Abstract
In allogeneic marrow transplantation, donor T cells that recognize recipient alloantigens prevent rejection but also cause graft-versus-host disease (GVHD). To evaluate whether the ability to prevent marrow graft rejection could be dissociated from the ability to cause GVHD, we generated a panel of four different CD8 cytotoxic T-lymphocyte clones specific for H2d alloantigens. Three of the clones caused no overt toxicity when as many as 20 × 106 cells were infused intravenously into irradiated H2d-positive recipients, and one clone caused acute lethal toxicity within 1 to 3 days after transferring 10 × 106cells into H2d-positive recipients. One clone that did not cause toxicity was able to prevent rejection of (C57BL/6J × C3H/HeJ)F1 marrow in 800 cGy-irradiated (BALB/cJ × C57BL/6J)F1 recipients without causing GVHD. Large numbers of cells and exogenously administered interleukin-2 were required to prevent rejection. These results with different CD8 clones suggest that GVHD and prevention of rejection could be separable effects mediated by distinct populations of donor T cells that recognize recipient alloantigens.
THE RISK OF graft-versus-host disease (GVHD) after allogeneic marrow transplantation can be decreased by removing T cells from the donor marrow, but the use of T-cell depletion has not improved disease-free survival because the benefit of decreased GVHD has been offset by an increased risk of graft failure and other complications.1 GVHD is initiated by the relatively small subset of donor T cells that recognize recipient alloantigens. In previous studies we have shown that donor T cells also prevent marrow graft rejection most effectively when they recognize an alloantigen expressed on recipient immune effectors that survive the pretransplant conditioning regimen.2 3 Whether rejection is prevented primarily by the same cells that cause GVHD is not known.
Recent studies have highlighted the role of proinflammatory cytokines in the pathogenesis of GVHD.4 Donor T cells activated by exposure to recipient alloantigens produce interferon-γ (IFN-γ), which primes macrophages. Stimulation of primed macrophages by lipopolysaccharide translocated from the gut induces release of tumor necrosis factor–α (TNF-α), which in turn causes cachexia and contributes to tissue injury. Cytokine-primed macrophages also produce nitric oxide, which contributes to immunosuppression associated with GVHD.5-7 The role of natural killer and T-cell cytotoxicity in causing GVHD has been controversial. However, experiments with Fas-ligand-defective and perforin-deficient donors have indicated that cytotoxic mechanisms do have some role in the pathogenesis of GVHD.8-10 The extent to which graft rejection is prevented by inflammatory cytokines or cytotoxic mechanisms has not been determined.
In the canine model, infusion of bulk-cultured cytotoxic T lymphocytes (CTL) specific for recipient alloantigens helped to prevent major histocompatibility complex (MHC)-mismatched marrow graft rejection, but all engrafted recipients died with acute GVHD.11 To determine whether prevention of rejection could be separated from GVHD, we generated a panel of donor-derived CD8 CTL clones specific for recipient alloantigens and tested their effects in vivo in murine marrow transplant models. Among these clones, we identified one that prevented allogeneic marrow graft rejection without causing overt toxicity in the recipient. Differences among the clones we tested suggest that the effector mechanisms involved in preventing rejection might be distinct from those that cause GVHD.
MATERIALS AND METHODS
Mice.
(C57BL/6J × SJL/J)F1 (B6SJL; H2b/s, Ly5b/a) males, (C57BL/6J × C3H/HeJ)F1 (B6C3; H2b/k) males, BALB/cJ (H2d, Ly5b) females, B6.C-H2bm1/ByJ (bm1; H2Kbm1, Ly5b) females, and (BALB/cJ × C57BL/6J)F1 (CB6; H2d/b) females were purchased from the Jackson Laboratory (Bar Harbor, ME). B6.Ly5.1;pep3b (B6.Ly5a) males and (C3H/HeJ × B6.Ly5a)F1 [(C3H × B6.Ly5a)F1; H2b/k, Ly5a/b] males were bred at the Fred Hutchinson Cancer Research Center (Seattle, WA). Founder B6.Ly5a males and females were kindly provided by Dr David Myers (Sloan Kettering Institute, New York, NY). Mice were housed in groups of five under microisolated, specific pathogen-free conditions with twice weekly cage changes and were administered sterilized chow and acidified water (pH 3.5) ad libitum. Four weeks after marrow transplantation, mice were transferred to conventional housing conditions. Experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center.
In vitro generation of CTL clones.
Responder B6C3 lymph node (LN) cells or splenocytes (2.0 × 106/mL) were stimulated with irradiated (30 Gy) CB6 or BALB/c spleen cells (1.0 × 106/mL) in culture medium containing a 1:1 mixture of RPMI 1640 medium and enriched Eagle's medium (Biofluids, Inc, Rockville, MD) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 10 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 5 × 10−5 mol/L 2-mercaptoethanol, and 5 mmol/L HEPES. Cultures were fed with fresh medium on the 5th day, and cells (1.0 × 106/well) were restimulated on the 10th day with irradiated (30 Gy) allogeneic splenocytes (5 × 105/well) in the presence of irradiated (30 Gy) syngeneic (responder strain) splenocytes (5 × 106/well) as filler cells in 24-well plates (2.0 mL/well). Three days after each stimulation, cultures were split 1:4 with fresh medium containing 5 U/mL human recombinant interleukin-2 (IL-2; Cetus, Emeryville, CA). After repeated cycles of restimulation at 10 to 21 day intervals, cells were cloned by limiting dilution in medium containing 10 U/mL IL-2 and 10 ng/mL murine recombinant IL-7 (R & D Systems, Minneapolis, MN), with irradiated (30 Gy) syngeneic splenocytes used as filler cells. T-cell clones were propagated by stimulation with irradiated allogeneic splenocytes (5 × 105/well) in the presence of irradiated (30 Gy) syngeneic splenocytes (5 × 106/well), 10 U/mL IL-2, and 1 to 10 ng/mL IL-7. T-cell clones were rested for at least 14 days between cycles of restimulation and for at least 7 days before testing in vitro or in vivo unless otherwise indicated.
In vitro cytotoxicity assays.
Targets were prepared from LN cells activated with 2.0 μg/mL ConA and then labeled with 51Cr. Cloned effectors and targets (10 × 103/well) were incubated in RPMI 1640 medium containing 5 mmol/L HEPES and 10% bovine serum for 4 hours at 37°C. Results represent the means of triplicate determinations in which the percent specific 51Cr release was calculated by standard methods. Spontaneous release values were less than 30%.
Cytokine production.
Cloned T cells (1.0 × 106/well) were stimulated in 2.0-mL culture wells with immobilized CD3-specific antibody 145-2C1112 (hamster IgG; hybridoma kindly provided by Dr Jeffrey Bluestone, University of Chicago, Chicago, IL). Culture supernatants were collected and stored at −20°C until testing. IL-2/IL-4 activity was determined by a biological assay using CTLL-2 cells, and other cytokines were measured with enzyme-linked immunosorbent assay kits and standards from Genzyme (Cambridge, MA) (IFN-γ, TNF-α, IL-4, and IL-1α) or from PerSeptive Diagnostics (League City, TX) (IL-6). Assay detection limits were 10 pg/mL for IL-4, 15 pg/mL for IL-1α, and 10 pg/mL for IL-6.
Immunofluorescent staining.
Cells were stained with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated monoclonal antibodies specific for CD3 (145-2C11, hamster IgG),12 T-cell receptor (TCR)-αβ (H57-597, hamster IgG; Pharmingen, San Diego, CA),13TCR-γδ (GL3, hamster IgG; Pharmingen),14 CD4 (GK1.5, rat IgG2b; hybridoma obtained from American Type Culture Collection [ATCC], Rockville, MD),15 CD8 (2.43, rat IgG2b; hybridoma obtained from ATCC),16 CD44 (IM7, rat IgG2b; Pharmingen),17 or CD62L (MEL 14, rat IgG2a; Pharmingen),18 each at optimal concentration. Stained cells were fixed in 1% paraformaldehyde and analyzed by flow cytometry with the use of a FACScan (Becton Dickinson and Co, Mountain View, CA).
Marrow transplantation.
Recipients 7 to 8 weeks of age were prepared by total body irradiation in a single fraction from dual-opposed 60Co sources at an exposure rate of 20 to 25 cGy/minute on the day before transplantation. Marrow obtained by femur flush was depleted of T lymphocytes by rabbit complement (1:10)-mediated lysis using a mixture of antibodies specific for CD4, CD8, and Thy-1.2 (30-H12, rat IgG2b; hybridoma kindly provided by Dr J.A. Ledbetter, Bristol-Myers-Squibb, Seattle, WA),19each at optimal concentration. Nylon wool-nonadherent T lymphocytes were obtained from pooled mesenteric, axillary, and femoral lymph nodes. CD8 blasts were enriched from mixed lymphocyte cultures (MLC) by complement-mediated lysis using a CD4-specific antibody. Mixtures containing T-cell–depleted donor marrow (5.0 × 106 cells/recipient) and purified T cells, CD8 blasts, or cloned CTL were injected into recipients via the lateral tail vein. In some experiments, recipients were administered recombinant human IL-2 (Cetus Co, Emeryville, CA) intraperitoneally (IP) on the day of transplant and for 6 to 13 consecutive days thereafter. IL-2 doses are expressed as U of activity for supporting the proliferation of murine CTLL-2 cells.
For assessment of chimerism after transplantation, heparinized blood obtained from the orbital venous plexus was lysed with NH4Cl buffer, and leukocytes were stained for two-color analysis with FITC-conjugated CD3-specific antibody 145-2C11 and with biotinylated antibodies specific for H2KkDk(16-1-2N, mouse IgG2a; hybridoma obtained from ATCC)20 or Ly5.1 (A20-1.7, mouse IgG2a; hybridoma kindly provided by Dr Shoji Kimura, Sloan Kettering Institute).21 Binding of the biotinylated antibody was assessed by staining with streptavidin-PE (Becton Dickinson). Stained cells were fixed in 1% paraformaldehyde and analyzed by flow cytometry with the use of a FACScan. Bit maps for lymphoid cells and granulocytes were defined by forward and side scatter characteristics, and the percent of donor cells within each window was enumerated. Results were rounded to the nearest integer and were not corrected for background staining. For each experiment, thresholds for delineating positive and negative cells were determined by staining samples from appropriate positive and negative controls. In the lymphoid and myeloid gates, respectively, negative control samples showed 0% to 2.5% (mean, 0.7%) and 0.1% to 16% (mean, 5.3%) background staining, whereas positive control samples showed 87.1% to 100% (mean, 99.2%) and 82.4% to 100% (mean, 99.0%) stained cells.
Recipients with less than or equal to 20% donor granulocytes in the blood at one month after transplantation were categorized as having rejected the graft. Except where noted, recipients with greater than 20% donor granulocytes at 1 month after transplantation were retested at 2 months after transplantation to assess the stability of donor myeloid engraftment. Recipients with less than or equal to 20% donor granulocytes in the blood at 2 months after transplantation were categorized as having rejected the graft, whereas those with greater than 20% donor granulocytes were categorized as durably engrafted. By this definition, rejection occurred in approximately 10% of recipients with greater than 20% donor granulocytes at 1 month after transplantation.
For three-color flow cytometry, cells were stained with an FITC-conjugated antibody specific for H2KkDk, PE-conjugated antibody specific for CD3 (145-2C11; Pharmingen), and biotin-conjugated antibody specific for Ly5.1. Binding of the biotin-conjugated antibody was assessed by staining with streptavidin-conjugated Tri-Color (Caltag Laboratories, San Francisco, CA).
RESULTS
Prevention of rejection by CD8 blasts specific for recipient alloantigens.
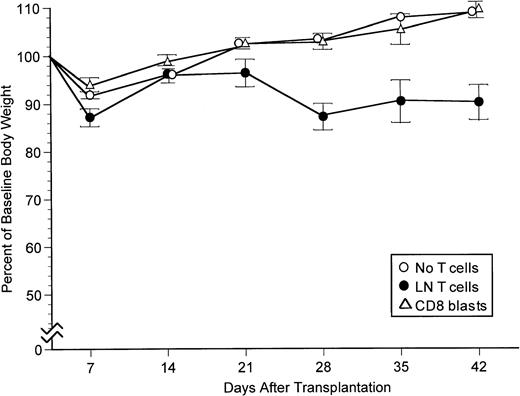
We have shown previously that donor CD8 cells isolated from lymph nodes can prevent rejection of T-cell–depleted B6C3 marrow and cause GVHD in irradiated (800 cGy) CB6 recipients.2 To determine whether cultured T cells can have similar effects in vivo, CD8 cells were recovered from 5-day mixed lymphocyte cultures in which B6C3 LN T cells were stimulated with irradiated host-specific CB6 spleen cells and tested for their ability to prevent allogeneic marrow graft rejection and cause GVHD in CB6 recipients. Rejection of B6C3 marrow cells in CB6 recipients was prevented by CB6-specific CD8 blasts, and these cells did not require exogenous IL-2 for optimal activity (Table1). At least 2.5 × 105CB6-specific CD8 blasts were needed to prevent rejection, suggesting that the cultured cells were substantially less effective than freshly isolated LN cells which could prevent rejection with as few as 5.0 × 104 CD8 cells.2 Recipients transplanted with grafts containing 1.0 to 1.25 × 106 freshly isolated LN CD8 cells developed readily apparent GVHD manifested by weight loss that began during the fourth week after transplantation as compared with recipients transplanted with T-cell–depleted marrow alone (Fig1). Recipients transplanted with grafts containing 1.0 to 1.25 × 106 cultured CD8 blasts did not develop overt GVHD manifested by weight loss when IL-2 was not administered after the transplant (Fig 1). Cultured CD8 blasts did cause weight loss in some experiments but not in others when IL-2 was administered after the transplant (data not shown).
Prevention of Marrow Graft Rejection by Cultured CD8 Blasts
| T Cells Added . | Number of T Cells . | IL-2 . | Recipients Tested . | Recipients Engrafted . | Percent Engrafted . |
|---|---|---|---|---|---|
| None | — | No | 10 | 0 | 0 |
| None | — | Yes | 10 | 0 | 0 |
| CD8 blasts | 5.0 × 104 | No | 5 | 0 | 0 |
| CD8 blasts | 5.0 × 104 | Yes | 10 | 0 | 0 |
| CD8 blasts | 2.5 × 105 | No | 9 | 3 | 33 |
| CD8 blasts | 2.5 × 105 | Yes | 15 | 6 | 40 |
| CD8 blasts | 1.0 to 1.2 × 106 | No | 9 | 9 | 100 |
| CD8 blasts | 1.0 to 1.2 × 106 | Yes | 10 | 10 | 100 |
| LN T | 1.0 to 1.2 × 106 | No | 9 | 9 | 100 |
| T Cells Added . | Number of T Cells . | IL-2 . | Recipients Tested . | Recipients Engrafted . | Percent Engrafted . |
|---|---|---|---|---|---|
| None | — | No | 10 | 0 | 0 |
| None | — | Yes | 10 | 0 | 0 |
| CD8 blasts | 5.0 × 104 | No | 5 | 0 | 0 |
| CD8 blasts | 5.0 × 104 | Yes | 10 | 0 | 0 |
| CD8 blasts | 2.5 × 105 | No | 9 | 3 | 33 |
| CD8 blasts | 2.5 × 105 | Yes | 15 | 6 | 40 |
| CD8 blasts | 1.0 to 1.2 × 106 | No | 9 | 9 | 100 |
| CD8 blasts | 1.0 to 1.2 × 106 | Yes | 10 | 10 | 100 |
| LN T | 1.0 to 1.2 × 106 | No | 9 | 9 | 100 |
Groups of five irradiated (800 cGy) CB6 recipients were transplanted with T-cell–depleted marrow (5.0 × 106cells) from B6C3 donors. Grafts contained no added T cells (negative control), 1.0 to 1.25 × 106 freshly isolated B6C3 lymph node T cells (LN T) (positive control), or the indicated numbers of CD8 blasts recovered on day 5 from MLC in which B6C3 LN T cells were stimulated with irradiated CB6 spleen cells. IL-2 (2,000 U IP) was administered daily for 6 days starting on the day of the transplant. Engrafted recipients had 86% to 99% (mean, 95.4%) H2k-positive donor-derived granulocytes in the blood at 2 months after transplantation, whereas recipients with rejection had 0% to 9% (mean, 1.9%) H2k-positive granulocytes when last tested at 1 to 2 months after transplantation. The table shows results pooled from three experiments.
Body weight profiles for CB6 recipients transplanted with B6C3 marrow cells alone, with 1.0 to 1.25 × 106 LN T cells added, or with 1.0 to 1.25 × 106 cultured CD8 blasts added. The figure summarizes results for recipients not treated with IL-2 in the experiments from Table 1. Bars indicated 1 SEM. At 28, 35, and 42 days after transplantation, body weight was significantly lower in the group transplanted with grafts containing LN T cells than in the other two groups (P < .02 for all comparisons).
Body weight profiles for CB6 recipients transplanted with B6C3 marrow cells alone, with 1.0 to 1.25 × 106 LN T cells added, or with 1.0 to 1.25 × 106 cultured CD8 blasts added. The figure summarizes results for recipients not treated with IL-2 in the experiments from Table 1. Bars indicated 1 SEM. At 28, 35, and 42 days after transplantation, body weight was significantly lower in the group transplanted with grafts containing LN T cells than in the other two groups (P < .02 for all comparisons).
Testing of CD8 CTL clones.
To evaluate whether the ability to prevent marrow graft rejection could be dissociated from the ability to cause GVHD, we generated a panel of four different B6C3 CD8 CTL clones specific for H2dalloantigens. Three of these clones caused no overt acute toxicity when as many as 20 × 106 cells were infused intravenously into irradiated CB6 recipients, even when exogenous IL-2 was administered to sustain viability and in vivo survival of the cells (data not shown). One clone caused acute lethal toxicity within 1 to 3 days after transferring 10 × 106 cells into recipients that expressed the alloantigen recognized by the clone. One clone (designated 14C3) that did not cause acute toxicity was able to prevent rejection of B6C3 marrow in CB6 recipients (Table2). The 14C3 cells prevented rejection only when IL-2 was administered after transplantation (Table 3, Exp 1), although there was some variation in the minimum amount of IL-2 needed for this effect (Table 3, Exp 1 and 2). At least 20 × 106 14C3 cells were needed to prevent rejection in this model (Table 3, Exp 3). Smaller numbers of 14C3 cells could not prevent rejection even when large doses of IL-2 were administered over an extended period of 13 days after transplantation in an attempt to sustain survival of the clone in vivo (Table 3, Exp 3).
Prevention of Rejection by a CTL Clone
| Donor Cells Added . | Percent Donor Cells (day 29) . | |
|---|---|---|
| T Cells . | Granulocytes . | |
| None | 1, 1, 1, 1 | 2, 2, 2, 3 |
| 20 × 106 14C3 CTL | 100, 100, 100, 0 | 99, 97, 98, 2 |
| 20 × 106 3F3 CTL | 1, 1, 1, 1 | 4, 3, 4, 4 |
| 20 × 106 7C11 CTL | 1, 1, 0, 2 | 75, 11, 4, 8 |
| 1.25 × 106 LN T | 100, 100, 100, 100 | 100, 100, 100, 100 |
| Donor Cells Added . | Percent Donor Cells (day 29) . | |
|---|---|---|
| T Cells . | Granulocytes . | |
| None | 1, 1, 1, 1 | 2, 2, 2, 3 |
| 20 × 106 14C3 CTL | 100, 100, 100, 0 | 99, 97, 98, 2 |
| 20 × 106 3F3 CTL | 1, 1, 1, 1 | 4, 3, 4, 4 |
| 20 × 106 7C11 CTL | 1, 1, 0, 2 | 75, 11, 4, 8 |
| 1.25 × 106 LN T | 100, 100, 100, 100 | 100, 100, 100, 100 |
Groups of four irradiated (800 cGy) CB6 recipients were transplanted with 5.0 × 106 T-cell–depleted B6C3 marrow cells with or without freshly isolated lymph node T cells (LN T) or cloned CTL added to the graft. All recipients were treated with IL-2 (30,000 U IP) on the day of transplant and for 6 consecutive days thereafter. On days 29 and 63 after transplantation, the percent of donor T cells and granulocytes in the blood was determined by two-color staining with CD3 and H2KkDk-specific antibodies. Data indicate results for individual recipients tested on day 29. Results of testing on day 63 were similar except that the recipient with 75% donor granulocytes on day 29 after administration of 7C11 cells had only 8% donor granulocytes on day 63 (not shown).
Requirement for IL-2 and Large Numbers of 14C3 Cells to Prevent Rejection
| Exp . | 14C3 Cells . | IL-2 Dose (U/d) . | IL-2 Days . | Percent Donor Cells . | |
|---|---|---|---|---|---|
| T Cells . | Granulocytes . | ||||
| 1 | 20 × 106 | None | 0, 1, 0, 1, 0 | 3, 9, 3, 5, 10 | |
| 80 | 0-6 | 1, 48, 64, 1, 1 | 9, 86, 100, 8, 7 | ||
| 400 | 0-6 | 4, 1, 0, 1, 1 | 24, 9, 8, 11, 8 | ||
| 2,000 | 0-6 | 5, 15, 92, 58, 96 | 14, 62, 99, 92, 97 | ||
| 2 | 20 × 106 | 240 | 0-6 | 100, 1, 100, 100 | 98, 7, 92, 88 |
| 1,200 | 0-6 | 100, 99, 99, 100, 100 | 93, 64, 86, 93, 97 | ||
| 6,000 | 0-6 | 100, 99, 99, 100, 100 | 97, 59, 53, 93, 90 | ||
| 3 | None | 30,000 | 0-13 | 0, 0, 0, 0, 0 | 2, 4, 5, 3, 3 |
| 5 × 106 | 30,000 | 0-13 | 0, 0, 0, 0, 0 | 6, 6, 4, 3, 4 | |
| 10 × 106 | 30,000 | 0-13 | 0, 0, 0, 0, 47 | 1, 4, 5, 5, 95 | |
| 20 × 106 | 30,000 | 0-13 | 31, 11, 97, 0, 100 | 77, 87, 95, 8, 98 | |
| Exp . | 14C3 Cells . | IL-2 Dose (U/d) . | IL-2 Days . | Percent Donor Cells . | |
|---|---|---|---|---|---|
| T Cells . | Granulocytes . | ||||
| 1 | 20 × 106 | None | 0, 1, 0, 1, 0 | 3, 9, 3, 5, 10 | |
| 80 | 0-6 | 1, 48, 64, 1, 1 | 9, 86, 100, 8, 7 | ||
| 400 | 0-6 | 4, 1, 0, 1, 1 | 24, 9, 8, 11, 8 | ||
| 2,000 | 0-6 | 5, 15, 92, 58, 96 | 14, 62, 99, 92, 97 | ||
| 2 | 20 × 106 | 240 | 0-6 | 100, 1, 100, 100 | 98, 7, 92, 88 |
| 1,200 | 0-6 | 100, 99, 99, 100, 100 | 93, 64, 86, 93, 97 | ||
| 6,000 | 0-6 | 100, 99, 99, 100, 100 | 97, 59, 53, 93, 90 | ||
| 3 | None | 30,000 | 0-13 | 0, 0, 0, 0, 0 | 2, 4, 5, 3, 3 |
| 5 × 106 | 30,000 | 0-13 | 0, 0, 0, 0, 0 | 6, 6, 4, 3, 4 | |
| 10 × 106 | 30,000 | 0-13 | 0, 0, 0, 0, 47 | 1, 4, 5, 5, 95 | |
| 20 × 106 | 30,000 | 0-13 | 31, 11, 97, 0, 100 | 77, 87, 95, 8, 98 | |
Groups of five irradiated (800 cGy) CB6 recipients were transplanted with 5.0 × 106 T-cell–depleted B6C3 marrow cells with or without the indicated numbers of 14C3 cells added to the graft. Recipients were treated with IL-2 administered IP daily according to the indicated doses and schedules. The percent of donor T cells and granulocytes in the blood of survivors at 2 months after transplantation was determined by two-color staining with CD3 and H2KkDk-specific antibodies. Data indicate results for individual recipients. Three recipients in Experiment 1 (Exp 1) remained stably engrafted with donor-derived T cells and granulocytes when last tested on day 117 after transplantation. Thirteen of the 14 recipients in Exp 2 remained stably engrafted when last tested on day 92 after transplantation. Five of the 20 recipients in Exp 3 remained stably engrafted when last tested on day 102 after transplantation.
Expansion and decline of 14C3 cell numbers after transplantation.
To evaluate the persistence of 14C3 cells after transplantation, irradiated CB6 recipients were transplanted with grafts containing 5.0 × 106 T-cell–depleted (B6.Ly5a × C3H)F1 marrow cells and 20 × 106 14C3 cells. Recipients were treated with IL-2 (30,000 U IP) on the day of transplant and for 6 consecutive days thereafter. In two recipients tested on day 7 after transplantation, 62% and 51% of LN cells were CD3-positive, H2k-positive, and Ly5.1-negative, indicating that they originated from the 14C3 clone and not from the Ly5.1-positive marrow graft or from the H2k-negative recipient. On day 7, the spleens in these two recipients respectively contained 3.4 × 106 and 2.6 × 106 14C3 cells. In two recipients tested on day 14 after transplantation, LN suspensions contained 10% and 4% 14C3 cells, and the spleens respectively contained 0.3 × 106 and 0.2 × 106 14C3 cells. Thus, 14C3 cells were able to traffic through lymphoid organs and persist for at least 2 weeks after transplantation, but the number of 14C3 cells decreased after treatment with IL-2 was discontinued on day 7 after transplantation. Longer periods of IL-2 administration did not prevent the decline in numbers of 14C3 cells in lymph nodes and the spleen after day 7 (data not shown), suggesting that in vivo survival of 14C3 cells was limited by factors other than the availability of IL-2.
Functional characteristics of CTL clones.
The 14C3 clone and the other two clones that did not cause acute lethal toxicity had similar characteristics, despite the differences in ability to prevent marrow graft rejection. All three clones expressed CD3 and TCR-αβ but not TCR-γδ, and all expressed CD8 but not CD4 (data not shown). None of these three CTL clones showed detectable CD62L expression at any time during the culture cycle. Clones 3F3 and 7C11 expressed CD44, but clone 14C3 showed little if any CD44 expression (data not shown). All three clones showed specific cytotoxic activity against H2d-positive BALB/c targets, and all three produced substantial amounts of IFN-γ and TNF-α after stimulation with immobilized CD3-specific antibody (Table4), but no IL-2, IL-4, IL-1α, or IL-6 could be detected (data not shown).
Cytotoxic Activity and Cytokine Production by CTL Clones
| Clone . | Percent Lysis* . | Cytokine Production3-151 . | ||
|---|---|---|---|---|
| BALB/c . | bm1 . | IFN-γ (ng/mL) . | TNF-α (ng/mL) . | |
| 14C3 | 623-152 | 1 | 2,500 | 4.8 |
| 3F3 | 28 | 1 | 18,750 | 45.0 |
| 7C11 | 56 | −1 | 6,500 | 10.6 |
| Clone . | Percent Lysis* . | Cytokine Production3-151 . | ||
|---|---|---|---|---|
| BALB/c . | bm1 . | IFN-γ (ng/mL) . | TNF-α (ng/mL) . | |
| 14C3 | 623-152 | 1 | 2,500 | 4.8 |
| 3F3 | 28 | 1 | 18,750 | 45.0 |
| 7C11 | 56 | −1 | 6,500 | 10.6 |
*Percent lysis was tested in a 4-hour 51Cr release assay with ConA blasts of the indicated strains. Results are shown at a 25:1 effector to target ratio.
Cytokine production was determined with supernatants from 2.0-mL cultures containing 1.0 × 106 cells in wells coated with CD3-specific antibody 145-2C11 (10 μg/mL in pH 9.4 buffer for 24 hours). Samples collected after 24 hours were stored at −20°C until testing.
The cytotoxic specificity of clone 14C3 was mapped to H2Kd by testing B10.D2 (R107) and B10.D2 (R103) targets.
Prevention of rejection by 14C3 cells requires recognition of a recipient alloantigen.
Three donor/recipient strain combinations were tested to determine the recognition requirements for prevention of marrow graft rejection by 14C3 cells. With B6C3 donors and CB6 recipients, 14C3 cells recognize an alloantigen expressed by recipient T cells (H2Kd). Rejection of B6C3 marrow in 800 cGy irradiated CB6 recipients was prevented by adding 2.5 × 105 B6C3 LN T cells to the graft and also by adding 20 × 106 B6C3-derived 14C3 cells to the graft (Table 5), confirming results shown in Tables 2 and 3. With B6SJL donors and CB6 recipients, 14C3 cells recognize an alloantigen expressed by recipient T cells (H2Kd), but they do not express H2salloantigens that provoke rejection of the B6SJL marrow graft and therefore cannot prevent rejection by interfering with the generation of cytotoxic responses against H2s alloantigens or by other passive mechanisms. Rejection of B6SJL marrow in 800 cGy irradiated CB6 recipients was prevented by adding 1.0 × 106B6SJL LN T cells to the graft and also by adding 20 × 106B6C3-derived 14C3 cells to the graft (Table 5). With B6.Ly5a donors and bm1 recipients, 14C3 cells do not recognize any alloantigens expressed by recipient T cells, but they do express the H2Kb alloantigens that provoke T-cell–mediated22 rejection of the B6.Ly5amarrow graft. Rejection of B6.Ly5a marrow in 550 cGy irradiated bm1 recipients was prevented by adding 2.5 × 105 B6.Ly5a LN T cells to the graft but not by adding 20 × 106 14C3 cells to the graft (Table 5). Results with B6.Ly5a donors and bm1 recipients were similar when 14C3 cells were activated either by stimulation with immobilized CD3-specific antibody for 1 day before transplantation or by adding 5.0 × 106 unirradiated T-cell–depleted CB6 marrow cells to the graft. Taken together, these results show that 14C3 must recognize an alloantigen on recipient cells to prevent marrow graft rejection.
Prevention of Rejection When 14C3 Cells Recognize Recipient Alloantigens
| Marrow Donor . | Recipient . | T Cells Added . | Recipients Tested . | Recipients Engrafted . | Percent Engrafted . |
|---|---|---|---|---|---|
| B6C3 | CB6 | None | 14 | 0 | 0 |
| LN T | 15 | 15 | 100 | ||
| 14C3 | 15 | 13 | 87 | ||
| B6SJL | CB6 | None | 10 | 1 | 10 |
| LN T | 8 | 8 | 100 | ||
| 14C3 | 10 | 9 | 90 | ||
| B6.Ly5a | bm1 | None | 14 | 2 | 14 |
| LN T | 11 | 9 | 82 | ||
| 14C3 | 15 | 3 | 20 |
| Marrow Donor . | Recipient . | T Cells Added . | Recipients Tested . | Recipients Engrafted . | Percent Engrafted . |
|---|---|---|---|---|---|
| B6C3 | CB6 | None | 14 | 0 | 0 |
| LN T | 15 | 15 | 100 | ||
| 14C3 | 15 | 13 | 87 | ||
| B6SJL | CB6 | None | 10 | 1 | 10 |
| LN T | 8 | 8 | 100 | ||
| 14C3 | 10 | 9 | 90 | ||
| B6.Ly5a | bm1 | None | 14 | 2 | 14 |
| LN T | 11 | 9 | 82 | ||
| 14C3 | 15 | 3 | 20 |
Groups of the five irradiated CB6 (800 cGy) or bm1 (550 cGy) recipients were transplanted with 5.0 × 106 T cell-depleted B6C3, B6SJL, or B6.Ly5a marrow cells alone (negative controls), with LN T cells (2.5 × 105 from B6C3 donors, 1.0 × 106 from B6SJL donors, and 2.5 × 105 from B6.Ly5a donors) added (positive controls), or with 20 × 106 14C3 cells added. All recipients were treated with IL-2 (2,000 U intraperitoneally) on the day of transplant and for 6 consecutive days thereafter. Engrafted recipients tested at 2 months after transplantation (n = 42) had 24% to 100% (mean, 91.5%) donor-derived granulocytes in the blood. Engrafted recipients last tested at 1 month after transplantation (n = 18) had 64% to 100% (mean, 96.3%) donor-derived granulocytes in the blood. Recipients with rejection had 1% to 13% (mean, 4.3%) donor-derived granulocytes when last tested at 1 to 2 months after transplantation. The table shows results pooled from three separate experiments.
Evaluation of toxicity caused by infusion of 14C3 cells in allogeneic recipients.
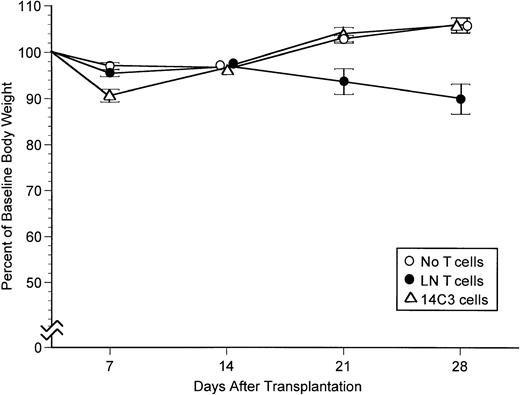
The ability of 14C3 cells to cause GVHD through recognition of H2Kd in irradiated CB6 recipients was evaluated by monitoring weight and by histological examination after transplantation of T-cell–depleted B6C3 marrow. During the first week after transplantation, recipients treated with 14C3 cells had slightly more weight loss than negative controls transplanted with grafts containing no added T cells (P < .001) (Fig2). After the first week, 14C3 recipients recovered completely and showed a weight gain profile identical to that of negative controls, whereas positive controls transplanted with grafts containing 2.5 × 105 LN T cells developed GVHD manifested by weight loss that began during the third week after transplantation as compared with recipients transplanted with T-cell–depleted marrow alone (P < .005 at day 21, andP < .001 at day 28). The transient weight loss caused by 14C3 cells was not accompanied by histological changes diagnostic of GVHD in the skin, liver, gut, or lung of recipients evaluated on day 7 after transplantation (data not shown). Increased weight loss during the first week after transplantation also occurred when 14C3 cells were administered with T-cell–depleted B6SJL marrow in CB6 recipients but not when 14C3 cells were administered with B6.Ly5a marrow in bm1 recipients (data not shown). Thus, weight loss did not occur in recipients lacking the H2Kd alloantigen recognized by 14C3 cells.
Body weight profiles for CB6 recipients transplanted with B6C3 marrow cells alone, with 2.5 × 105 LN T cells added, or with 20 × 106 14C3 cells added. The figure summarizes results in experiments from Table 5. All recipients were treated with IL-2 (2,000 U IP) on the day of transplant and for 6 consecutive days thereafter. Bars indicate 1 SEM.
Body weight profiles for CB6 recipients transplanted with B6C3 marrow cells alone, with 2.5 × 105 LN T cells added, or with 20 × 106 14C3 cells added. The figure summarizes results in experiments from Table 5. All recipients were treated with IL-2 (2,000 U IP) on the day of transplant and for 6 consecutive days thereafter. Bars indicate 1 SEM.
DISCUSSION
Results in the present study have shown two notable findings. First, bulk-cultured, alloantigen-stimulated CD8 cells and certain cloned CD8 cytotoxic effector cells caused little or no overt acute toxicity after adoptive transfer into irradiated recipients expressing antigen(s) recognized by the CTL. Second, one of the clones we generated was able to prevent allogeneic marrow graft rejection without causing GVHD. These results suggest that GVHD and prevention of rejection could be separable functional effects mediated by distinct populations of donor T cells that recognize recipient alloantigens.
The ability of 14C3 cells to prevent marrow graft rejection was exceptional among the clones we tested. We have not identified characteristics of 14C3 cells that distinguish this clone from others that did not prevent rejection. It is possible that rejection would have been prevented if we had given larger numbers of cells from clones that did not cause toxicity. All of the CD8 clones we tested recognized an alloantigen in the recipient, had cytotoxic function, and produced type 1 cytokines. The clones that did not cause toxicity had closely similar cell surface phenotypes and functional responses as determined by in vitro testing. The functional heterogeneity we observed could reflect differences in TCR avidity.23 However, in preliminary experiments 14C3 cells and 7C11 showed similar susceptibility to inhibition of cytotoxic activity by CD8-specific antibody (unpublished observations, June 1995). By this criterion,24 differences in TCR avidity for these two clones were not apparent. The requirement for exogenous IL-2 administration for prevention of rejection with 14C3 cells but not with alloactivated CD8 blasts is consistent with the inability of 14C3 cells to produce IL-2 after activation.
Although donor T cells can prevent rejection even when they do not recognize an alloantigen expressed by recipient T cells,3,25,26 adoptive transfer of 14C3 cells did not prevent rejection in recipients lacking the H2Kdalloantigen recognized by the clone. Because previous studies have shown that CTL clones inhibit or “veto” the generation of cytotoxic responses against their own antigens,27-29 and because 14C3 cells express H2Kb antigens, we had expected that they would be able to prevent rejection of B6.Ly5amarrow by bm1 recipients. In this model, rejection occurs entirely through recognition of H2Kb antigens, presumably resulting in the generation of cytotoxic effectors that destroy the graft. In further studies we have found that 14C3 cells do not mediate veto effects that inhibit the generation of cytotoxic responses against their own antigens (unpublished observations, January 1997). We have not identified an explanation for the inability of 14C3 cells to mediate veto activity. This unusual characteristic of 14C3 cells made it impossible for us to determine whether a CTL clone with veto activity could prevent marrow graft rejection in recipients not expressing the antigen recognized by the clone.
The cellular receptors involved in preventing marrow graft rejection through recognition of alloantigens expressed by recipient T cells have not been fully defined. Our current results with 14C3 cells strongly implicate TCR-αβ receptors as an allorecognition mechanism by which donor T cells can prevent rejection. The 14C3 CTL clone expresses TCR-αβ but not TCR-γδ or NK1.1 and was able to prevent rejection only in recipients that express the H2Kdalloantigen recognized by the clone. These results are consistent with findings reported by Drobyski and Majewski,30 who found that T cells from TCR-αβ–deficient donors had a greatly reduced ability to prevent marrow graft rejection as compared with T cells from TCR-γδ–deficient donors.
By comparison with freshly isolated T cells, cultured CD8 blasts and cloned CD8 CTL had a greatly reduced ability to prevent marrow graft rejection. If the precursor frequency for MHC class I alloantigens is estimated at 1/100 to 1/1,000,31,32 our previous observation that rejection can be prevented by as few as 5 × 104 lymph node T cells implies that 50 to 500 cells specific for recipient alloantigens could be responsible for this effect.2 In the same model, at least 25 × 104cultured CD8 blasts were necessary to prevent rejection. If the host-specific CTL precursor frequency in this population is estimated at 1/10 to 1/100, then these results imply that freshly isolated LN CD8 cells have 10- to 100-fold more activity for preventing rejection than short-term cultured CD8 blasts. With the 14C3 CTL clone, 20 × 106 cells were needed to prevent rejection, implying that freshly isolated CD8 cells have on the order of 200,000-fold more activity for preventing rejection than 14C3 cells.
Previous studies have shown that murine T-cell clones often have limited survival and abnormal migration patterns, which could explain their inefficient function after adoptive transfer in vivo.33-37 Poor survival could result from insufficient production of critical autocrine cytokines such as IL-2, and limited ability to migrate into lymph nodes could reflect the absence of L-selectin (CD62L) expression by cultured murine T-cell clones.33 The ability of donor T cells to recognize host effectors responsible for causing rejection could be impaired if the alloantigen recognized on host effectors is also expressed by many other tissues. The killing of target cells by CTL can be decreased by competitive inhibition, and high-affinity interactions with alloantigen might downregulate TCR and CD8 expression,38 induce anergy, or cause apoptosis,39 thereby disabling the clone. Activation-induced cell death after unremitting antigen stimulation has been described in other CD8 adoptive transfer systems,39 40and could explain why 14C3 cells could not be sustained beyond 2 weeks in vivo, despite the exogenous administration of IL-2.
Acute lethal toxicity in adoptive transfer experiments has been described for cultured CD4 clones,41,42 but not for CD8 clones. In recipients with acute lethal toxicity caused by our CD8 clones, histopathologic examination showed evidence of pulmonary vascular leak as the predominant abnormality (unpublished observations, October 1994), similar to the results described earlier for CD4 clones. The induction of pulmonary vascular leak by CD4 clones required recognition of an activating alloantigen in the recipient but did not require CTL activity or secondary host-derived inflammatory mechanisms.42 Correlations between acute toxicity after adoptive transfer and the ability to produce large quantities of TNF-α suggested that inflammatory mediators released by activated CD4 cells can directly influence vascular permeability.41 We have not performed extensive experiments to assess potential mechanisms that might explain differences among CD8 clones in their ability to cause acute toxicity after adoptive transfer in recipients that express the alloantigen recognized by the clone.
Several explanations might account for the limited ability of 14C3 cells and other CD8 clones to cause toxicity after adoptive transfer into recipients that express the antigen recognized by the clone. First, the limited survival of 14C3 cells after adoptive transfer might constrain their ability to cause chronic toxicity. Second, although the clones do produce certain inflammatory cytokines such as TNF-α and IFN-γ, they might not produce other cytokines necessary for causing acute lethal toxicity. Further studies to delineate the cytokines uniquely produced by clones that caused toxicity are in progress. The transient weight loss observed during the first week after transfer of 20 × 106 14C3 cells into CB6 recipients suggests that this clone might have caused more apparent toxicity if larger numbers of cells had been administered.
In clinical marrow transplantation, GVHD occurs more frequently than rejection. This observation and the reciprocal decrease in risk of GVHD and increase in risk of rejection associated with T-cell depletion of the donor marrow could suggest a simple quantitative relationship between the two effects. Thus, the prevention of rejection by donor T cells could be interpreted as a clinically limited form of GVHD. However, our previous studies have shown that murine CD4 cells that cause severe GVHD have a limited ability to prevent rejection, presumably because the recipient effectors that cause rejection do not express class II alloantigens recognized by donor CD4 cells.2 Thus, the recognition requirements for preventing rejection are more stringent than those for causing GVHD in that rejection is prevented primarily through recognition of antigens on recipient immune effectors, whereas GVHD is caused through recognition of antigens potentially having more diverse distributions of tissue expression. Our current results showing marked differences among CD8 clones that recognize recipient alloantigens in their ability to prevent rejection or cause toxicity suggest that the primary donor T-cell effector mechanisms responsible for preventing rejection might be distinct from those that cause tissue damage in GVHD. Such qualitative differences in recognition and function suggest the possibility that selected T-cell populations could be used clinically to prevent marrow graft rejection without causing GVHD in humans.
ACKNOWLEDGMENT
The experiments described in this study were performed with assistance from Kelli McIntyre, and the manuscript was prepared with assistance from Alison Sell. The authors thank Dr Michael A. Bean for helpful discussions and Dr Martin A. Cheever and Dr Ilonna Rimm for critical reading of the manuscript.
Supported by US Public Health Service Grants No. AI-27951 and HL-55257. Y.K.'s work was performed during a sabbatical leave supported by the Radiation Effects Research Foundation, Hiroshima, Japan.
Address reprint requests to Paul J. Martin, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-100, PO Box 19024, Seattle, WA 98109-1024.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal