the LSAB Kit, respectively.
Effects of TPO on the size of SCF + GM-CSF–dependent GM colonies. Mean values and SD for 20 GM colonies grown by SCF + GM-CSF (□) or SCF + GM-CSF + TPO (▧) are indicated.
Effects of TPO on the size of SCF + GM-CSF–dependent GM colonies. Mean values and SD for 20 GM colonies grown by SCF + GM-CSF (□) or SCF + GM-CSF + TPO (▧) are indicated.
Twenty GM colonies from each of the patients and controls were obtained and pooled. Cell types were determined by differential counting of the Cytospin preparations stained with May-Grünwald-Giemsa, ANB, and NASDCA. The results are presented in Figs 2 and4. In the normal control group, a large part of the constituent cells of the GM colonies grown by SCF + GM-CSF were positive for NASDCA. The addition of TPO resulted in no change in the percentage of NASDCA+ cells. In the JCML cultures, however, approximately 80% to 90% of the constituent cells of the SCF + GM-CSF–dependent GM colonies were positive for ANB with or without TPO. It is possible that the generation of megakaryocytes contributes to the TPO-mediated increase in the SCF + GM-CSF–dependent GM colony size, based on the presence of undifferentiated cells. As presented in Fig 2E and F, the immunocytochemical studies showed that the mean percentages of CD41b+ cells generated by normal BM CD34+CD38high cells on day 12 were 1.3% in the presence of SCF + GM-CSF and 6.3% in the presence of SCF + GM-CSF + TPO. The addition of TPO to the culture containing SCF + GM-CSF caused an approximately sevenfold increase in the absolute number of CD41b+ cells. In contrast, no CD41b+ cells were observed in the cultures containing JCML BM cells and SCF + GM-CSF with or without TPO.
Differential counts of pooled GM colonies generated by SCF + GM-CSF or SCF + GM-CSF + TPO. Twenty GM colonies from each JCML patient and normal control were obtained and pooled. ANB+ cells (▪), NASDCA+ cells (▨), undifferentiated cells (□).
Differential counts of pooled GM colonies generated by SCF + GM-CSF or SCF + GM-CSF + TPO. Twenty GM colonies from each JCML patient and normal control were obtained and pooled. ANB+ cells (▪), NASDCA+ cells (▨), undifferentiated cells (□).
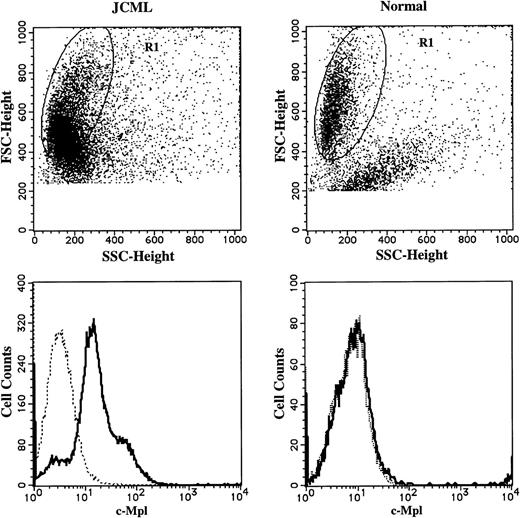
To elucidate the mechanism underlying the TPO-mediated enlargement of JCML GM colonies supported by SCF + GM-CSF, we examined the surface expression of CD34, CD13, and c-Mpl on the cultured cells, using a three-color FACScan analysis. CD13 was used as a marker for the myeloid differentiation. The results are presented in Fig5. At the beginning of the culture, 6.3% ± 3.2% (n = 3) of the JCML BM CD34+CD38high cells expressed c-Mpl, whereas 3.1% ± 2.9% (n = 3) of the normal CD34+CD38high cells reacted with anti–c-Mpl MoAb. In the cultures with SCF + GM-CSF, most of the progenies generated by the JCML BM cells expressed CD13 antigen, and a small percentage of CD13+ cells were positive for CD34 until day 8, as shown in Fig 5. CD34+ cells were not detectable on day 12. Most intriguing was the c-Mpl expression on one third of the JCML CD13+ cells on day 5, and the percentage of c-Mpl+ cells in the CD13+ cells increased with culture (approximately 80% on day 12). In contrast, the c-Mpl expression was not detected on CD13+ cells from normal CD34+CD38high cells on day 12, as shown in Fig6.
Time course of c-Mpl and CD34 expression on CD13+ cells grown by SCF + GM-CSF from JCML CD34+CD38high cells. The cells generated by the JCML CD34+CD38high cells in the presence of SCF + GM-CSF were labeled with anti–c-Mpl MoAb, and then stained with FITC-conjugated GAM. After treatment with mouse serum, the cells were stained with PE-conjugated CD13 MoAb and PE-Cy 5–conjugated CD34 MoAb. Surface marker expression was analyzed on days 5, 8, and 12. The quadrants were determined by the negative controls using isotype-matched Ig.
Time course of c-Mpl and CD34 expression on CD13+ cells grown by SCF + GM-CSF from JCML CD34+CD38high cells. The cells generated by the JCML CD34+CD38high cells in the presence of SCF + GM-CSF were labeled with anti–c-Mpl MoAb, and then stained with FITC-conjugated GAM. After treatment with mouse serum, the cells were stained with PE-conjugated CD13 MoAb and PE-Cy 5–conjugated CD34 MoAb. Surface marker expression was analyzed on days 5, 8, and 12. The quadrants were determined by the negative controls using isotype-matched Ig.
Comparison of c-Mpl expression on CD13+cells generated by JCML or normal CD34+CD38high cells in the presence of SCF + GM-CSF. The cells generated by JCML or normal CD34+CD38high cells on day 12 were stained with anti–c-Mpl MoAb (—) or mouse IgG1, (---), and then stained with FITC-conjugated GAM. After treatment with mouse serum, the cells were stained with PE-conjugated anti-CD13 MoAb. Surface-marker expression on the cells in R1 region was analyzed.
Comparison of c-Mpl expression on CD13+cells generated by JCML or normal CD34+CD38high cells in the presence of SCF + GM-CSF. The cells generated by JCML or normal CD34+CD38high cells on day 12 were stained with anti–c-Mpl MoAb (—) or mouse IgG1, (---), and then stained with FITC-conjugated GAM. After treatment with mouse serum, the cells were stained with PE-conjugated anti-CD13 MoAb. Surface-marker expression on the cells in R1 region was analyzed.
Effects of TPO on the production of GM colony-forming cells by JCML CD34+CD38neg/low cells.
Next we examined the effects of TPO on JCML primitive hematopoietic progenitors using a serum-deprived suspension culture, as described by Kobayashi et al.9 The CD34+CD38neg/low cells sorted by the FACStar plus flow cytometer were incubated with TPO, SCF, GM-CSF, alone or in combination for 7 days. GM colony-forming cells were assayed in clonal cultures containing SCF, GM-CSF, IL-3, and G-CSF. As shown in Table2, production of GM colony-forming cells by CD34+CD38neg/low population was significantly increased by the addition of TPO to the culture containing SCF + GM-CSF in two JCML patients and by the addition of TPO to the culture containing SCF in the remaining patient. The combination of SCF + TPO or SCF + GM-CSF + TPO generated 11 to 39 GM colony-forming cells from 100 JCML CD34+CD38neg/low cells. On the other hand, only 2 to 15 GM colony-forming cells were produced by 2,000 JCML CD34+CD38high cells under stimulation with the two-factor or three-factor combination. The stimulatory effects of TPO on the SCF-dependent or SCF + GM-CSF–dependent production of GM colony-forming cells were either absent or present at variable levels.
Effects of TPO on the Production of GM Colony-Forming Cells by JCML CD34+CD38neg/low Cells and CD34+CD38high Cells in Suspension Culture
| . | No. of Cells per Well . | SCF . | TPO . | GMCSF . | SCF + TPO . | SCF + GM-CSF . | SCF + GM-CSF + TPO . |
|---|---|---|---|---|---|---|---|
| CD34+CD38neg/low | |||||||
| Case 2 | 100 | 0 | 0 | 0 | 2 ± 2 | 0 | 11 ± 2 |
| Case 4 | 100 | 0 | 0 | 0 | 0 | 1 ± 1 | 34 ± 2 |
| Case 5 | 100 | 4 ± 1 | 3 ± 1 | 0 | 39 ± 7 | 9 ± 2 | 39 ± 3 |
| CD34+CD38high | |||||||
| Case 2 | 2,000 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 2 |
| Case 4 | 2,000 | 0 | 1 ± 1 | 2 ± 1 | 5 ± 1 | 4 ± 1 | 7 ± 1 |
| Case 5 | 2,000 | 4 ± 1 | 2 ± 1 | 3 ± 1 | 14 ± 2 | 13 ± 1 | 15 ± 2 |
| . | No. of Cells per Well . | SCF . | TPO . | GMCSF . | SCF + TPO . | SCF + GM-CSF . | SCF + GM-CSF + TPO . |
|---|---|---|---|---|---|---|---|
| CD34+CD38neg/low | |||||||
| Case 2 | 100 | 0 | 0 | 0 | 2 ± 2 | 0 | 11 ± 2 |
| Case 4 | 100 | 0 | 0 | 0 | 0 | 1 ± 1 | 34 ± 2 |
| Case 5 | 100 | 4 ± 1 | 3 ± 1 | 0 | 39 ± 7 | 9 ± 2 | 39 ± 3 |
| CD34+CD38high | |||||||
| Case 2 | 2,000 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 2 |
| Case 4 | 2,000 | 0 | 1 ± 1 | 2 ± 1 | 5 ± 1 | 4 ± 1 | 7 ± 1 |
| Case 5 | 2,000 | 4 ± 1 | 2 ± 1 | 3 ± 1 | 14 ± 2 | 13 ± 1 | 15 ± 2 |
One hundred CD34+/CD38neg/low cells or 2,000 CD34+/CD38high cells in 3 patients with JCML were plated per well in serum-deprived culture media containing designated cytokines and incubated for 7 days. The cultured cells in each well were replated in clonal cultures containing SCF, GM-CSF, IL-3, and G-CSF. After 14 days, GM colonies were scored. Data represent the mean ± SD of GM colonies per plate in triplicate suspension cultures.
DISCUSSION
The apparent GM colony growth in the absence of hematopoietic factors is a hallmark of JCML. The endogenous production of IL-1, GM-CSF, or tumor necrosis factor-α by monocytes has been shown to account for the spontaneous GM colony growth in JCML.19-21In addition, FBS is a potential endogenous source of hematopoietic factors, as described previously.22 Therefore, we used a serum-deprived single-cell culture technique to clarify the direct effects of TPO on the growth of hematopoietic progenitors in JCML CD34+CD38high cells. The present study showed that SCF or GM-CSF alone yielded lower numbers of GM colonies, as compared with our previous results.12 This may be due to a difference in the target cells and/or the culture system.
We have previously reported the favorable response of GM progenitors to SCF + GM-CSF in JCML.12 The present results showed that, in normal BM cells, the addition of TPO to cultures containing SCF + GM-CSF resulted in increases in both the number and size of GM colonies. On the other hand, in the case of JCML, TPO failed to increase the number of GM colonies supported by SCF + GM-CSF, but enlarged GM colonies to a significantly greater extent, as compared with the increase in the size of GM colonies grown by normal CD34+CD38high cells. There was no difference in the type of constituent cells of GM colonies with or without TPO grown by JCML BM cells. Although the c-Mpl expression on JCML BM CD34+CD38high cells was at a low level in JCML, as in normal BM CD34+CD38high cells, most of the JCML myeloid progenies in the cultures expressed c-Mpl at a higher level compared with the normal myeloid progenies. Thus, the combined effect of SCF and GM-CSF appears to be optimal for the initial stage of the proliferation of JCML GM progenitors. Alternatively, TPO may not stimulate the entry of SCF + GM-CSF–dependent GM progenitors to the proliferative process, but it can enhance their subsequent growth, with no influence on the differentiation into the myeloid lineage.
In addition to a high level of the c-Mpl expression on the JCML CD13+ cultured cells generated by SCF + GM-CSF, one third of the circulating CD13+ cells of the JCML patients reacted with anti–c-Mpl MoAb. However, c-Mpl expression was not detected on normal circulating CD13+ cells (unpublished data, January 1998). Thus, a higher level of the c-Mpl expression implies an essential abnormality in JCML myeloid cells.
It is generally held that the CD34+CD38−immunophenotype defines a primitive subpopulation of progenitors in fetal liver, fetal BM, cord blood, and adult BM.23-29Lapidot et al30 indicated that JCML stem cells are also present in a CD34+CD38− fraction, based on the results of the transplantation into severe combined immune deficient mice. In the present study, the addition of TPO to the culture containing SCF or SCF + GM-CSF caused a significant increase in the production of GM colony-forming cells by JCML CD34+CD38neg/low population. These results suggest that primitive hematopoietic progenitors in JCML can be responsive to TPO.
In the presence of TPO alone, pure megakaryocyte colony growth was not found in the cultures containing normal or JCML BM CD34+CD38high cells. Our serum-deprived liquid culture allowed the generation of a large number of megakaryocytes from cord blood CD34+ cells.18 However, in the serum-deprived single-cell culture of normal BM CD34+cells, only 4% of the wells contained two to four megakaryocytes by stimulation with TPO alone, whereas half of wells contained various combinations of progenies including megakaryocytes in the presence of SCF, GM-CSF, G-CSF, IL-3, TPO, and erythropoietin (unpublished data, January 1998). Therefore, the present results may be explained by a low frequency of TPO-responsive megakaryocytic progenitors in BM CD34+CD38highcells rather than the inappropriate culture condition. The normal BM cells generated a significant number of megakaryocytes as well as myeloid cells in response to the combination of SCF, GM-CSF, and/or TPO on day 12. In contrast, megakaryocytic cells were barely produced by the JCML progenitors. These findings suggest that the thrombocytopenia in JCML, in part, results from a defective megakaryocyte production.
In conclusion, our results may provide a fundamental insight for the clinical application of TPO in JCML, suggesting that the administration of TPO augments the aberrant growth of GM progenitors rather than the recovery of megakaryocytopoiesis.
ACKNOWLEDGMENT
We are deeply indebted to Prof A. Komiyama (Department of Pediatrics, Shinshu University School of Medicine) for helpful comments. We also thank Dr S. Ito (Blood Transfusion Service, Shinshu University Hospital) for his excellent technical assistance. We are grateful to Drs H. Miyazaki and T. Kato of Kirin Brewery Co, Ltd, for supplying recombinant TPO and other growth factors.
Supported by Grants-in-Aid No. 09670796 and 09770537 from the Ministry of Education of Japan.
Address reprint requests to Kenichi Koike, MD, Department of Pediatrics, Shinshu University School of Medicine, 3-1-1, Asahi, Matsumoto, 390, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal