Abstract
Colony-stimulating factor-1 (CSF-1), the primary regulator of mononuclear phagocyte (M▹) production, exists as either a circulating or cell surface, membrane-spanning molecule. To establish transplacental transfer of maternal CSF-1, gestational day-17 mothers were injected intravenously with 125I-mouse CSF-1 or human rCSF-1, and the 125I-cpm or human CSF-1 concentrations were measured in fetal tissue, placenta, and fetal/maternal sera. Biologically active CSF-1 crossed the placenta and peaked in fetal tissue, placenta, and serum 10 minutes after injection. The role of CSF-1 in perinatal M▹ development was examined by studying the CSF-1–deficient osteopetrotic (csfmop/csfmop) mouse. Fetal/neonatal mice, derived from matings of either +/csfmopfemales with csfmop/csfmop males or the reciprocal pairings, were genotyped and tissue M▹ identified and quantified. In the presence of circulating maternal CSF-1 (+/csfmop mother), M▹ development incsfmop/csfmop liver was essentially complete at birth relative to +/csfmoplittermates, but significantly reduced in spleen, kidney, and lung. In the absence of circulating maternal CSF-1 (csfmop/csfmop mother), M▹ numbers at birth were reduced in csfmop/csfmopliver relative to the offspring of +/csfmopmothers, but were similar in spleen, kidney, and lung. We conclude that CSF-1 is required for the perinatal development of most M▹ in these tissues. Compensation for total absence of local CSF-1 production by circulating, maternal CSF-1 is tissue-specific and most prominent in liver, the first fetal organ perfused by placental blood. However, because some M▹ developed in the complete absence of CSF-1, other factors must also be involved in the regulation of macrophage development.
COLONY-STIMULATING factor-1 (CSF-1) is a hematopoietic growth factor that regulates the survival, proliferation, and differentiation of the mononuclear phagocyte (reviewed in Pixley and Stanley1). Whereas endothelial cells lining blood vessels appear to be the primary source of circulating CSF-1 in vivo (P. Roth and E.R. Stanley, unpublished observations), numerous other cell types, including fibroblasts, monocytes, bone marrow stromal cells, osteoblasts, thymic epithelial cells, keratinocytes, astrocytes, myoblasts, mesothelial cells, liver parenchymal cells, and epithelial cells of the pregnant uterus, have been shown to produce this growth factor (reviewed in Stanley2). Isolation and sequencing of both human and murine CSF-1 cDNA clones3-10 coupled with studies of CSF-1 biosynthesis and expression have shown that CSF-1 exists in several biologically active forms, including a long-lived (t½ = 7 hours) membrane-spanning, cell surface glycoprotein, a secreted glycoprotein, and a secreted chondroitin sulfate-containing proteoglycan.11 The proteoglycan form may be localized to specific types of extracellular matrix.11,12 The existence of diverse forms of CSF-1 makes it well suited to exert its effects both locally as well as at distant sites via the circulation. Some of the major biological properties that have been attributed to CSF-1 include the regulation of mononuclear phagocyte production in vivo, fetal osteoclast development, regulation of cells in the female reproductive tract during pregnancy (reviewed in Stanley2and Pollard and Stanley13), and regulation of testicular macrophages involved in male reproductive functions.14,15In addition, CSF-1 seems to preferentially regulate the development of tissue macrophages, which are involved in tissue remodelling and organogenesis during fetal and neonatal life.16 Whereas CSF-1 appears to play an important role in mononuclear phagocyte development, there are no data regarding its ability to cross the placenta during fetal life. While the passage of IgG from mother to fetus is the most striking and has been the most extensively studied, other proteins may be transferred to a much more limited extent.17 In particular, granulocyte colony-stimulating factor (G-CSF), another hematopoietic growth factor, has been shown to cross the placenta after maternal administration and induce myelopoietic activity in the fetal rat.18
A useful model for the study of the biologic role of CSF-1 is the osteopetrotic (csfmop/csfmop) mutant mouse, which is deficient in bone resorption due to a paucity of bone osteoclasts19; has markedly reduced bone marrow macrophages, blood monocytes, and serosal macrophages20-22; displays defects in both male14,15 and female fertility (reviewed in Pollard and Stanley13 and Pollard et al23); and exhibits abnormalities in neural processing.24 These abnormalities are the result of a null mutation in the CSF-1 gene caused by a single thymidine insertion in exon 4 leading to a frame-shift at base pair 262 with translational termination 21 bp downstream.25 The resultant 63 amino acid truncated translation product is totally devoid of biological activity.21,25 Thecsfmop/csfmop mouse has been extremely helpful in defining mononuclear phagocyte populations that are dependent on CSF-1 for their development. Administration of CSF-1 to newborn csfmop/csfmop mice cured their osteopetrosis and substantially corrected the deficiencies in osteoclasts, bone marrow cellularity, and blood monocytes, although having little effect on resident macrophages at serosal surfaces.16,26-28 These observations suggest that dependence on circulating CSF-1 for development is limited to specific macrophage populations. In fact, a detailed analysis of the postnatal development of various tissue macrophage populations incsfmop/csfmop mice and their normal littermates indicates that distinct populations may be subject to complete dependence on, partial dependence on, and complete independence of CSF-1 for their development and maintenance.16 In general, macrophages in tissues, which undergo significant remodelling and morphogenesis during the perinatal period, seemed most dependent on CSF-1 and failed to develop incsfmop/csfmop mice. These cells are thought to act like osteoclasts as scavengers or produce trophic factors in these tissues, such as the dermis and synovial membranes, which are hypoplastic and hypotrophic in mutantcsfmop/csfmop mice,16 and testes, in which the function of closely associated Leydig cells is affected in csfmop/csfmopmice.14,15 In contrast, the appearance of macrophages, which are important in immunologic and inflammatory responses, in the epidermis (Langerhans cells), thymus, and lymph node is independent of CSF-1.16 29-31 Becausecsfmop/csfmop mice are totally devoid of CSF-1, they are well suited to study the role of this growth factor in the appearance of macrophages during perinatal development.
In the present study, we demonstrate that maternal CSF-1 crosses the placenta and that CSF-1 is required for the perinatal development of most macrophages in liver, spleen, kidney, and lung. We also show that circulating maternal CSF-1 can compensate for the absence of embryonic/fetal CSF-1 in csfmop/csfmopfetuses and regulate normal macrophage development in liver but not spleen, kidney, or lung.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were obtained from the National Cancer Institute and housed in the animal care facility of the Albert Einstein College of Medicine according to established guidelines. Osteopetroticcsfmop/csfmop mice on a (C57BL/6 × C3HeB/FeJLe) F1 background (originally obtained from Jackson Laboratories, Bar Harbor, ME) and littermate controls were bred and maintained in isolated units of the Albert Einstein College of Medicine animal care facility as described previously.16 23These latter mice were fed ad libitum with powdered chow and infant milk formula (Enfamil; Mead-Johnson, Evansville, IN). Animals were bred and females were checked daily for the appearance of vaginal copulation plugs. Gestational age was determined by counting days from the appearance of plugs, with the day of appearance of the plug designated as day 1. Mice were studied on gestational days 13 through 18 as well as on postnatal days 1 through 7. Blood obtained at the time of death was collected in capillary tubes and centrifuged, and the serum was further handled as described below. Tissues obtained at the time of death were handled as described below in the different experiments.
Identification of csfmop/csfmopand +/csfmopoffspring.
Matings were performed between +/csfmop females andcsfmop/csfmop males as well as the reciprocal pairing of csfmop/csfmopfemales with +/csfmop males. Distinction ofcsfmop/csfmop and+/csfmop offspring was accomplished by radioimmunoassay (RIA) and/or polymerase chain reaction (PCR). When RIA was used, tails from fetal and neonatal mice were removed before tissue fixation for immunohistochemistry, homogenized, and heated at 56°C for 30 minutes. CSF-1 levels in tail extracts were measured by an RIA that detects biologically active mouse growth factor32 and the presence or absence of CSF-1 was used to distinguish +/csfmop andcsfmop/csfmop mice, respectively. Alternatively, PCR was used to amplify the segment of exon 4 containing the op mutation in DNA purified from the tails of fetal and neonatal mice, as previously described.14 3335S-dATP-labeled PCR products were run on sequencing gels, which were dried and subjected to autoradiography. The wild-type fragment is 59 bp in length, compared with the mutant fragment, which is 60 bp in length due to the single thymidine insertion. Consequently,+/csfmop heterozygotes display both bands.
Transplacental transfer of CSF-1.
Pregnant C57BL/6 female mice on day 17 of gestation were injected intravenously with approximately 1 × 106cpm 125I-labeled CSF-1 in 0.1 mL physiologic saline. Pure mouse L-cell CSF-1 was radioiodinated to high specific activity (∼3 × 105 cpm/ng protein) and confirmed to be greater than 95% immunoprecipitable, as previously described.34Animals were killed 5 and 10 minutes after injection, and125I-cpm in fetal and placental tissues was determined. In addition, immunoprecipitable 125I-CSF-1 cpm were measured in serum using an antibody that detects only biologically active growth factor, correcting for counts precipitated by preimmune serum, as previously described.34 In separate experiments, day-17 pregnant mice were injected intravenously with 12 μg of human recombinant CSF-1, which is active on mouse CSF-1 receptors35 and was a generous gift from Chiron Corp (Emeryville, CA), in 0.1 mL physiologic saline. Animals were killed at 1 minute, 10 minutes, 1 hour, 2 hours, or 24 hours, respectively, after CSF-1 injections and both sera and tissues were stored at −80°C before analysis. CSF-1 was extracted from tissues by homogenization and heating at 56°C for 30 minutes, as previously described.32 Human CSF-1 levels were measured in tissue extracts and sera by an RIA that detects biologically active growth factor at concentrations ≥120 pg/mL, does not cross-react with mouse CSF-1, and has interassay and intra-assay variabilities of approximately 10%.36 CSF-1 concentrations in tissue extracts were converted to picograms per milligram of tissue by multiplying concentrations (in picograms per milliliter) by the volume of RIA extraction buffer added per milligram of wet tissue weight (milliliter per milligram).
Tissue macrophage immunohistochemistry.
Whole fetuses (13 to 18 days of gestation) and tissues excised from neonatal (days 1 through 7)csfmop/csfmop and+/csfmop mice were fixed for 6 hours in periodate-lysine-2% paraformaldehyde-0.05% glutaraldehyde, pH 7.4 (PLPG).37-39 Tissues were then dehydrated and embedded in polyester wax. Sections of 5 μm were cut and air-dried on gelatin-coated slides. Immunostaining was performed with the macrophage-specific rat monoclonal antibody F4/80,37 as previously described,16 using the indirect peroxidase-conjugated streptavidin procedure.22 38 Rat gamma globulin (5 μg/mL) was used as a control.
At each age, at least two csfmop/csfmopand two normal mice were examined. F4/80-positive cells were counted under high power (40×) light microscopy with the aid of a video monitor (Sony Trinitron, Sony Corp, Tokyo, Japan) by scoring at least 5 to 10 fields (0.03 mm2/field) and were expressed in cells per square millimeter. Cell densities were averages derived from at least two animals and standard deviations for multiple counts (n > 5) were less than 10% of the means.
Statistical analysis.
Data are expressed as the means ± standard error of the mean. Comparisons were made using the Student's t-test and nonparametric Tukey analysis for multiple comparisons, where appropriate.40
RESULTS
Transplacental transfer of CSF-1.
To determine whether CSF-1 is transferred across the placenta, trace amounts of 125I-CSF-1 labeled to high specific activity were administered intravenously to pregnant female mice on day 17 of gestation. Results of a representative experiment demonstrating125I-cpm transferred to whole fetuses and placentas are shown in Table 1. At 10 minutes postinjection, approximately 5% of the injected cpm was transferred across the placenta to the 10 fetuses in this particular experiment. Determination of immunoprecipitable counts (due to intact125I-CSF-1 only) showed that approximately 30% of fetus-associated cpm were due to intact growth factor (data not shown). This observation is not surprising in view of the fact that greater than 80% of an intravenously injected dose of 125I-CSF-1 is normally taken up and rapidly degraded by the maternal liver, leaving a relatively smaller amount available for transplacental transfer.34
125I-CSF-1 Transfer to Fetal/Placental Tissues After Maternal Intravenous Administration
| Tissue . | Time Postinjection-150 . | |
|---|---|---|
| 5 min . | 10 min . | |
| Fetal | 0.27 ± 0.03 (n = 10) | 0.48 ± 0.07 (n = 10) |
| Placental | 0.45 ± 0.07 (n = 10) | 0.38 ± 0.06 (n = 10) |
| Tissue . | Time Postinjection-150 . | |
|---|---|---|
| 5 min . | 10 min . | |
| Fetal | 0.27 ± 0.03 (n = 10) | 0.48 ± 0.07 (n = 10) |
| Placental | 0.45 ± 0.07 (n = 10) | 0.38 ± 0.06 (n = 10) |
Results are expressed as the percentage of injected cpm ± SEM.
Day-17 gestation pregnant female mice received 1 × 106cpm 125I-CSF-1 (∼300,000 cpm/ng) intravenously and were killed at the indicated times after the injection. 125I-cpm in whole fetuses and placentas was determined.
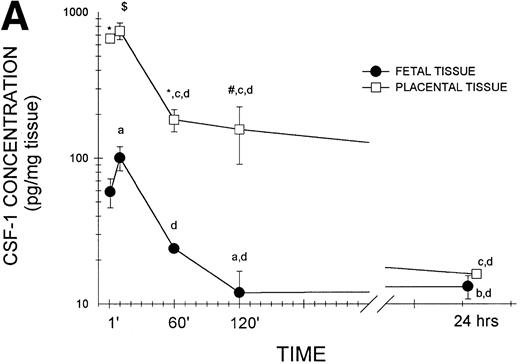
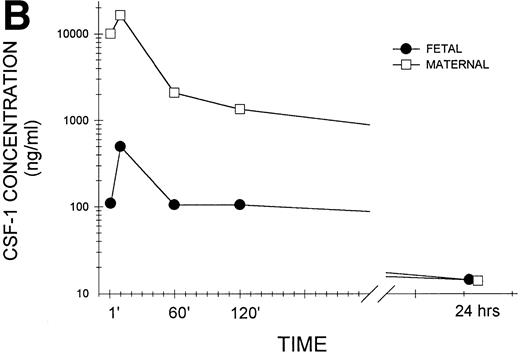
To confirm that biologically active CSF-1 was, in fact, transferred across the placenta, day-17 gestation pregnant female mice were injected intravenously with human recombinant CSF-1, which also interacts with mouse CSF-1 receptors.35 The mice were killed at various times after injection and growth factor levels were measured in placental and fetal tissues by a radioimmunoassay that detects only biologically active growth factor and does not cross-react with mouse CSF-1.36 As shown in the representative experiment in Fig 1A, biologically active CSF-1 was already detectable at 1 minute, peaked at 10 minutes, and was still detectable at 24 hours after injection in both fetal and placental issues. Placental CSF-1 concentrations on a wet tissue weight basis exceeded those in the fetus from 1 to 120 minutes by 7.4- to 13.2-fold. Interestingly, the amount of human CSF-1 transferred to each fetus corresponds closely to the amount predicted from125I-CSF-1 experiments. Because each fetus weighs approximately 550 mg (Roth and Stanley, unpublished data), the peak human CSF-1 concentration of 100 pg/mg fetal tissue corresponds to 55 ng/fetus, which equals 0.46% of the injected dose of 12 μg. Although virtually identical to the 0.5% of the injected dose predicted by the 125I-CSF-1 experiments (Table 1), it represents a fourfold increase in biologically active (immunoreactive) CSF-1, which is not surprising considering the rapid saturation of maternal clearance and degradation of CSF-1 by the liver at elevated circulating CSF-1 concentrations. Examination of pooled sera (Fig 1B) again showed detectable CSF-1 at 1 minute, peak values at 10 minutes, and levels that could still be detected at 24 hours in both maternal and fetal sera. Although following similar kinetics, large differences were apparent between circulating levels in the maternal and fetal animals in this representative and other similar experiments.
Transplacental transfer of human recombinant CSF-1. Gestational day-17 pregnant mice were injected intravenously with human recombinant CSF-1 and killed at the indicated times postinjection, and human CSF-1 levels were measured by a specific RIA in (A) whole fetuses and placentas, with at least five of each measured at each time point in this one of several representative experiments, and (B) fetal sera pooled from individual litters at each time point and the corresponding maternal serum, with the data representing one of several experiments. Where indicated, placental tissue CSF-1 concentrations differed from those measured in fetal tissues at #P = .06, $P< .01, and *P < .001, respectively. Multiple comparisons over time showed differences, where indicated, ofaP < .025, bP < .005, andcP < .001, respectively, versus 1 minute anddP < .001 versus 10 minutes.
Transplacental transfer of human recombinant CSF-1. Gestational day-17 pregnant mice were injected intravenously with human recombinant CSF-1 and killed at the indicated times postinjection, and human CSF-1 levels were measured by a specific RIA in (A) whole fetuses and placentas, with at least five of each measured at each time point in this one of several representative experiments, and (B) fetal sera pooled from individual litters at each time point and the corresponding maternal serum, with the data representing one of several experiments. Where indicated, placental tissue CSF-1 concentrations differed from those measured in fetal tissues at #P = .06, $P< .01, and *P < .001, respectively. Multiple comparisons over time showed differences, where indicated, ofaP < .025, bP < .005, andcP < .001, respectively, versus 1 minute anddP < .001 versus 10 minutes.
Appearance of F4/80-positive macrophages in perinatal tissues.
To assess the role of circulating CSF-1 in the development and maintenance of F4/80-positive macrophages, several representative tissues were studied in csfmop/csfmopmice and their normal +/csfmop littermates born to both phenotypically normal +/csfmop females (with normal levels of circulating CSF-1 available for transplacental passage) and csfmop/csfmop mothers (with no circulating CSF-1).
Liver.
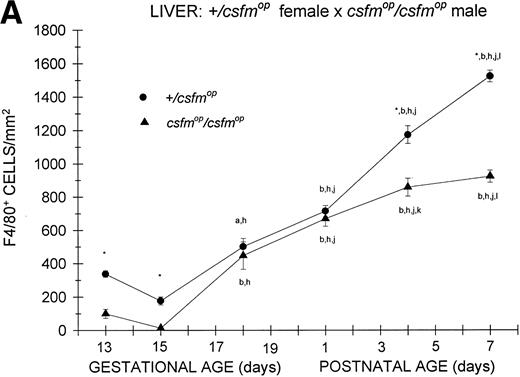
In animals born to +/csfmop mothers, F4/80-positive cells were present at the earliest time points studied (Fig 2A) in bothcsfmop/csfmop and+/csfmop offspring. Whereas the densities of F4/80-positive cells were somewhat diminished incsfmop/csfmop mice compared with their+/csfmop littermates during fetal life (gestational days 13 and 15), there was no difference shortly after birth (postnatal day 1). However, during the first postnatal week, F4/80-positive liver macrophages continue to increase in the +/csfmopmice, in contrast to the csfmop/csfmopmice, in which they have plateaued.
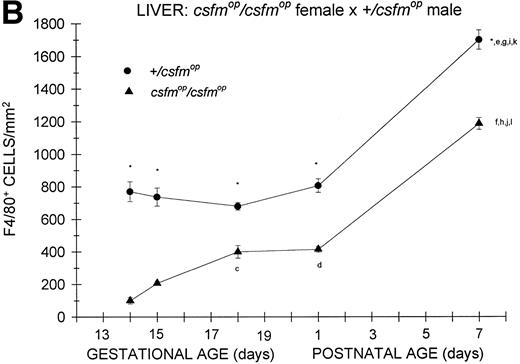
Appearance of F4/80-positive cells in fetal and neonatal liver. Tissues were stained for F4/80, a macrophage-specific marker, as described in the Materials and Methods and positive cells were quantified. Results are expressed as the mean ± SEM for+/csfmop (•) andcsfmop/csfmop (▴) offspring after matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice, respectively. Where indicated,+/csfmop differed fromcsfmop/csfmop at *P < .001. Results of multiple comparisons over time, where indicated, showedaP < .01 and bP < .001 versus day13; cP < .05, dP< .025, eP < .01, and fP< .001 versus day 14; gP < .01 andhP < .001 versus day 15; iP< .005 and jP < .001 versus day 18; and kP < .005 and lP < .001 versus postnatal day 1, respectively.
Appearance of F4/80-positive cells in fetal and neonatal liver. Tissues were stained for F4/80, a macrophage-specific marker, as described in the Materials and Methods and positive cells were quantified. Results are expressed as the mean ± SEM for+/csfmop (•) andcsfmop/csfmop (▴) offspring after matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice, respectively. Where indicated,+/csfmop differed fromcsfmop/csfmop at *P < .001. Results of multiple comparisons over time, where indicated, showedaP < .01 and bP < .001 versus day13; cP < .05, dP< .025, eP < .01, and fP< .001 versus day 14; gP < .01 andhP < .001 versus day 15; iP< .005 and jP < .001 versus day 18; and kP < .005 and lP < .001 versus postnatal day 1, respectively.
As was the case for the offspring of +/csfmopfemales, F4/80-positive cells were present at the earliest time points studied in both csfmop/csfmop and+/csfmop mice born tocsfmop/csfmop mothers (Fig 2B). Althoughcsfmop/csfmop mice had diminished liver macrophage densities compared with their +/csfmoplittermates during fetal life, they also had reduced numbers shortly after birth (postnatal day 1), in contrast to the situation observed for the offspring of +/csfmop females (Fig 2A). Once again, the density of F4/80-positive cells in+/csfmop mice increased over the first week of life; but, surprisingly, the density of these cells on postnatal day 7 incsfmop/csfmop mice relative to that observed in +/csfmop littermates was 70%, which was very similar to the relationship observed in the offspring of+/csfmop females. Interestingly,+/csfmop fetal mice growing incsfmop/csfmop mothers appeared to have higher densities of F4/80-positive cells between gestational days 13 and 15 than those growing in +/csfmop mothers.
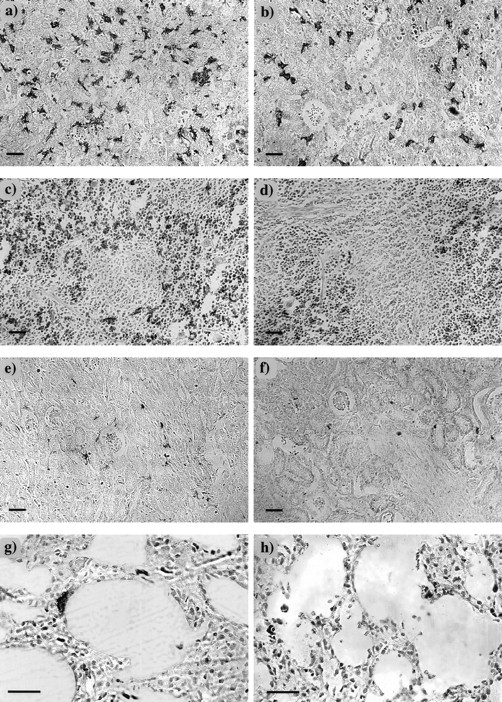
Despite the differences described above, the histologic appearance of F4/80-positive cells in the livers ofcsfmop/csfmop and+/csfmop mice did not depend on whether they were the offspring of +/csfmop orcsfmop/csfmop mothers (data not shown). In both +/csfmop andcsfmop/csfmop neonatal livers, F4/80-positive cells were dendritic and intimately associated with islands of hematopoietic cells (Fig 3a and b). However, there was an obvious reduction in the density of F4/80-positive cells in the neonatal csfmop/csfmop liver (Fig 3b).
F4/80-positive cells in neonatal tissues. Neonatal tissues (postnatal day 7) were stained for the macrophage marker, F4/80, as described in Fig 2, and counterstained with hematoxylin in liver (a and b), spleen (c and d), kidney (e and f), and lung (g and h) of +/csfmop (a, c, e, and g) and csfmop/csfmop (b, d, f, and h) newborn mice born to +/csfmop mothers. Original magnifications are ×110 (a through f) and ×230 (g and h), respectively. Bar = 50 μm.
F4/80-positive cells in neonatal tissues. Neonatal tissues (postnatal day 7) were stained for the macrophage marker, F4/80, as described in Fig 2, and counterstained with hematoxylin in liver (a and b), spleen (c and d), kidney (e and f), and lung (g and h) of +/csfmop (a, c, e, and g) and csfmop/csfmop (b, d, f, and h) newborn mice born to +/csfmop mothers. Original magnifications are ×110 (a through f) and ×230 (g and h), respectively. Bar = 50 μm.
Spleen.
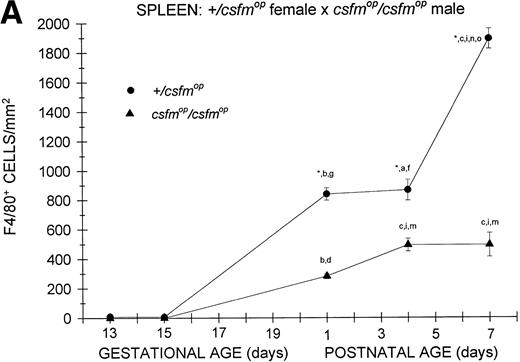
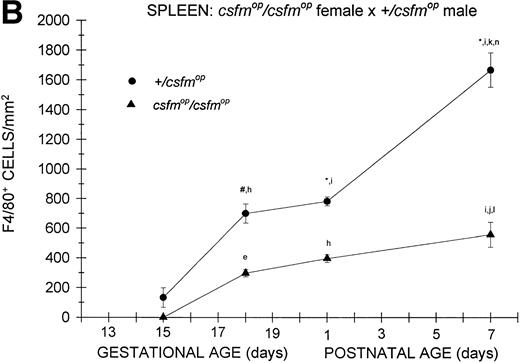
Although F4/80-positive cells were detectable in the spleens of bothcsfmop/csfmop and+/csfmop mice during fetal life, they were present at extremely low levels (Fig 4). In the+/csfmop offspring of +/csfmopmothers, splenic F4/80-positive macrophages steadily increased in density over the first week of life. Incsfmop/csfmop offspring, although positive cells were present in substantial numbers, they were significantly lower in density compared with+/csfmop littermates at birth (postnatal day 1) and during the first week of life (Fig 4A). Despite the absence of transplacental transfer of CSF-1, similar densities of F4/80-positive cells were evident at birth and during the first postnatal week in+/csfmop born tocsfmop/csfmop females (Fig 4B) as in those born to phenotypically normal +/csfmopfemales (Fig 4A). Furthermore, although F4/80-positive cells were present in significantly lower numbers incsfmop/csfmop mice born tocsfmop/csfmop mothers compared with+/csfmop littermates (Fig 4B), they nevertheless were present in similar numbers to those seen incsfmop/csfmop born to+/csfmop females.
Appearance of F4/80-positive cells in fetal and neonatal spleen. Tissues were stained, positive cells were quantified, and results are expressed for +/csfmop (•) andcsfmop/csfmop (▴) offspring after matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice as in Fig 2. Where indicated,+/csfmop differed fromcsfmop/csfmop at #P < .01 and *P < .001, respectively. Results of multiple comparisons over time, where indicated, showed aP < .025,bP < .01, and cP < .001 versus day13; dP = .08, eP< .05, fP < .025, gP < .01, hP < .005, and iP < .001 versus day 15; jP < .01 andkP < .001 versus day 18; lP < .05, mP < .025, and nP < .001 versus postnatal day 1; and oP < .005 versus postnatal day 4, respectively.
Appearance of F4/80-positive cells in fetal and neonatal spleen. Tissues were stained, positive cells were quantified, and results are expressed for +/csfmop (•) andcsfmop/csfmop (▴) offspring after matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice as in Fig 2. Where indicated,+/csfmop differed fromcsfmop/csfmop at #P < .01 and *P < .001, respectively. Results of multiple comparisons over time, where indicated, showed aP < .025,bP < .01, and cP < .001 versus day13; dP = .08, eP< .05, fP < .025, gP < .01, hP < .005, and iP < .001 versus day 15; jP < .01 andkP < .001 versus day 18; lP < .05, mP < .025, and nP < .001 versus postnatal day 1; and oP < .005 versus postnatal day 4, respectively.
On histological examination, differences were evident betweencsfmop/csfmop and+/csfmop fetal/neonatal mouse spleens independent of the maternal genotype (Fig 3c and d and data not shown). Splenic F4/80-positive cells were located exclusively in the red pulp and were both stellate and rounded in +/csfmop mice (Fig3c). In addition to the reduction in density, F4/80-positive cells incsfmop/csfmop spleen were predominantly rounded cells (Fig 3d).
Kidney.
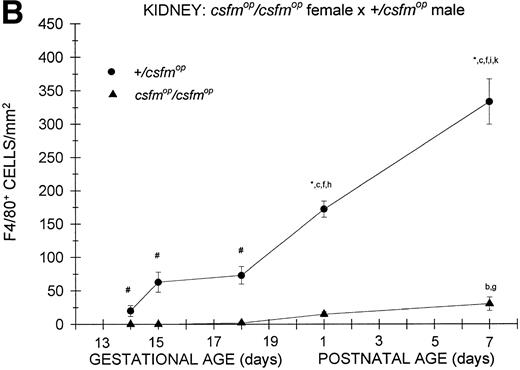
During fetal life, there were minimal to no F4/80-positive cells in the kidneys of +/csfmop mice born to eithercsfmop/csfmop or +/csfmopmothers (Fig 5). However, postnatally, the densities were higher at birth and continued to increase over the first week of life in these same mice (Fig 5). In contrast, there was a virtual absence of F4/80-positive cells in kidneys of csfmop/csfmop mice born to both +/csfmop (Fig 5A) andcsfmop/csfmop (Fig 5B) mothers. Consequently, there were many more F4/80-positive macrophages in+/csfmop compared withcsfmop/csfmop kidneys throughout early postnatal development (Fig 5).
Appearance of F4/80-positive cells in fetal and neonatal kidney. Tissues were stained, positive cells were quantified, and results are expressed for +/csfmop (•) andcsfmop/csfmop (▴) offspring after matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice as in Fig 2. Where indicated,+/csfmop differed fromcsfmop/csfmop at #P < .01 and *P < .001, respectively. Results of multiple comparisons over time, where indicated, showed aP = .09,bP < .05, and cP < .001 versus day14; dP < .025, eP< .005, and fP < .001 versus day 15;gP < .05, hP < .025, andiP < .001 versus day 18; jP< .05, and kP < .001 verus postnatal day 1; andlP < .005 versus postnatal day 4, respectively.
Appearance of F4/80-positive cells in fetal and neonatal kidney. Tissues were stained, positive cells were quantified, and results are expressed for +/csfmop (•) andcsfmop/csfmop (▴) offspring after matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice as in Fig 2. Where indicated,+/csfmop differed fromcsfmop/csfmop at #P < .01 and *P < .001, respectively. Results of multiple comparisons over time, where indicated, showed aP = .09,bP < .05, and cP < .001 versus day14; dP < .025, eP< .005, and fP < .001 versus day 15;gP < .05, hP < .025, andiP < .001 versus day 18; jP< .05, and kP < .001 verus postnatal day 1; andlP < .005 versus postnatal day 4, respectively.
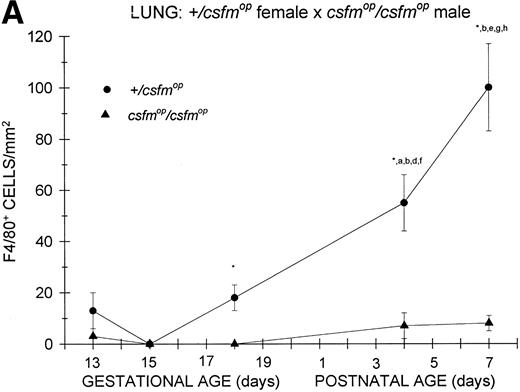
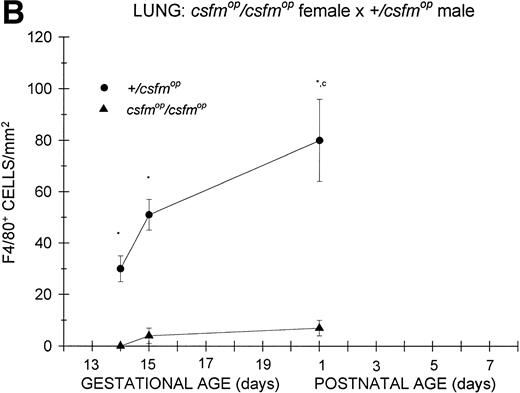
Appearance of F4/80-positive cells in fetal and neonatal lung. Tissues were stained, positive cells were quantified, and results are expressed for +/csfmop (•) andcsfmop/csfmop (▴) after following matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice as in Fig 2. Because of technical difficulties, Fig 6b includes only data through postnatal day 1. Where indicated, +/csfmop differed fromcsfmop/csfmop at *P< .001. Results of multiple comparisons over time, where indicated, showed aP = .07 andbP < .001 versus day13;cP < .025 versus day 14; dP< .005 and eP < .001 versus day 15;fP < .05 and gP < .001 verus day 18; and hP < .025 versus postnatal day 4, respectively.
Appearance of F4/80-positive cells in fetal and neonatal lung. Tissues were stained, positive cells were quantified, and results are expressed for +/csfmop (•) andcsfmop/csfmop (▴) after following matings of (A) +/csfmop female ×csfmop/csfmop male and (B)csfmop/csfmop female ×+/csfmop male mice as in Fig 2. Because of technical difficulties, Fig 6b includes only data through postnatal day 1. Where indicated, +/csfmop differed fromcsfmop/csfmop at *P< .001. Results of multiple comparisons over time, where indicated, showed aP = .07 andbP < .001 versus day13;cP < .025 versus day 14; dP< .005 and eP < .001 versus day 15;fP < .05 and gP < .001 verus day 18; and hP < .025 versus postnatal day 4, respectively.
Lung.
Similar to the kidney, there was a virtual absence of F4/80-positive cells in the lung of csfmop/csfmop mice born to either +/csfmop (Fig 6A) orcsfmop/csfmop (Fig 6B) mothers. In contrast, F4/80-positive cells were present during fetal life in+/csfmop mice born to either maternal genotype (Fig6A and B). Whereas there was a clear increase in lung macrophages during the first postnatal week in +/csfmopoffspring (Fig 6A), technical difficulties in preserving lung macrophages during the fixation process incsfmop/csfmop offspring beyond 1 day of age made it impossible to draw any conclusions regarding later time points (Fig 6B). Nevertheless, the differences between mutant mice and their normal littermates were readily detectable shortly after birth.
DISCUSSION
Growth factors that are capable of crossing the placenta during gestation, as we have demonstrated for CSF-1 in this report, have the potential to exert significant effects on developing fetal cell populations. Homologous CSF-1, radiolabeled to high specific activity and administered to pregnant mice in trace quantities that do not disturb steady-state concentrations, is detectable in fetal tissues and serum shortly after injection. The presence of radioactivity in fetal tissues and serum associated not only with intact but also degraded CSF-1 is consistent with previous data,34 in which we demonstrated that a large proportion of injected labeled CSF-1 is taken up rapidly by the liver, in the present case that of the pregnant mother, and rapidly degraded in that organ and released back into the bloodstream. Furthermore, use of pharmacologic doses of heterologous (human) CSF-1, which is capable of interacting with mouse CSF-1 receptors35 and is detectable by an RIA that specifically detects biologically active human CSF-1 and not mouse CSF-1,36 confirms the transplacental passage of this hematopoietic growth factor, which can then potentially interact with CSF-1 receptor bearing fetal cells. These data are similar to those reported for G-CSF, which is detectable in the fetal circulation within minutes after maternal administration.18 Furthermore, as in the case of CSF-1 in this report, fetal levels of G-CSF were far lower than maternal levels. Nevertheless, significant effects were still observed on fetal myelopoiesis.18
The csfmop/csfmop mouse is well suited for the study of the requirements of different tissues for circulating versus locally produced CSF-1 for the establishment of mononuclear phagocyte populations.16,28 Although the presence of biologically active concentrations of both the proteoglycan and glycoprotein forms of CSF-1 in the circulation11 and of biologically active cell surface-associated CSF-1 glycoprotein41 have been described in normal animals, none of these species can be detected incsfmop/csfmop mice. Previous data on the postnatal administration of exogenous CSF-1 tocsfmop/csfmop mice suggest that the response of mononuclear phagocytes to circulating growth factor is tissue specific, with complete restoration in tissues such as liver, spleen, and kidney; partial restoration in gastrointestinal tissues, bladder, salivary glands, and dermis; and no effect in muscle, synovium, and tendon.16 Thus, although in some tissues regulation of mononuclear phagocyte production may be mediated by circulating CSF-1, in others, it may require growth factor locally, as either the cell surface form or the sequestered proteoglycan.
In the current report, differences between +/csfmopand csfmop/csfmop animals in the appearance of F4/80-positive mononuclear phagocytes suggest that CSF-1 is responsible for the perinatal development of a very large proportion of mononuclear phagocytes in the liver, spleen, kidney, and lung. Furthermore, the ability of maternally derived circulating CSF-1 to compensate for the total absence of local CSF-1 production during fetal and early neonatal life is tissue specific. This phenomenon is most striking in the liver, in which the densities of F4/80-positive cells are equivalent shortly after birth in +/csfmop andcsfmop/csfmop mice born to+/csfmop mothers, but not in similar littermate pairs born to csfmop/csfmop mothers. Thus, circulating CSF-1, which is present in normal concentrations in phenotypically normal +/csfmop females, appears to be capable of crossing the placenta and promoting the normal appearance of hepatic mononuclear phagocytes in theircsfmop/csfmop offspring. This observation is not so surprising, because nearly 80% of hepatic blood flow in the fetus consists of oxygen-enriched blood derived directly from the umbilical vein42 (reviewed in Polin and Fox43). Therefore, mononuclear phagocyte progenitors in the fetal liver can be expected to be exposed to higher concentrations of maternally derived circulating CSF-1 than any other tissue.
In contrast to the liver, splenic F4/80-positive cells were reduced by similar proportions in csfmop/csfmopcompared with +/csfmop littermates, regardless of whether they were born to csfmop/csfmopor +/csfmop mothers, reflecting the failure of transplacental CSF-1 to significantly alter F4/80-positive cell density. Whereas the subnormal but substantial development of mononuclear phagocytes in the spleen is consistent with the partial ability of circulating CSF-1 to restore this population,16the near total absence of F4/80-positive cells in the developing perinatal csfmop/csfmop kidney and lung intimate that local CSF-1 production is required for their development. Unlike postnatal restoration of circulating CSF-1 concentrations through exogenous administration, which readily increased the densities of splenic and kidney F4/80-positive cells to normal levels,16 circulating maternal CSF-1 was unable to effect similar results. Unlike the liver, blood flow to the spleen in utero is derived from the descending aorta distal to the ductus arteriosus and thus consists of a mixture of relatively enriched umbilical vein/ductus venosus and depleted fetal venous return blood.43Consequently, the spleen may be exposed to CSF-1 concentrations substantially lower than those achieved in the experiments described by Cecchini et al.16 In fact, those investigators demonstrate a requirement for the establishment of supraphysiologic concentrations of growth factor to fully restore thecsfmop/csfmop spleen. However, this requirement was not the case in the kidney, which was extremely responsive to restoration of its mononuclear phagocyte populations by lower doses of exogenous CSF-1 in their experiments.16 The apparent discrepancy between this observation and the failure of maternally derived circulating CSF-1 to promote macrophage development in this tissue during fetal life may actually be attributed to alterations in renal blood flow that occur postnatally. During fetal life, not only does the kidney receive its blood supply via the postductal descending aorta, but it also is characterized by markedly elevated vascular resistance. As a result, the kidney receives approximately 2% of cardiac output in utero compared with the 15% to 18% it receives postnatally.43 Thus, under these conditions, perinatal kidney macrophages appear to depend on local production of CSF-1 for their development. Similar to the kidney, the lung receives a far smaller proportion of cardiac output during fetal life (5% to 10%) compared with postnatal life. Whereas no data exist regarding the responsiveness of lung mononuclear phagocytes to postnatal administration of exogenous CSF-1, our data clearly indicate that, in the absence of local CSF-1 production, the available maternally derived circulating CSF-1 fails to foster the development of either interstitial or alveolar macrophages in this tissue. Finally, with the emergence of the fetal mononuclear phagocyte population in the liver, it would be expected that these cells would play an increasing role in clearance of growth factor from the circulation as it makes its first pass through the fetal circulation, thereby reducing the amount of growth factor available to other fetal tissues, including the spleen, kidney, and lung.
Interestingly, our data on the density of F4/80-positive mononuclear phagocytes in the liver, kidney, and spleen shortly after birth (postnatal day 1) agree very well with those of Cecchini et al16 (postnatal day 2). Based on a combination of our data and that of those investigators, it is apparent that, in these tissues, the density of mononuclear phagocytes peaks at 1 week of age (current report) and that, by 2 weeks,16 it has already begun to decrease.
Although the current data point to the importance of CSF-1 in mononuclear phagocyte development, they also suggest that other factors are involved. For example, the data on both hepatic and splenic mononuclear phagocytes, demonstrating the appearance of substantial numbers of cells despite the total absence of CSF-1, are compatible with the involvement of other hematopoietic growth factors or a growth factor-independent pathway for mononuclear phagocyte development. However, from the experiments described here, it is impossible to determine whether such pathways are compensatory, depending on factors other than CSF-1 that operate exclusively in its absence, or normal, in that they operate even when CSF-1 is available in+/csfmop mice. The degree to which such pathways are operative may be responsible in part for the increased density of F4/80-positive cells in fetal liver of +/csfmopmice developing in csfmop/csfmopmothers relative to those developing in +/csfmopmothers as well as the increase in F4/80-positive cells seen in postnatal livers of csfmop/csfmop mice born to csfmop/csfmop mothers allowing them to achieve a similar percentage of positive cells relative to+/csfmop littermates at 1 week of age. Previous data suggest that this cell compartment develops under the influence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3) in addition to CSF-1.44 Although exogenous GM-CSF corrects many of the macrophage deficiencies incsfmop/csfmop mice, it is still unable to induce osteoclast production and thereby correct the underlying osteopetrosis.45 Furthermore, mice that are doubly deficient in GM-CSF and CSF-1 still have substantial numbers of mononuclear phagocytes, suggesting that a significant contribution to production of these cells may derive from other factors, such as IL-3 and IL-2/IL-4, the inducible production of which is elevated incsfmop/csfmop mice.21,46Thus, it appears that mononuclear phagocyte production will occur to the greatest degree in the presence of CSF-1 even in the absence of both GM-CSF and IL-3 activity, as demonstrated in mice with inactive receptors for GM-CSF and IL-5 (due to a mutation in their common βC chain) and with a targeted inactivating mutation in the IL-3 gene, which have normal numbers of mononuclear phagocytes.47 However, when CSF-1 is absent, even when GM-CSF and IL-3 are both present, as is the case in thecsfmop/csfmop mouse, mononuclear phagocyte production occurs, but to a much reduced level. Finally, when IL-3 alone of the three mononuclear phagocyte growth factors is present, as in the case of the mouse doubly deficient for CSF-1 and GM-CSF, development of this cell compartment occurs to even a lesser degree46 and possibly under the additional influence of as yet undetermined additional factors. It is possible that each factor is capable of stimulating a particular subpopulation of target cells and may take on different relative importance in the face of deficiencies of the other respective mononuclear phagocyte growth factors. Specific in situ data may be helpful in elucidating the contributions of these other potential factors in the absence of local CSF-1 expression or the total absence of all CSF-1 expression, respectively.
Supported by National Institutes of Health Grant No. CA32551, by a grant from the Lucille P. Markey Charitable Trust (to E.R.S.), and by Albert Einstein Core Cancer Grant No. P30-CA13330.
Address reprint requests to Philip Roth, MD, PhD, Division of Neonatology-4 East, Staten Island University Hospital, 475 Seaview Ave, Staten Island, NY 10305.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal