Abstract
Both tumor necrosis factor α (TNFα) and Fas ligand (FasL) have been implicated in the pathogenesis of graft-versus-host disease (GVHD). In this study, we examined the ameliorating effects of neutralizing anti-FasL and/or anti-TNFα monoclonal antibody (MoAb) in a lethal acute GVHD model in mice. Whereas the treatment with either anti-FasL or anti-TNFα MoAb alone significantly delayed the mortality and improved the body weight, a complete protection was achieved by the administration of both MoAbs. Pathological examination indicated differential effects of anti-FasL or anti-TNFα MoAb on GVHD-associated pathologies. Hepatic lesion was improved by anti-FasL but not anti-TNFα MoAb. In contrast, intestinal lesion was improved by anti-TNFα but not anti-FasL MoAb. Cutaneous and splenic lesions were improved by either MoAb. The combination of both MoAbs improved all these lesions. These results indicate that FasL and TNFα differentially contribute to the GVHD pathologies and a complete protection from mortality can be achieved by neutralization of both FasL and TNFα.
ALLOGENEIC bone marrow transplantation (BMT) has been a clinical treatment modality for hematopoietic disorders and hematologic malignancies.1 The success rate of BMT has steadily increased in recent years, but graft-versus-host disease (GVHD) is still a major cause of posttransplant mortality.2,3 An acute lethal form of GVHD is caused by activation of the host-reactive donor T cells as represented by a murine model that is caused by transfusion of C57BL/6 splenic T cells into (DBA/2 × C57BL/6)F1 or (BALB/c × C57BL/6)F1 mice.4 Acute GVHD affects the skin, liver, gastrointestinal tract, and lymphoid tissues where inflammatory reactions characterized by mononuclear cell infiltration and histopathologic damage take place, which lead to erythroderma, diarrhea, wasting, and finally death. The effector mechanisms leading to the GVHD-associated tissue damage have not been fully clarified.
Tumor necrosis factor α (TNFα) has been implicated in the pathogenesis of GVHD. TNFα has been identified as a principal mediator of cachexia in rodents5 and is a potent mediator of various inflammatory diseases.6 It has been shown that serum levels of TNFα were increased in patients undergoing GVHD after allogeneic BMT7 and that administration of anti-TNFα antibody markedly reduced the weight loss and mortality in a mouse model of acute GVHD.8 Some beneficial effects of an anti-TNFα monoclonal antibody (MoAb) for the treatment of refractory acute GVHD have been obtained in the phase I-II clinical trials.9 These observations substantiate that TNFα is an important target for the clinical treatment of GVHD. Furthermore, a recent study showed that TNF receptor p55 (TNFRp55)-deficient recipients of allogeneic T cells exhibited a reduced mortality as compared with wild-type recipients, indicating a critical contribution of host TNFRp55 to the GVHD mortality.10
Recently, the ligand for Fas (FasL) has been also implicated in the pathogenesis of GVHD. Fas (APO-1, CD95) is a member of the TNF receptor family and transmits an apoptotic cell death signal upon ligation by FasL.11 Fas is expressed in various tissues, including the skin, liver, and intestine, that are target tissues of GVHD.12 A recent study using allogenic T cells from FasL-defective gld (generalized lymphoproliferative disease) mice clearly showed that FasL plays a critical role in the pathogenesis of acute GVHD, especially in the development of hepatic and cutaneous lesions.13 In a different murine model of acute GVHD, others also showed the involvement of FasL in the lymphoid organ damage.14 However, the contribution of FasL to the mortality varied among previous studies.13-15
In the present study, we compared the ameliorating effects of neutralizing anti-FasL and anti-TNFα MoAbs in a lethal acute GVHD model in mice. Whereas the treatment with either MoAb alone was effective in delaying the mortality, a complete protection was achieved by the combination of both MoAbs. Histological examination indicated differential effects of anti-FasL and anti-TNFα MoAbs on GVHD pathologies. Pathogenic and clinical implications are discussed.
MATERIALS AND METHODS
Mice.
Six-week-old female BALB/c (H-2d), C57BL/6 (B6; H-2b), and (BALB/c × C57BL/6)F1 (CBF1; H-2b/d) mice were purchased from SLC (Shizuoka, Japan) and maintained in our animal facilities.
Reagents.
A neutralizing antimouse FasL MoAb, K10 (mouse IgG2b, κ), was prepared as described previously.16 A neutralizing antimouse TNFα MoAb (MP-6 XT22) and control mouse or rat IgG were obtained from PharMingen (San Diego, CA).
Induction of lethal acute GVHD.
CBF1 mice (10 mice in each group) were intravenously (IV) injected with 1 × 108 spleen cells from B6 mice on days 0 and 7. Some mice received intraperitoneally (IP) 2 mg of anti-FasL MoAb and/or 1 mg of anti-TNFα MoAb on days 0, 4, 8, and 12. Some mice received IP 2 mg of control mouse IgG and 1 mg of control rat IgG on the same schedule. Survival was monitored until day 60. The body weight of the survivors was measured weekly until day 60. On day 19 for the GVHD group or day 21 for the other groups, 3 mice in each group were killed and their ear skin, livers, small intestines, spleens, and bone marrow were subjected to histopathological examination.
Histopathology.
Tissues were fixed in 10% buffered formalin and paraffin-embedded. Sections were stained with hematoxylin and eosin and examined under microscopy.
Flow cytometric analysis.
Splenocytes were prepared from normal CBF1, GVHD, or MoAb-treated mice on day 21 and stained with fluorescein isothiocyanate (FITC)-conjugated anti-H-2Kd (SF1-1.1; PharMingen), biotin-conjugated anti-H-2Kb (AF6-88.5; PharMingen), and phycoerythrin (PE)-conjugated anti-CD4 (RM4-5; PharMingen), anti-CD8 (53-6.7; PharMingen), or anti-B220 (RA3-6B2; PharMingen) MoAbs followed by APC-conjugated avidin (PharMingen). Cells (1 × 104) were analyzed on FACS Vantage and analyzed by Cell Quest program (Becton Dickinson, San Jose, CA). Recipient and donor lymphocytes were identified as H-2Kd+Kb+ and H-2Kd−Kb+ cells, respectively. Cell numbers of CD4+ T, CD8+ T, and B220+ B cells of recipient or donor origin were calculated from the total numbers of splenocytes recovered, and the percentages of each subpopulation were determined by the three-color analysis.
Statistical analysis.
Significant differences between experimental groups were determined using the Mann-Whitney U test for the survival rate or using the Student′s t-test for the body weight. Pvalues less than .05 were considered statistically significant.
RESULTS
Effect of anti-FasL and/or anti-TNFα MoAb on GVHD-induced mortality and weight loss.
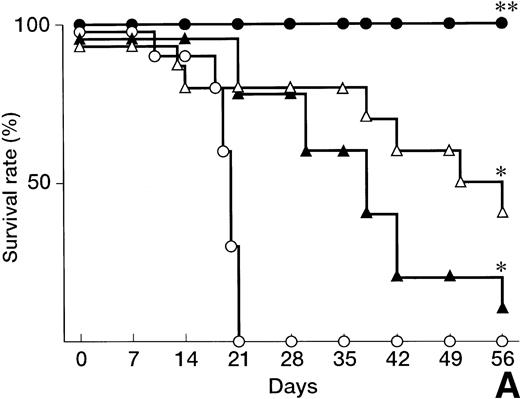
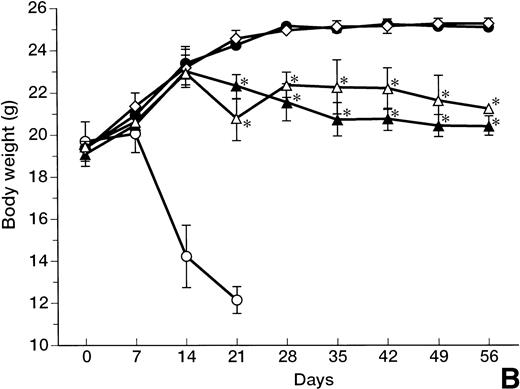
A lethal acute type of GVHD was induced by IV injection of B6 splenocytes into CBF1 mice. As represented in Fig 1A, all the recipients administrated with control IgG died within 21 days. In these mice, clinical symptoms of acute GVHD, such as hair ruffling, lesser mobility, and weight loss (Fig 1B), became apparent within 2 weeks. Administration of either anti-FasL MoAb or anti-TNFα MoAb alone significantly delayed but not completely reduced the mortality, and 1 of 10 or 4 of 10 mice survived at day 60, respectively (Fig 1A). In these surviving mice, no significant weight loss was observed as compared with the age-matched normal mice until day 14, but their growth was retarded after the discontinuation of the treatment at day 14 (Fig 1B) with clinical symptoms of GVHD.
Prevention of lethal acute GVHD by anti-FasL and/or anti-TNFα antibodies. Lethal acute GVHD was induced by IV injection of B6 splenocytes into CBF1 mice on days 0 and 7. Ten mice in each group received IP 2 mg anti-FasL MoAb (▴), 1 mg anti-TNFα MoAb (▵), 2 mg anti-FasL MoAb and 1 mg anti-TNFα MoAbs (•), or control IgG (○) on days 0, 4, 8, and 12. Survival (A) was monitored every day until day 56. Body weight (B) was measured at the indicated days is indicated as the mean ± standard deviation (SD) of 5 to 10 mice. In (B), the body weight of age-matched normal CBF1 (◊) is also plotted. In (A), *P < .05 and **P < .01 compared with the GVHD group. In (B), *P < .05 compared with the normal CBF1 group.
Prevention of lethal acute GVHD by anti-FasL and/or anti-TNFα antibodies. Lethal acute GVHD was induced by IV injection of B6 splenocytes into CBF1 mice on days 0 and 7. Ten mice in each group received IP 2 mg anti-FasL MoAb (▴), 1 mg anti-TNFα MoAb (▵), 2 mg anti-FasL MoAb and 1 mg anti-TNFα MoAbs (•), or control IgG (○) on days 0, 4, 8, and 12. Survival (A) was monitored every day until day 56. Body weight (B) was measured at the indicated days is indicated as the mean ± standard deviation (SD) of 5 to 10 mice. In (B), the body weight of age-matched normal CBF1 (◊) is also plotted. In (A), *P < .05 and **P < .01 compared with the GVHD group. In (B), *P < .05 compared with the normal CBF1 group.
In contrast, all the recipient mice treated with both anti-FasL and anti-TNFα MoAbs survived over 60 days (Fig 1A). Even after the discontinuation of the treatment at day 14, no apparent clinical symptoms of acute GVHD were observed in these mice and their growth was comparable to that of the age-matched normal mice (Fig 1B).
Effect on GVHD-associated histopathologies.
In the liver from the control mice undergoing GVHD, a massive infiltration of mononuclear cells and fibrosis were observed mainly in the periportal areas (Fig 2A). A similar hepatic pathology was observed in the liver from the anti-TNFα–treated mice (Fig 2B). In contrast, such inflammatory changes were minimal in the liver from the anti-FasL–treated mice (Fig2C). The gut from the control mice undergoing GVHD exhibited a dilatation, flattening of the villi, and elevation and atrophy of the crypts, which are characteristics of intestinal GVHD (Fig 2F). Similar changes were observed in the gut of anti-FasL–treated mice, although structural integrity of the villi was partially improved as compared with the GVHD control (Fig 2H). In contrast, all these lesions were almost absent in the anti-TNFα–treated mice (Fig 2G).
Histopathological examination. Induction of lethal acute GVHD and administration of anti-FasL and/or anti-TNFα MoAb were performed as described in Fig 1. On day 19 for the GVHD group or day 21 for the other groups, 3 mice in each group were killed. Paraffin section of the liver (A through E), intestine (F through J), skin (K through O), and spleen (P through T) were stained by hematoxylin and eosin. Sections from age-matched normal CBF1 are also represented. The specimens shown are representatives of 3 mice in each group with similar histology. Original magnification × 100.
Histopathological examination. Induction of lethal acute GVHD and administration of anti-FasL and/or anti-TNFα MoAb were performed as described in Fig 1. On day 19 for the GVHD group or day 21 for the other groups, 3 mice in each group were killed. Paraffin section of the liver (A through E), intestine (F through J), skin (K through O), and spleen (P through T) were stained by hematoxylin and eosin. Sections from age-matched normal CBF1 are also represented. The specimens shown are representatives of 3 mice in each group with similar histology. Original magnification × 100.
The skin from the control mice undergoing GVHD exhibited severe inflammatory infiltrates with intraepidermal lymphocytes and dyskeratotic cells, ulceration, loss of hair follicles, and destruction of rete ridges (Fig 2K). Such changes were not observed in either anti-FasL–treated (Fig 2M) or anti-TNFα–treated (Fig 2L) mice.
The spleen from the control GVHD mice showed a marked lymphoid atrophy, structural disorganization, and focal necrosis (Fig 2P). Such changes were minimal in either anti-FasL–treated (Fig 2R) or anti-TNFα–treated (Fig 2Q) mice. In the recipients treated with both anti-FasL and anti-TNFα MoAbs, no apparent lesion was observed in the liver (Fig 2D), intestine (Fig 2I), skin (Fig 2N), or spleen (Fig 2S) as compared with normal mice (Fig 2E, J, O, and T).
Effect on GVHD-associated lymphoid hypoplasia.
Cell numbers of CD4+ T, CD8+ T, and B220+ B cells of recipient (H-2Kd+Kb+) or donor (H-2Kd−Kb+) origin in the spleen of normal CBF1, GVHD, or MoAb-treated mice on day 21 were calculated from the total numbers of recovered and the percentages of each subpopulation were determined using three-color flow cytometric analysis (Table 1). In the splenocytes from GVHD mice, both CD4+ and CD8+ T cells and B220+ B cells of host origin were severely decreased as compared with normal CBF1 mice. The treatment with either anti-FasL or anti-TNFα MoAb alone partially but substantially prevented the loss of all these lymphocyte subpopulations, and almost complete protection was achieved by the treatment with both MoAbs. It was also noted that donor-derived CD4+ and CD8+ T cells were increased in the anti-FasL– and/or anti-TNFα–treated mice as compared with the GVHD mice, representing a chimeric state of these recipients. As represented in Table 1, 60% to 67% of T cells and 47% to 58% of B cells were donor origin. This chimeric state appeared to be stable, because no further change in the numbers of host and donor lymphocytes was observed on day 60 in the recipients treated with both anti-FasL and anti-TNFα MoAbs (not shown).
Effect of Anti-FasL and/or Anti-TNFα Antibodies on GVHD-Associated Lymphoid Hypoplasia
| Mice . | Cell No. (×107) . | |||||
|---|---|---|---|---|---|---|
| H-2Kd+Kb+ . | H-2Kd−Kb+ . | |||||
| CD4+ . | CD8+ . | B220+ . | CD4+ . | CD8+ . | B220+ . | |
| Normal | 2.1 ± 0.11 | 1.5 ± 0.57 | 2.8 ± 0.80 | — | — | — |
| GVHD | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.7 ± 0.06 | 0.4 ± 0.06 | 0.3 ± 0.07 | 1.3 ± 0.60 |
| Anti-FasL | 1.2 ± 0.05 | 1.7 ± 0.06 | 1.8 ± 0.33 | 2.2 ± 0.70 | 2.5 ± 0.84 | 1.6 ± 0.52 |
| Anti-TNFα | 1.2 ± 0.46 | 1.1 ± 0.29 | 1.4 ± 0.02 | 2.4 ± 0.30 | 2.0 ± 0.30 | 1.9 ± 0.10 |
| Anti-FasL + anti-TNFα | 1.6 ± 0.20 | 1.9 ± 0.45 | 2.0 ± 0.65 | 3.3 ± 0.27 | 3.3 ± 0.39 | 2.8 ± 0.90 |
| Mice . | Cell No. (×107) . | |||||
|---|---|---|---|---|---|---|
| H-2Kd+Kb+ . | H-2Kd−Kb+ . | |||||
| CD4+ . | CD8+ . | B220+ . | CD4+ . | CD8+ . | B220+ . | |
| Normal | 2.1 ± 0.11 | 1.5 ± 0.57 | 2.8 ± 0.80 | — | — | — |
| GVHD | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.7 ± 0.06 | 0.4 ± 0.06 | 0.3 ± 0.07 | 1.3 ± 0.60 |
| Anti-FasL | 1.2 ± 0.05 | 1.7 ± 0.06 | 1.8 ± 0.33 | 2.2 ± 0.70 | 2.5 ± 0.84 | 1.6 ± 0.52 |
| Anti-TNFα | 1.2 ± 0.46 | 1.1 ± 0.29 | 1.4 ± 0.02 | 2.4 ± 0.30 | 2.0 ± 0.30 | 1.9 ± 0.10 |
| Anti-FasL + anti-TNFα | 1.6 ± 0.20 | 1.9 ± 0.45 | 2.0 ± 0.65 | 3.3 ± 0.27 | 3.3 ± 0.39 | 2.8 ± 0.90 |
Cell numbers of CD4+ T, CD8+ T, and B220+ B cells of host (H-2Kd+Kb+) or donor (H-2Kd−Kb+) origin in the splenocytes from normal CBF1, GVHD, or anti-FasL antibody-treated and/or anti-TNFα antibody-treated mice on day 21 were calculated from the total numbers of splenocytes recovered, and the percentages of each subpopulation were determined using three-color flow cytometric analysis. Data represent the mean ± SD of 5 mice in each group.
DISCUSSION
In this study, we explored the ameliorating effects of neutralizing MoAbs against FasL and TNFα, both of which have been implicated in the pathogenesis of GVHD, in a murine model of lethal acute GVHD. Whereas the treatment with either anti-FasL or anti-TNFα MoAb alone significantly delayed the mortality and improved the weight loss, a complete protection was achieved by the combination of both MoAbs. Histological examination indicated differential effects of these MoAbs on GVHD-associated pathologies.
Recent studies have implied that FasL plays a critical role in the development of hepatic and cutaneous lesions and lymphoid atrophy. Baker et al13 showed that, when the FasL-deficientgld mice were used as the T-cell donor in a major histocompatibility complex (MHC)-matched but minor-mismatched allogenic BMT model of acute GVHD, only minimal signs of hepatic and cutaneous GVHD pathology were observed and the lymphoid atrophy in the spleen was improved. However, intestinal GVHD was not abrogated and neither weight loss nor mortality was improved. In contrast, Braun et al15 reported a significantly delayed mortality in the recipients of FasL-defective T cells in a MHC-mismatched spleen cell transfer model. We used the parent (B6) to F1 (CBF1) spleen cell transfer model and found that the treatment with anti-FasL MoAb delayed the mortality and improved the weight loss, consistent with the observation by Braun et al.15 The apparent discrepancy from the observation by Baker et al13in the FasL contribution to mortality remains to be resolved by testing the effect of anti-FasL MoAb in the BMT model. Our histological observations are consistent with those by Baker et al,13indicating a critical contribution of FasL to the development of hepatic and cutaneous, but not intestinal, lesions and splenic atrophy.
The ameliorating effect of anti-TNFα treatment observed in this study was essentially consistent with that reported by Piguet et al.8 They described that the administration of an anti-TNFα polyclonal antibody reduced the mortality at day 40 by 50% and abolished the weight loss on day 18. In our present study, the administration of an anti-TNFα MoAb similarly reduced the mortality and abolished the weight loss. They also showed that the GVHD-associated pathologies in the skin and gut, but not those in the liver, were prevented by the anti-TNFα treatment. These observations are also consistent with ours, indicating a critical contribution of TNFα to the development of intestinal and cutaneous, but not hepatic, lesions.
When both anti-FasL and anti-TNFα MoAbs were administered in combination, all of these histological lesions in the liver, intestine, skin, and spleen were minimal. It was notable that all the recipients survived more than 60 days and grew well as normal mice without growth retardation observed in the recipients treated with either anti-TNFα or anti-FasL MoAb alone, which may result from hepatic or intestinal damage, respectively. These results verified that FasL and TNFα differentially contribute to the GVHD pathologies as follows: (1) hepatic GVHD is predomonantly mediated by FasL; (2) intestinal GVHD is predominantly mediated by TNFα; and (3) cutaneous GVHD, splenic atrophy, weight loss, and mortality are mediated by both FasL and TNFα. Importantly, FasL and TNFα in combination appear to mostly account for all these GVHD-associated pathologies observed in the present study.
In the histological examination, the treatment with either anti-FasL or anti-TNFα MoAb improved the splenic atrophy. Flow cytometric analysis for the lymphocyte subpopulations indicated that the GVHD-associated elimination of host lymphocytes (both T and B cells) was prevented partially by either MoAb alone and almost completely by the combination of both MoAbs. This suggests that both TNFα and FasL contribute to cytotoxic elimination of host lymphocytes by host-reactive donor T cells. Alternatively, this may be also due to blocking of suppressive effects of TNFα and FasL on hematopoiesis17-20 (our unpublished data). It was also noted that donor-derived T cells were increased in the anti-FasL– and/or anti-TNFα–treated mice as compared with the GVHD mice. This may result from inhibition of activation-induced apoptosis, in which both FasL and TNFα have been implicated.21 The preservation of both host and donor lymphocytes represents a chimeric state of the recipients, which appears to be stable over 60 days in the anti-FasL/TNFα-treated mice. It remains to be determined whether a tolerance to the host alloantigen has been established in the donor T cells.
In conclusion, a complete protection was achieved by administration of both anti-FasL and anti-TNFα MoAbs in a murine model of lethal acute GVHD. Although our observations were made in a parent into F1 model in which no cytotoxic conditioning was used before the transplant and thus cannot necessarily be directly extrapolated to allogeneic transplantation as performed clinically today, our present findings may provide insights that would be useful for the treatment of GVHD. The phase I-II clinical trials with a humanized anti-TNFα MoAb for the treatment of refractory acute GVHD have resulted in limited success.9 We recently succeeded to generate a humanized version of antihuman FasL MoAb (manuscript in preparation), which may be useful for the clinical treatment of severe acute GVHD patients in combination with the anti-TNFα MoAb.
ACKNOWLEDGMENT
The authors thank C. Ushiyama for technical assistance and helpful suggestions.
Supported by grants from the Ministry of Education, Science and Culture, and the Ministry of Health, Japan.
Address reprint requests to Ko Okumura, MD, Department of Immunology, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal