Abstract
Erythropoietin (EPO) is a factor essential for erythroid cell proliferation, differentiation, and survival. The production of EPO by the kidneys in response to hypoxia and anemia is well documented. To determine whether EPO is also produced by hematopoietic cells, we analyzed the expression of EPO in normal human hematopoietic progenitors and in their progeny. Undifferentiated CD34+lin− hematopoietic progenitors do not have detectable EPO mRNA. Differentiating CD34+ cells that are stimulated with recombinant human EPO in serum-free liquid cultures express both EPO and EPO receptor (EPOR). Because CD34+ cells represent a heterogeneous cell population, we analyzed individual burst-forming units–erythroid (BFU-E) and nonerythroid colony-forming unit–granulocyte-macrophage colonies for EPO mRNA. Only BFU-E colonies were positive for EPO mRNA. Lysates from pooled BFU-E colonies stained positively for EPO by immunoblotting. To further confirm the intrinsic nature of erythroid EPO, we replaced extrinsic EPO in erythroid colony cultures with EPO-mimicking peptide (EMP). We show EPO expression in the EMP-stimulated BFU-Es at both mRNA and protein levels. Stimulation of bone marrow mononuclear cells (BMMCs) with EMP upregulated EPO expression. Furthermore, we found EPO and EPOR mRNAs as well as EPO protein in K562 cells, a human erythroleukemia cell line. Stimulation of K562 cells with EMP upregulated EPO expression. We suggest that EPO of erythroid origin may have a role in the regulation of erythropoiesis.
ERYTHROPOIETIN (EPO) is a specific stimulator of erythropoiesis, produced in the kidneys during adult life1-4 or in the liver during fetal and neonatal life.5 Its receptor, EPOR, is expressed on colony forming unit-erythroid and burst forming unit-erythroid (BFU-E) progenitors.6 EPO stimulates erythroid cell proliferation,7,8 differentiation,9,10 and inhibits apoptosis.11 Homozygous deletion of EPO or EPOR alleles in mice leads to severe anemia and death at 11 to 15 days of gestation.12-14 In these animals, erythroid commitment takes place, but terminal erythroid differentiation does not proceed. Hermine et al15 described the expression of EPO mRNA in normal mouse bone marrow cells. Experiments with EPO and EPOR antisense oligonucleotides showed that the inhibition of bone marrow EPO expression affects the commitment of hematopoietic cells.15EPO expression was also detected in several human erythroleukemia cell lines.16 17
It has been recently shown that EPOR can be activated by small EPO-mimicking peptides (derived from random phage display peptide libraries) that have no amino acid homology with EPO. These peptides stimulate erythroid differentiation of normal human bone marrow progenitors as well as myeloid cell lines stably transfected with EPOR.18-20
We studied EPO and EPOR expression in normal human undifferentiated and differentiated early hematopoietic progenitors and in erythroid and nonerythroid precursors derived from these cells.
MATERIALS AND METHODS
Tissue Culture of CD34+ cells, BFU-Es, and cell lines.
Peripheral blood mononuclear cells (PBMCs) or bone marrow mononuclear cells (BMMCs) from normal volunteers were isolated on Ficoll-Histopaque (Sigma Chemical Co, St Louis, MO) gradient. Informed consent from studied human subjects was obtained. Early hematopoietic progenitors (CD34+ cells)21 were separated by using a CD34+ cell magnetic sorting kit (Miltenyi Biotec, Auburn, CA).22 The CD34+ cell fraction was cultured for 12 days in StemPro-34SFM serum-free media (Life Technologies Inc, Gaithersburg, MD) with 3 IU/mL EPO and 20 ng/mL stem cell factor. Aliquots of 105 cells were harvested every other day for isolation of total RNA.
BFU-E and colony-forming unit–granulocyte-macrophage (CFU-GM) colonies were cultured in clonogenic cultures in 35-mm Petri dishes by using semisolid medium (Methocult H4531; StemCell Technologies, Vancouver, BC, Canada) with EPO (0.125-3 IU/mL; Life Technologies) or EPO-mimicking peptide 1 (EMP1; 0.1-10 μmol/L; a kind gift of Amgen and Dr D. Johnson, R.W. Johnson Pharmaceutical Research Institute). Cultures were maintained in humidified atmosphere at 5% CO2 and 21% O2 at 37°C. BFU-E colonies were scored at day 7 and 14 with standard criteria.23 24K562, Hep3B, and HepG2 cell lines (ATCC, Rockville, MD) were cultured under the standard protocols.
Single colony reverse transcription-polymerase chain reaction (RT-PCR).
Individual BFU-E and CFU-GM colonies containing approximately 500 to 2,000 cells were harvested under the control of a microscope and lysed in guanidium isothiocyanate-based lysis buffer. Total RNA was isolated by using phenol/chloroform extraction. After DNase treatment, synthesis of the corresponding cDNA was accomplished by using Superscript II reverse transcriptase (Life Technologies). EPO, EPOR, and G6PD cDNAs were amplified with Taq polymerase (Life Technologies) in a Model 480 thermocycler (Perkin-Elmer Inc, Norwalk, CT) for 20 to 30 cycles.25 Sequences of specific primers for RT-PCR were as follows: upstream /downstream primers, EPO (5′ATCACGACGGGCTGTGCTGAACAC/GGGAGATGGCTTCCTTCTGGGCTC3′) cDNA fragment containing 2-5 exon, 289 bp; EPOR (5′GGGAGCGTACAGAGGGTGGAGAT/AGAGCCCGGCGGTGGGAGAGCAG3′) cDNA fragment containing 4-7 exon, 263 bp; G6PD (5′AGGCTGCAGTTCCATGATGT/GCAGCATGGGGTGAAAATA3′) cDNA fragment containing 10-12 exon, 280 bp, genomic fragment 490 bp.26 Each cycle consisted of 30 seconds denaturation at 94°C, 30 seconds annealing at 58°C and 30 seconds extension at 72°C. Specificity of PCR amplification was confirmed by restriction digestion of PCR product with Pst I (23, 175, and 90 bp fragments) or Pvu II (201 and 87 bp).
Immunoblotting of the BFU-E colony lysates.
Individual BFU-E colonies were harvested from semisolid cultures at days 7 to 14, pooled, washed in phosphate-buffered saline (PBS), and lysed in lysis buffer containing 300 nmol/L NaCl, 50 mmol/L Tris HCl, pH 7.6, and 0.5% Triton X-100 (Sigma Chemical Co) for 30 to 45 minutes on ice. After 15 minutes centrifugation at 10,000g, the lysate was denatured for 5 minutes at 95°C in 1 × Laemli buffer and separated by 10% sodium dodecyl sulfate-olyacrylamide gel electrophoresis (SDS-PAGE).27 The gel was electrophoretically transferred onto a 0.45-μm nitrocellulose membrane (Schleicher and Schuell, Keene, NH) by using a semidry blotter (Bio-Rad Laboratory, Hercules, CA).28 The membrane was blocked with 5% nonfat milk in 1 × PBS/0.05% Tween-20 overnight at 4°C. Subsequent washing and dilutions were with 1 × PBS/0.05% Tween-20 at room temperature. The primary rabbit anti-EPO polyclonal antibody, a kind gift from Dr Goldwasser (University of Chicago, Chicago, IL) and Dr Fingerova (Palacky University at Olomouc, Olomouc, Czech Republic) or purchased from Genzyme (Cambridge, MA), was detected by peroxidase-linked antirabbit antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA). All three anti-EPO antibodies used in our experiments gave comparable results. Activated chemiluminescence was detected on radiograph film (Eastman Kodak Co, Rochester, NY) and developed in an RP X-omat processor (Eastman Kodak Co). The specificity of immunostaining was verified by preincubation of primary antibody with 50 IU of recombinant human (rh) EPO. The sensitivity of immunostaining was determined to be 5 mU of rhEPO.
Enzyme-linked immunosorbent assay (ELISA).
EPO was detected by using monoclonal anti-EPO antibody as the capture antibody and revealed with rabbit anti-EPO polyclonal IgG (Genzyme Cambridge, MA) followed by antirabbit IgG horseradish peroxidase antibodies respectively. The color reaction was activated with 3,3′,5,5′-tetramethylbenzidin (TMB) substrate and stopped with 1 mol/L H2SO4. Optical density of samples was detected at 450 nm on ELISA reader (Bio-Tek Instruments Inc, Winooski, VT).29
RESULTS
Differentiating CD34+lin+ cells express EPO.
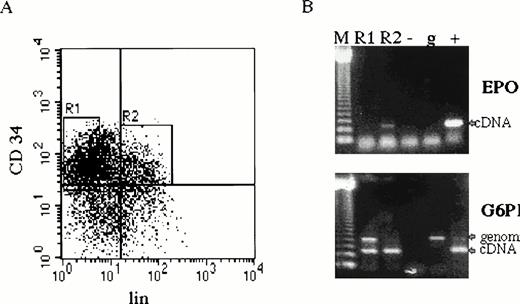
CD34+ cells were cultured in serum-free medium under conditions favoring proliferation and differentiation of erythroid cells.30,31 EPO and EPOR mRNAs were detected in CD34+ cultured cells on days 0 to 12 of in vitro culture by RT-PCR25,26 (Fig 1). Because the CD34+ cells represent a heterogeneous cell population, we have further separated them into undifferentiated (CD34+lin−) and differentiated hematopoietic progenitors (CD34+lin+) from three normal subjects by fluorescence-activated cell sorting30 (Fig 2A). EPO mRNA was detected by RT-PCR in CD34+lin+ cells but not in CD34+lin− cells (Fig 2B).
Cultured CD34+ cells express EPO and EPOR mRNAs. CD34+ cells prepared by magnetic sorting22 were cultured with EPO to preferentially favor differentiation of erythroid progenitors and precursors.30Aliquots of cells were taken at days 0, 2, 4, 6, 8, and 12; total RNA was isolated; and EPO and EPOR cDNAs were generated by RT-PCR by using specific primers. Size marker 123 bp ladder (M), negative control (−), genomic DNA (g), EPO cDNA (+) upper panel, EPO cDNA (+) lower panel.
Cultured CD34+ cells express EPO and EPOR mRNAs. CD34+ cells prepared by magnetic sorting22 were cultured with EPO to preferentially favor differentiation of erythroid progenitors and precursors.30Aliquots of cells were taken at days 0, 2, 4, 6, 8, and 12; total RNA was isolated; and EPO and EPOR cDNAs were generated by RT-PCR by using specific primers. Size marker 123 bp ladder (M), negative control (−), genomic DNA (g), EPO cDNA (+) upper panel, EPO cDNA (+) lower panel.
EPO expression in CD34+ cells. (A) Bone marrow cells were first enriched for CD34+ cells by magnetic sorting.22 Cells were further separated by fluorescence-activated cell sorting by using phycoerythrin-labeled anti-34 antibody recognizing a different CD34 epitope and cocktail of anti-lin (CD2, CD14, CD15, CD16, CD19, and glycophorin) antibodies labeled with fluorescein isothiocyanate. CD34+lin− (R1) and CD34+lin+ (R2). (B) Total RNA from separated cells (4 × 104 of CD34+lin− and 1 × 104 of CD34+lin+) was prepared for RT-PCR (35 cycles) with EPO and G6PD primers (CD34+lin− cDNA template was prepared from 4 times more cells than CD34+lin+ cDNA, which resulted in higher genomic DNA contamination detected by PCR). CD34+lin+ cells (lane R2) contain EPO mRNA, whereas CD34+lin− do not (lane R1). Size marker 123 bp ladder (M), negative control (−), genomic DNA (g), EPO and G6PD cDNA (+).
EPO expression in CD34+ cells. (A) Bone marrow cells were first enriched for CD34+ cells by magnetic sorting.22 Cells were further separated by fluorescence-activated cell sorting by using phycoerythrin-labeled anti-34 antibody recognizing a different CD34 epitope and cocktail of anti-lin (CD2, CD14, CD15, CD16, CD19, and glycophorin) antibodies labeled with fluorescein isothiocyanate. CD34+lin− (R1) and CD34+lin+ (R2). (B) Total RNA from separated cells (4 × 104 of CD34+lin− and 1 × 104 of CD34+lin+) was prepared for RT-PCR (35 cycles) with EPO and G6PD primers (CD34+lin− cDNA template was prepared from 4 times more cells than CD34+lin+ cDNA, which resulted in higher genomic DNA contamination detected by PCR). CD34+lin+ cells (lane R2) contain EPO mRNA, whereas CD34+lin− do not (lane R1). Size marker 123 bp ladder (M), negative control (−), genomic DNA (g), EPO and G6PD cDNA (+).
BFU-E colony cells express EPO mRNA.
In vitro clonogenic cultures23 24 were used to grow individual erythroid (BFU-E) and nonerythroid (CFU-GM) colonies in the presence of rhEPO. Individual colonies (500-2,000 cells/colony) were analyzed by RT-PCR for EPO and EPOR expression. We analyzed individual BFU-E (n ≥ 15) and CFU-GM (n ≥ 15) colonies from each of three normal subjects and pooled BFU-E and CFU-GM colonies from each of eight additional normal subjects. EPO and EPOR mRNAs were detected in BFU-E colonies at different stages of erythroid differentiation but not in nonerythroid colonies (Fig 3A). EPO RT-PCR products were verified by restriction digestion. The same restriction pattern was detected from each of the analyzed samples as well as from the RT-PCR product amplified from control EPO cDNA clone (Fig 3B).
Individual BFU-E but not CFU-GM colonies express EPO. (A) Individual erythroid BFU-E and nonerythroid CFU-GM colonies stimulated with EMP were harvested and analyzed by RT-PCR for EPO, EPOR, and G6PD. BFU-E colonies (lanes 1-4) contain EPO and EPOR mRNAs, and CFU-GM colonies (lanes 5-8) were negative. The housekeeping gene G6PD is expressed in both types of colonies. Size marker 123 bp ladder (M), negative control (−), genomic DNA (g), EPO, EPOR, or G6PD cDNA (+). (B) Restriction digestion analysis. EPO RT-PCR products prepared from individual BFU-E colonies (lanes 2-4) were digested (lanes 6-8) with either PstI (upper panel) or PvuII (lower panel). cDNA EPO template PCR product (lane 1) and its digest (lane 5). A similar restriction pattern in respect to the size of digested DNA (PstI digest, 175, 90, and 23 bp; PvuII digest, 201 and 87 bp) was obtained from all analyzed samples.
Individual BFU-E but not CFU-GM colonies express EPO. (A) Individual erythroid BFU-E and nonerythroid CFU-GM colonies stimulated with EMP were harvested and analyzed by RT-PCR for EPO, EPOR, and G6PD. BFU-E colonies (lanes 1-4) contain EPO and EPOR mRNAs, and CFU-GM colonies (lanes 5-8) were negative. The housekeeping gene G6PD is expressed in both types of colonies. Size marker 123 bp ladder (M), negative control (−), genomic DNA (g), EPO, EPOR, or G6PD cDNA (+). (B) Restriction digestion analysis. EPO RT-PCR products prepared from individual BFU-E colonies (lanes 2-4) were digested (lanes 6-8) with either PstI (upper panel) or PvuII (lower panel). cDNA EPO template PCR product (lane 1) and its digest (lane 5). A similar restriction pattern in respect to the size of digested DNA (PstI digest, 175, 90, and 23 bp; PvuII digest, 201 and 87 bp) was obtained from all analyzed samples.
BFU-E colony cells express EPO on protein level.
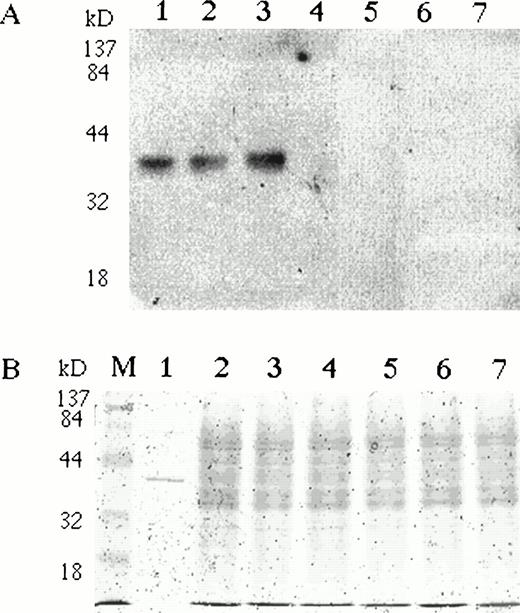
To study EPO expression in erythroid cells at the protein level, we analyzed pooled BFU-Es with specific anti-EPO antibodies on Western blots. Normal BMMCs were stimulated with rhEPO in semisolid cultures. BFU-E colonies were harvested at day 10, repeatedly washed, and analyzed by immunoblotting. A specific single band was detected in BFU-E colony lysates with comparable electrophoretic mobility to rhEPO (Fig 4). To rule out the possibility that the EPO detected in erythroid cells represented internalized extrinsic rhEPO, we stimulated BMMCs with an EMP. BFU-E colonies were harvested, pooled, and analyzed on Western blots for EPO.32 Endogenous EPO protein was detected in cells stimulated with EMP (Fig 4). Specific immunostaining was completely blocked by the preincubation of anti-EPO antibodies with rhEPO (data not shown).
BFU-E colonies express EPO protein. Bone marrow mononuclear cells were plated in semisolid media with either EPO or EMP. (A) BFU-E and CFU-GM colonies were pooled, washed, and lysed with Triton X-100–based lysis buffer. Lysates (20 μg per lane) were separated on 10% SDS-PAGE, transferred onto nitrocellulose membrane, and incubated with rabbit polyclonal anti-EPO antibody. A single band with comparable electrophoretic mobility to rhEPO (lane 1) was detected in BFU-E cells generated with EMP1 (lane 2) or with rhEPO (lane 3). The EPO-specific band was absent in Epstein-Barr virus–transformed lymphocytes (lane 4), granulocytes (lane 5), monocytes (lane 6), and CFU-GM colonies (lane 7). The same membrane was reprobed with antibodies against abundantly expressed angiotensin receptor type 1 (also expressed in BFU-E colony cells)26and a specific band of comparable intensity was detected in all samples. (B) Coomassie staining of protein lysates used on Western blots. To see electrophoretic migration rhEPO was loaded in excess of 50 U/lane.
BFU-E colonies express EPO protein. Bone marrow mononuclear cells were plated in semisolid media with either EPO or EMP. (A) BFU-E and CFU-GM colonies were pooled, washed, and lysed with Triton X-100–based lysis buffer. Lysates (20 μg per lane) were separated on 10% SDS-PAGE, transferred onto nitrocellulose membrane, and incubated with rabbit polyclonal anti-EPO antibody. A single band with comparable electrophoretic mobility to rhEPO (lane 1) was detected in BFU-E cells generated with EMP1 (lane 2) or with rhEPO (lane 3). The EPO-specific band was absent in Epstein-Barr virus–transformed lymphocytes (lane 4), granulocytes (lane 5), monocytes (lane 6), and CFU-GM colonies (lane 7). The same membrane was reprobed with antibodies against abundantly expressed angiotensin receptor type 1 (also expressed in BFU-E colony cells)26and a specific band of comparable intensity was detected in all samples. (B) Coomassie staining of protein lysates used on Western blots. To see electrophoretic migration rhEPO was loaded in excess of 50 U/lane.
EMP upregulates EPO expression in bone marrow cells.
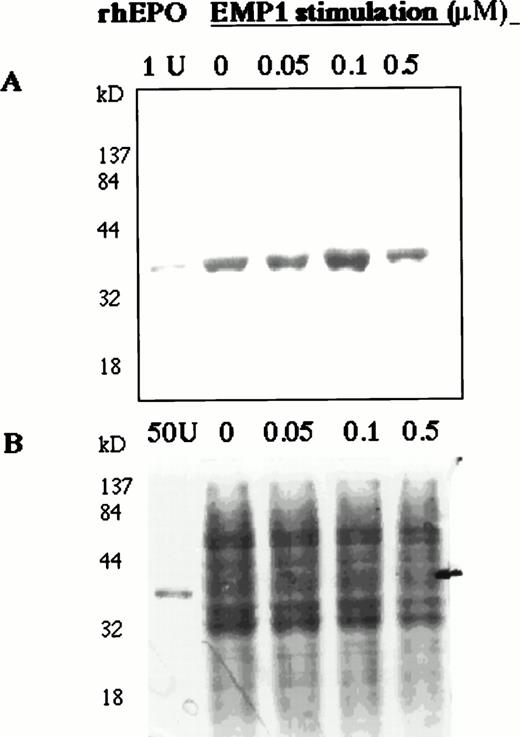
To study the regulation of EPO production in human bone marrow, BMMCs were stimulated with EMP in serum-free medium for 6 hours and EPO in cell lysates was detected on Western blots. EMP upregulated EPO expression (Fig 5A).
EMP1 upregulates EPO expression in BMMCs. Normal BMMCs were cultured for 2 hours in serum-free medium and then stimulated for 6 hours with EMP1 (0, 0.05, 0.1, and 0.5 μmol/L). Cell lysates (10 μg/lane) were analyzed for EPO on Western blots (A). EMP1 upregulates EPO expression in BMMCs with increasing EMP1 concentration up to 0.1 μmol/L. No carrier protein was added to rhEPO in lane 1 and, thus, the intensity of rhEPO band is not comparable with the intensities of EPO bands detected in cell lysates. (B) Coomassie staining of 10% SDS-PAGE separated cell lysates (10 μg per lane). In the EPO lane, 50 U of rhEPO was used.
EMP1 upregulates EPO expression in BMMCs. Normal BMMCs were cultured for 2 hours in serum-free medium and then stimulated for 6 hours with EMP1 (0, 0.05, 0.1, and 0.5 μmol/L). Cell lysates (10 μg/lane) were analyzed for EPO on Western blots (A). EMP1 upregulates EPO expression in BMMCs with increasing EMP1 concentration up to 0.1 μmol/L. No carrier protein was added to rhEPO in lane 1 and, thus, the intensity of rhEPO band is not comparable with the intensities of EPO bands detected in cell lysates. (B) Coomassie staining of 10% SDS-PAGE separated cell lysates (10 μg per lane). In the EPO lane, 50 U of rhEPO was used.
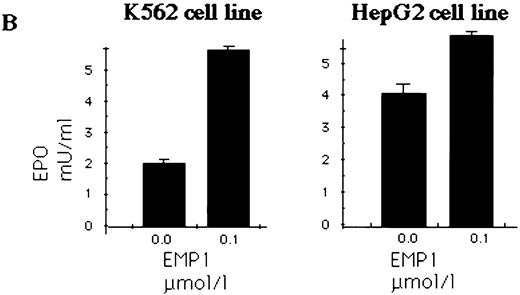
EMP upregulates secretion of EPO by erythroleukemia and hepatocarcinoma cell lines.
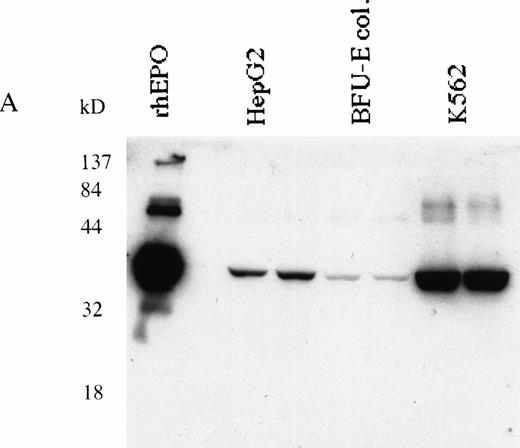
HepG233,34 and K562 human erythroleukemia cells31 were used to determine EPO production in response to EPOR stimulation. Expression of EPO in K562 and HepG2 cells was detected by RT-PCR (data not shown) or immunoblotting (Fig 6A). K562 and HepG2 cells were cultured in serum-free medium and stimulated with EMP for 16 hours; the EPO in supernatants was quantitated by ELISA.29 34 We show that EPO production was upregulated by EMP (Fig 6B).
(A) K562 erythroleukemia cells express EPO. Total protein cell lysates (10 μg/lane) from K562 cells, from rhEPO stimulated BFU-E colonies, and from HepG2 cells were analyzed by immunoblotting with anti-EPO antibodies. rhEPO (1 U/lane) is shown on lane 1. (B) K562 cells secrete EPO in response to stimulation with EMP. EMP1 was used (0.1 μmol/L represents optimal stimulatory concentration) for stimulation of K562 and HepG2 cells (5 × 106 cells/mL) in serum-free media for 16 hours. The EPO concentration was measured in supernatant by ELISA29 (EMP1 does not crossreact with anti-EPO antibodies). Error bars represent EPO concentration ± standard deviation.
(A) K562 erythroleukemia cells express EPO. Total protein cell lysates (10 μg/lane) from K562 cells, from rhEPO stimulated BFU-E colonies, and from HepG2 cells were analyzed by immunoblotting with anti-EPO antibodies. rhEPO (1 U/lane) is shown on lane 1. (B) K562 cells secrete EPO in response to stimulation with EMP. EMP1 was used (0.1 μmol/L represents optimal stimulatory concentration) for stimulation of K562 and HepG2 cells (5 × 106 cells/mL) in serum-free media for 16 hours. The EPO concentration was measured in supernatant by ELISA29 (EMP1 does not crossreact with anti-EPO antibodies). Error bars represent EPO concentration ± standard deviation.
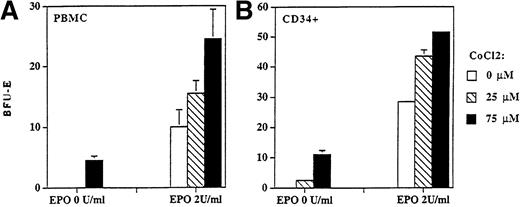
Hypoxia or cobalt chloride upregulates EPO erythroid expression.
The effect of hypoxia on EPO expression (determined by immunoblotting) was measured in K562 and HepG2 cell lines. The incubation of these cells on 1% of oxygen increased EPO expression at the protein level 1.2 and 1.5 times, respectively. Similar augmentation of EPO production was seen when these cells were incubated with cobalt chloride (data not shown). To further study the effect of cobalt chloride on regulation of erythropoiesis we enumerated BFU-E colonies derived from PBMCs or CD34+ cells in the absence and in the presence of a suboptimal stimulatory concentration of rhEPO (0 and 2 U/mL) under different concentrations of cobalt chloride. We show that cobalt chloride stimulated the formation of BFU-E colonies in the absence of EPO and increased their numbers when a submaximal concentration of EPO was used (Fig 7). The stimulatory effect of cobalt chloride on BFU-E colony formation (derived from CD34+) was statistically significant for 25 μmol/L (P < .05) or 75 μmol/L (P < .01) according to the Student's t-test.
BFU-E colonies derived from PBMCs (A) and CD34+ (B) cells were stimulated with cobalt chloride in semisolid cultures. Cobalt chloride stimulated formation of BFU-E colonies derived from PBMCs (2 × 105/mL) and CD34+ cells (104/mL) in the absence (P < .01) and in the presence of suboptimal concentration of rhEPO (2 U/mL; P < .05) in semisolid cultures. Colony numbers are expressed as mean ± standard deviation.
BFU-E colonies derived from PBMCs (A) and CD34+ (B) cells were stimulated with cobalt chloride in semisolid cultures. Cobalt chloride stimulated formation of BFU-E colonies derived from PBMCs (2 × 105/mL) and CD34+ cells (104/mL) in the absence (P < .01) and in the presence of suboptimal concentration of rhEPO (2 U/mL; P < .05) in semisolid cultures. Colony numbers are expressed as mean ± standard deviation.
DISCUSSION
We report that early human hematopoietic progenitors, CD34+lin+ cells but not uncommitted CD34+lin− progenitors, express EPO. With the progressive commitment of human differentiating hematopoietic progenitors, EPO and EPOR are expressed only in erythroid, but not in nonerythroid progenitors. EPO production was previously detected in some erythroleukemia cells,16,17 and the EPO transcript was found in mouse unseparated bone marrow cells.15 It was also reported that murine macrophages can express EPO mRNA.35,36Here we show that normal human CFU-GM colonies derived from CD34+ hematopoietic progenitors or BMMCs have undetectable EPO and EPOR mRNAs under the conditions used. Additionally, human peripheral blood macrophages isolated by adherence on plastic showed no detectable EPO on Western blots. EPO expression, either at the mRNA or protein level, has not been previously reported in normal human erythroid precursors. Because erythroid precursors differentiate from early hematopoietic progenitors in the presence of EPO, we could not distinguish in our cultures an extrinsic or intrinsic origin of EPO detected in erythroid precursors. We then used EMP, EMP1,18-20 and differentiated early hematopoietic progenitors to erythroid precursors in vitro (BFU-E colonies) in the absence of extrinsic EPO. EPO detected in BFU-E colony cells by immunoblotting clearly showed the intrinsic nature of EPO that is newly synthesized by erythroid precursors.
We show that the EPO production increases with increasing stimulation of EPOR by EMP in the hepatocarcinoma cell line HepG2, which has been used as a model of EPO production and its regulation.37-40We also show upregulation of EPO expression by EMP in normal bone marrow cells as well as in the human erythroleukemia cell line K562, which was not previously reported to produce EPO.
It has been recently reported that BFU-Es exposed to hypoxia in the presence of EPO have formed larger numbers of BFU-E colonies compared with normoxic control. Hypoxia was shown to increase proliferation of BFU-Es whereas their terminal differentiation was inhibited.41 We showed that cobalt chloride, a known stimulator of EPO expression, augmented the formation of BFU-E colonies from either PBMCs or CD34+ cells in the absence and in the presence of EPO. These experiments suggest the important role of intrinsic erythroid EPO expression in the regulation of erythroid differentiation.
The results reported here, suggesting a role of erythroid EPO in terminal erythroid differentiation, are supported by studies in EPO or EPOR knock-out mice, in which the erythroid commitment takes place but erythroid differentiation does not proceed beyond the level of BFU-E progenitors.12-14 This contention is also supported by our previous work showing that neutralizing anti-EPO or anti-EPOR antibodies block erythroid differentiation of early BFU-Es.42,43 However, the addition of these antibodies to cultures of cells at later stages of erythroid differentiation does not inhibit BFU-E colony formation.42 The upregulation of erythroid EPO production by EPOR stimulation suggested that EPO expression in hematopoietic cells may play an important supportive role in erythropoiesis. However, the physiological role of intrinsic EPO and its precise role in the regulation of erythropoiesis has to be defined and further studies on genetically engineered cell lines and animals will be required.
ACKNOWLEDGMENT
We thank the R.W. Johnson Pharmaceutical Research Institute for allowing us to use the peptide mimicking EPO and especially Dr Dana L. Johnson for information concerning the use of EMP peptide. We thank Drs V. Divoky, T. Townes, E. Goldwasser, and R. Kralovics for helpful discussion and M. Divoka for technical assistance.
Supported by a Veterans Administration Hospital Merit Grant, the United States Public Health Service, National Institutes of Health Grants No. HL51650 and HL50077, and by Internal Grant Agency of Czech Republic Grant No. 4917-3.
Address reprint requests to J.T. Prchal, MD, 1900 University Blvd, #513 THT, Birmingham, AL 35294.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal