Abstract
Improved survival in aplastic anemia (AA) has shown a high incidence of late clonal marrow disorders. To investigate whether accelerated senescence of hematopoietic stem cells might underlie the pathophysiology of myelodysplasia (MDS) or paroxysmal nocturnal hemoglobinuria (PNH) occurring as a late complication of AA, we studied mean telomere length (TRF) in peripheral blood leukocytes from 79 patients with AA, Fanconi anemia, or PNH in comparison with normal controls. TRF lengths in the patient group were significantly shorter for age than normals (P < .0001). Telomere shortening was apparent in both granulocyte and mononuclear cell fractions, suggesting loss at the level of the hematopoietic stem cell. In patients with acquired AA with persistent cytopenias (n = 40), there was significant correlation between telomere loss and disease duration (r = −.685; P < .0001), equivalent to progressive telomere erosion at 216 bp/yr, in addition to the normal age-related loss. In patients who had achieved normal full blood counts (n = 20), the rate of telomere loss had apparently stabilised. There was no apparent association between telomere loss and secondary PNH (n = 13). However, of the 5 patients in the study with TRF less than 5.0 kb, 3 had acquired cytogenetic abnormalities, suggesting that telomere erosion may be relevant to the pathogenesis of MDS in aplastic anemia.
THE PROGNOSIS OF patients with aplastic anemia (AA) has been greatly improved by treatment with antilymphocyte globulin (ALG).1-5 However, despite achieving red blood cell and platelet transfusion independence, many treated patients still show evidence of impaired hematopoiesis, in the form of persistent peripheral blood cytopenias and macrocytosis.6-8 A residual hematopoietic defect can also be inferred from in vitro bone marrow culture results. Marrows from AA patients who have responded to treatment may still display a relative deficiency of early progenitor cells, affecting both total CD34+ cells9-11 and the CD34+33− subset.10 The production of committed progenitor cells (colony-forming units [CFU]) is subnormal, and there is a shorter duration of CFU production than normals in long-term bone marrow culture.12-15
With prolonged survival has also come the increasing recognition that AA patients with autologous marrow recovery are at significant risk of developing late clonal marrow disorders.8,16-20 The combined cumulative risk of developing either paroxysmal nocturnal hemoglobinuria (PNH) or myelodysplastic syndrome (MDS) has been calculated to be as high as 42% at 15 years after ALG.19Although an association between AA and PNH has long been recognised,21,22 to date it has not been possible to predict which patients are at highest risk of developing secondary PNH.6,7 By contrast, age has been identified as an important prognostic factor in the development of secondary MDS, with the risk of MDS increasing with age as a continuous variable.23 This led us to hypothesise that accelerated senescence of a reduced population of hematopoietic stem cells could contribute to the pathogenesis of secondary MDS in AA.
Hematopoietic cells, in common with other somatic cells,24-30 show evidence of progressive telomere shortening with age in vivo24,31,32 as well as in vitro.32-34 Loss of telomeric DNA can therefore be used as an indicator of hematopoietic aging. In addition, genetic instability associated with age-related loss of telomeric DNA in hematopoietic stem cells may be important in the pathogenesis of de novo MDS,35 which is predominantly a disease of the elderly.36 We therefore studied an otherwise unselected group of patients with AA, Fanconi anemia (FA), or PNH, looking at telomere length in DNA extracted from peripheral blood leukocytes.
We have found evidence for significant telomeric shortening in AA, affecting both granulocyte and mononuclear cell fractions, and therefore probably occurring at the level of the hematopoietic stem cell. Our results suggest that the rate of telomere loss stabilizes after complete hematological recovery, although progressive telomere erosion probably continues in those patients who do not fully regain normal hematopoiesis, with continuing neutropenia, thrombocytopenia, and macrocytosis. According to the classic model of the role of telomeres in replicative senescence and immortalization,37-39 we would predict the subgroup of patients in whom telomere length was approaching a critical point to be at highest risk of developing MDS. In keeping with this, the AA patients in our study with acquired cytogenetic abnormalities were among those with the shortest telomere lengths. However, there was no apparent association between telomere loss and the development of secondary PNH. It is also unlikely that telomere erosion plays a direct role in the pathogenesis of bone marrow failure in acquired AA; in all but a small minority of patients in this study, the extent of telomere loss did not approach that associated with replicative cell senescence in in vitro cultures of somatic cells.29,30,38 40
MATERIALS AND METHODS
Patients
Blood samples were collected from 79 unselected patients with a diagnosis of AA, FA, or PNH at times of routine clinic visit or admission to the ward for treatment. Sixty-eight patients (33 male and 35 female patients; 12 to 81 years of age) had idiopathic acquired AA (Table 1). A further 2 patients with apparently acquired AA (Table 1) gave a history of aplastic anemia in first-degree relatives and are here classified as familial AA, with FA having been excluded by induced chromosome breakage studies. Six patients (4 to 18 years of age) had FA (including 2 pairs of siblings). Four patients with acquired AA were PNH-positive at presentation (2 with hemolytic PNH and 2 without hemolysis but with deficiency in glycosyl-phosphatidylinositol [GPI]-anchored proteins on fluorescence-activated cell sorting [FACS] analysis). Three patients had de novo hemolytic PNH without AA.
Details of Patients With AA
| . | Age/ Sex . | Diagnosis-150 . | Duration-151 . | Specific Therapy . | MCV . | ANC-152 . | plts-152 . | PNH/ Interval-153 . | TRF kb . |
|---|---|---|---|---|---|---|---|---|---|
| Acquired AA | |||||||||
| 1 | 23/F | NSAA | 33 | ALG, CyA | 89 | 2.9 | 154 | Y/23 | 9.27 |
| 2 | 28/F | NSAA | 42 | ALGx2, CyA, androgens | 105 | 1.7 | 148 | Yh/pres | 6.80 |
| 3 | 33/M | VSAA | 1 | None | TD | 0.1 | 5 | Y/pres | 8.36 |
| 4 | 62/F | — | — | — | TD | 2.3 | 33 | — | 7.02 |
| 5 | 61/M | SAA | 22 | CyA | 104 | 2.5 | 96 | N | 6.87 |
| 6 | 32/F | SAA | 132 | ALGx2, CyA | 90 | 3.2 | 131 | N | 6.82 |
| 7 | 12/M | VSAA | 39 | ALGx2, CyA | 95 | 2.1 | 192 | N | 9.22 |
| 8 | 16/F | VSAA | 12 | ALGx2, G-CSF | 100 | 1.3 | 40 | N | 8.05 |
| 9 | 61/F | NSAA | 40 | ALG, CyA | 100 | 5.4 | 201 | N | 5.79 |
| 10 | 16/M | NSAA | 58 | ALG, CyA | 110 | 1.4 | 73 | Yh/58 | 7.19 |
| 11 | 17/F | VSAA | 6 | ALG, CyA | 104 | 4.4 | 124 | N | 8.00 |
| 12 | 69/F | VSAA | 1 | None | TD | 0.0 | 15 | N | 6.97 |
| 13 | 34/F | NSAA | 18 | ALG, CyA | 98 | 5.7 | 72 | N | 9.03 |
| 14 | 49/M | NSAA | 76 | ALG, androgens | 112 | 1.7 | 19 | N | 4.51¶ |
| 15 | 22/F | SAA | 34 | BMT (autologous recovery) | 101 | 3.9 | 183 | Y/34 | 8.32 |
| 16 | 24/F | NSAA | 35 | BMT (donor hematopoiesis) | 94 | 4.9 | 200 | N | 6.24 |
| 17 | 22/M | NSAA | 10 | ALG, CyA | 116 | 0.6 | 13 | N | 8.44 |
| 18 | 62/M | NSAA | 10 | ALGx2, CyA, androgens, G-CSF | TD | 3.0 | 4 | N | 7.51 |
| 19 | 44/F | NSAA | 36 | CyA | 113 | 0.8 | 18 | N | 7.15 |
| 20 | 65/M | NSAA | 7 | None | TD | 1.1 | 9 | N | 6.12 |
| 21 | 66/F | SAA | 31 | ALGx2, CyA | 100 | 1.5 | 39 | Y/26 | 6.52 |
| 22 | 16/M | VSAA | 25 | ALGx2, CyA, androgens, G-CSF | TD | 0.1 | 10 | N | 7.27 |
| 23 | 32/M | NSAA | 47 | CyA | 107 | 2.3 | 176 | Yh/pres | 6.88 |
| 24 | 36/F | NSAA | 15 | CyA | TD | 1.6 | 28 | N | 6.60 |
| 25 | 29/M | SAA | 94 | ALG, androgens | 96 | 2.5 | 147 | Y/76 | 7.36 |
| 26 | 25/M | NSAA | 79 | ALGx2, CyA, androgens | 102 | 5.2 | 165 | Y/61 | 6.97 |
| 27 | 45/F | NSAA | — | — | 105 | 4.7 | 103 | N | 4.60 |
| 28 | 30/F | NSAA | 99 | CyA | 108 | 1.4 | 15 | N | 7.13 |
| 29 | 69/F | NSAA | 29 | ALG, CyA | 95 | 6.7 | 199 | N | 5.40 |
| 30 | 28/F | — | 174 | BMT (donor hematopoiesis) | 89 | 2.8 | 160 | N | 7.25 |
| 31 | 27/M | NSAA | 1 | None | TD | 1.7 | 12 | N | 8.27 |
| 32 | 23/M | SAA | 17 | ALG, CyA, G-CSF | 98 | 7.8 | 218 | N | 7.65 |
| 33 | 29/F | SAA | 13 | ALG, CyA, G-CSF | 101 | 2.3 | 79 | N | 8.46 |
| 34 | 32/F | NSAA | 36 | ALG, CyA | 113 | 2.4 | 86 | N | 6.68 |
| 35 | 47/F | NSAA | 22 | ALG, CyA, pyridoxine | 94 | 2.8 | 122 | N | 7.50 |
| 36 | 38/M | VSAA | 172 | CyA, androgens | 109 | 0.1 | 30 | N | 6.94 |
| 37 | 27/F | NSAA | 30 | ALGx2, CyA, G-CSF, IL-6 | 115 | 1.6 | 24 | N | 8.78 |
| 38 | 13/F | SAA | 4 | BMT (donor hematopoiesis) | 107 | 8.7 | 235 | N | 7.67 |
| 39 | 22/M | NSAA | 164 | Androgens | 99 | 1.8 | 35 | Y/129(t) | 3.58¶ |
| 40 | 54/F | NSAA | 26 | ALG, CyA | 100 | 2.8 | 50 | Y/pres | 6.51 |
| 41 | 17/M | SAA | 9 | ALGx2, CyA | TD | 0.6 | 40 | N | 8.80 |
| 42 | 33/M | SAA | 41 | ALGx2, CyA, androgens | 108 | 0.8 | 66 | N | 7.35 |
| 43 | 26/M | SAA | 10 | ALGx2, CyA, androgens | 111 | 2.3 | 22 | Y/4(t) | 8.49 |
| 44 | 20/M | — | — | CyA | TD | 0.2 | 43 | Y/13 | 7.76 |
| 45 | 67/M | SAA | 40 | ALGx2 | 88 | 1.5 | 81 | N | 5.96 |
| 46 | 40/M | NSAA | 48 | ALGx3, CyA, androgens | 100 | 2.3 | 49 | N | 6.82 |
| 47 | 47/F | SAA | 42 | ALGx2, CyA | 120 | 2.0 | 58 | N | 6.89 |
| 48 | 20/F | SAA | 1 | None | TD | 0.2 | 19 | N | 9.37 |
| 49 | 41/F | NSAA | 21 | ALGx2, CyA, G-CSF, BMT (autol) | TD | 4.4 | 41 | N | 6.28 |
| 50 | 32/M | SAA | 54 | ALGx2, CyA, andro, BMT (autol) | 110 | 1.5 | 121 | N | 7.26 |
| 51 | 33/M | SAA | 50 | ALG, androgens | 110 | 1.7 | 137 | N | 6.60 |
| 52 | 55/F | NSAA | 372 | Androgens | 94 | 2.2 | 245 | N | 7.42 |
| 53 | 57/M | NSAA | 25 | ALGx2, CyA | 99 | 2.5 | 91 | Y/4 | 7.50 |
| 54 | 28/M | NSAA | 11 | ALG, BMT (donor hematopoiesis) | 93 | 2.2 | 30 | N | 8.52 |
| 55 | 14/F | NSAA | 46 | BMT (donor hematopoiesis) | 84 | 2.6 | 309 | N | 8.05 |
| 56 | 31/M | NSAA | 16 | ALG, CyA, G-CSF | 104 | 1.3 | 66 | N | 7.32 |
| 57 | 45/F | SAA | 1 | None | TD | 0.4 | 30 | N | 8.10 |
| 58 | 65/M | NSAA | 88 | ALGx2, CyA | 88 | 3.1 | 131 | Y/71 | 5.78 |
| 59 | 43/F | NSAA | 53 | ALG, CyA | 90 | 3.8 | 218 | N | 6.29 |
| 60 | 61/M | VSAA | 77 | ALG | 96 | 2.4 | 145 | N | 6.21 |
| Age/ Sex | Diagnosis-150 | Duration-151 | Specific Therapy | MCV | ANC-152 | plts | PNH/ Interval-153 | TRF kb | |
| 61 | 64/M | NSAA | 56 | ALG | 107 | 1.9 | 59 | Y/32 | 6.72 |
| 62 | 60/F | VSAA | 7 | ALGx2, G-CSF | TD | 10.8 | 27 | N | 7.31 |
| 63 | 81/M | NSAA | 108 | ALG | 103 | 2.7 | 111 | Y/108 | 6.83 |
| 64 | 38/M | NSAA | 65 | ALG, androgens | 100 | 3.0 | 114 | N | 6.69 |
| 65 | 79/F | NSAA | 69 | CyA | 87 | 3.8 | 335 | N | 5.98 |
| 66 | 74/F | NSAA | 107 | ALGx2 | 90 | 2.1 | 163 | N | 5.53 |
| 67 | 71/F | VSAA | 4 | ALG | TD | 1.8 | 61 | N | 6.97 |
| 68 | 37/M | SAA | 4 | ALG, CyA | TD | 0.3 | 25 | N | 7.69 |
| Familial AA | |||||||||
| 1 | 36/F | NSAA | 73-155 | None | 101 | 1.3 | 117 | N | 4.98¶ |
| 2 | 15/F | NSAA | 24-155 | None | 110 | 1.2 | 41 | N | 4.72 |
| . | Age/ Sex . | Diagnosis-150 . | Duration-151 . | Specific Therapy . | MCV . | ANC-152 . | plts-152 . | PNH/ Interval-153 . | TRF kb . |
|---|---|---|---|---|---|---|---|---|---|
| Acquired AA | |||||||||
| 1 | 23/F | NSAA | 33 | ALG, CyA | 89 | 2.9 | 154 | Y/23 | 9.27 |
| 2 | 28/F | NSAA | 42 | ALGx2, CyA, androgens | 105 | 1.7 | 148 | Yh/pres | 6.80 |
| 3 | 33/M | VSAA | 1 | None | TD | 0.1 | 5 | Y/pres | 8.36 |
| 4 | 62/F | — | — | — | TD | 2.3 | 33 | — | 7.02 |
| 5 | 61/M | SAA | 22 | CyA | 104 | 2.5 | 96 | N | 6.87 |
| 6 | 32/F | SAA | 132 | ALGx2, CyA | 90 | 3.2 | 131 | N | 6.82 |
| 7 | 12/M | VSAA | 39 | ALGx2, CyA | 95 | 2.1 | 192 | N | 9.22 |
| 8 | 16/F | VSAA | 12 | ALGx2, G-CSF | 100 | 1.3 | 40 | N | 8.05 |
| 9 | 61/F | NSAA | 40 | ALG, CyA | 100 | 5.4 | 201 | N | 5.79 |
| 10 | 16/M | NSAA | 58 | ALG, CyA | 110 | 1.4 | 73 | Yh/58 | 7.19 |
| 11 | 17/F | VSAA | 6 | ALG, CyA | 104 | 4.4 | 124 | N | 8.00 |
| 12 | 69/F | VSAA | 1 | None | TD | 0.0 | 15 | N | 6.97 |
| 13 | 34/F | NSAA | 18 | ALG, CyA | 98 | 5.7 | 72 | N | 9.03 |
| 14 | 49/M | NSAA | 76 | ALG, androgens | 112 | 1.7 | 19 | N | 4.51¶ |
| 15 | 22/F | SAA | 34 | BMT (autologous recovery) | 101 | 3.9 | 183 | Y/34 | 8.32 |
| 16 | 24/F | NSAA | 35 | BMT (donor hematopoiesis) | 94 | 4.9 | 200 | N | 6.24 |
| 17 | 22/M | NSAA | 10 | ALG, CyA | 116 | 0.6 | 13 | N | 8.44 |
| 18 | 62/M | NSAA | 10 | ALGx2, CyA, androgens, G-CSF | TD | 3.0 | 4 | N | 7.51 |
| 19 | 44/F | NSAA | 36 | CyA | 113 | 0.8 | 18 | N | 7.15 |
| 20 | 65/M | NSAA | 7 | None | TD | 1.1 | 9 | N | 6.12 |
| 21 | 66/F | SAA | 31 | ALGx2, CyA | 100 | 1.5 | 39 | Y/26 | 6.52 |
| 22 | 16/M | VSAA | 25 | ALGx2, CyA, androgens, G-CSF | TD | 0.1 | 10 | N | 7.27 |
| 23 | 32/M | NSAA | 47 | CyA | 107 | 2.3 | 176 | Yh/pres | 6.88 |
| 24 | 36/F | NSAA | 15 | CyA | TD | 1.6 | 28 | N | 6.60 |
| 25 | 29/M | SAA | 94 | ALG, androgens | 96 | 2.5 | 147 | Y/76 | 7.36 |
| 26 | 25/M | NSAA | 79 | ALGx2, CyA, androgens | 102 | 5.2 | 165 | Y/61 | 6.97 |
| 27 | 45/F | NSAA | — | — | 105 | 4.7 | 103 | N | 4.60 |
| 28 | 30/F | NSAA | 99 | CyA | 108 | 1.4 | 15 | N | 7.13 |
| 29 | 69/F | NSAA | 29 | ALG, CyA | 95 | 6.7 | 199 | N | 5.40 |
| 30 | 28/F | — | 174 | BMT (donor hematopoiesis) | 89 | 2.8 | 160 | N | 7.25 |
| 31 | 27/M | NSAA | 1 | None | TD | 1.7 | 12 | N | 8.27 |
| 32 | 23/M | SAA | 17 | ALG, CyA, G-CSF | 98 | 7.8 | 218 | N | 7.65 |
| 33 | 29/F | SAA | 13 | ALG, CyA, G-CSF | 101 | 2.3 | 79 | N | 8.46 |
| 34 | 32/F | NSAA | 36 | ALG, CyA | 113 | 2.4 | 86 | N | 6.68 |
| 35 | 47/F | NSAA | 22 | ALG, CyA, pyridoxine | 94 | 2.8 | 122 | N | 7.50 |
| 36 | 38/M | VSAA | 172 | CyA, androgens | 109 | 0.1 | 30 | N | 6.94 |
| 37 | 27/F | NSAA | 30 | ALGx2, CyA, G-CSF, IL-6 | 115 | 1.6 | 24 | N | 8.78 |
| 38 | 13/F | SAA | 4 | BMT (donor hematopoiesis) | 107 | 8.7 | 235 | N | 7.67 |
| 39 | 22/M | NSAA | 164 | Androgens | 99 | 1.8 | 35 | Y/129(t) | 3.58¶ |
| 40 | 54/F | NSAA | 26 | ALG, CyA | 100 | 2.8 | 50 | Y/pres | 6.51 |
| 41 | 17/M | SAA | 9 | ALGx2, CyA | TD | 0.6 | 40 | N | 8.80 |
| 42 | 33/M | SAA | 41 | ALGx2, CyA, androgens | 108 | 0.8 | 66 | N | 7.35 |
| 43 | 26/M | SAA | 10 | ALGx2, CyA, androgens | 111 | 2.3 | 22 | Y/4(t) | 8.49 |
| 44 | 20/M | — | — | CyA | TD | 0.2 | 43 | Y/13 | 7.76 |
| 45 | 67/M | SAA | 40 | ALGx2 | 88 | 1.5 | 81 | N | 5.96 |
| 46 | 40/M | NSAA | 48 | ALGx3, CyA, androgens | 100 | 2.3 | 49 | N | 6.82 |
| 47 | 47/F | SAA | 42 | ALGx2, CyA | 120 | 2.0 | 58 | N | 6.89 |
| 48 | 20/F | SAA | 1 | None | TD | 0.2 | 19 | N | 9.37 |
| 49 | 41/F | NSAA | 21 | ALGx2, CyA, G-CSF, BMT (autol) | TD | 4.4 | 41 | N | 6.28 |
| 50 | 32/M | SAA | 54 | ALGx2, CyA, andro, BMT (autol) | 110 | 1.5 | 121 | N | 7.26 |
| 51 | 33/M | SAA | 50 | ALG, androgens | 110 | 1.7 | 137 | N | 6.60 |
| 52 | 55/F | NSAA | 372 | Androgens | 94 | 2.2 | 245 | N | 7.42 |
| 53 | 57/M | NSAA | 25 | ALGx2, CyA | 99 | 2.5 | 91 | Y/4 | 7.50 |
| 54 | 28/M | NSAA | 11 | ALG, BMT (donor hematopoiesis) | 93 | 2.2 | 30 | N | 8.52 |
| 55 | 14/F | NSAA | 46 | BMT (donor hematopoiesis) | 84 | 2.6 | 309 | N | 8.05 |
| 56 | 31/M | NSAA | 16 | ALG, CyA, G-CSF | 104 | 1.3 | 66 | N | 7.32 |
| 57 | 45/F | SAA | 1 | None | TD | 0.4 | 30 | N | 8.10 |
| 58 | 65/M | NSAA | 88 | ALGx2, CyA | 88 | 3.1 | 131 | Y/71 | 5.78 |
| 59 | 43/F | NSAA | 53 | ALG, CyA | 90 | 3.8 | 218 | N | 6.29 |
| 60 | 61/M | VSAA | 77 | ALG | 96 | 2.4 | 145 | N | 6.21 |
| Age/ Sex | Diagnosis-150 | Duration-151 | Specific Therapy | MCV | ANC-152 | plts | PNH/ Interval-153 | TRF kb | |
| 61 | 64/M | NSAA | 56 | ALG | 107 | 1.9 | 59 | Y/32 | 6.72 |
| 62 | 60/F | VSAA | 7 | ALGx2, G-CSF | TD | 10.8 | 27 | N | 7.31 |
| 63 | 81/M | NSAA | 108 | ALG | 103 | 2.7 | 111 | Y/108 | 6.83 |
| 64 | 38/M | NSAA | 65 | ALG, androgens | 100 | 3.0 | 114 | N | 6.69 |
| 65 | 79/F | NSAA | 69 | CyA | 87 | 3.8 | 335 | N | 5.98 |
| 66 | 74/F | NSAA | 107 | ALGx2 | 90 | 2.1 | 163 | N | 5.53 |
| 67 | 71/F | VSAA | 4 | ALG | TD | 1.8 | 61 | N | 6.97 |
| 68 | 37/M | SAA | 4 | ALG, CyA | TD | 0.3 | 25 | N | 7.69 |
| Familial AA | |||||||||
| 1 | 36/F | NSAA | 73-155 | None | 101 | 1.3 | 117 | N | 4.98¶ |
| 2 | 15/F | NSAA | 24-155 | None | 110 | 1.2 | 41 | N | 4.72 |
Abbreviations: Acquired AA: idiopathic aplastic anemia; familial AA: non-Fanconi AA with family history of AA in first-degree relative; age, at time of study; —, data unavailable; ALG, antilymphocyte globulin; CyA, cyclosporin A; BMT (autol/autologous recovery), autologous marrow recovery after failed allogeneic BMT; BMT (donor hematopoiesis), successful engraftment with hematopoiesis of donor origin; none, supportive care only; MCV, mean cell volume in femtoliters; TD, red blood cell transfusion-dependent; ANC, absolute neutrophil count (×109/L); plts, platelet count (×109/L); Y, PNH positive; N, negative Ham test and normal expression of GPI-anchored proteins; (t), transient; Yh, hemolytic PNH; Y/pres, PNH-positive at presentation.
Definition of severity of AA3,67: nonsevere (NSAA), neutrophils >0.5 × 109/L; severe (SAA), neutrophils 0.2 to 0.5 × 109/L; very severe (VSAA), neutrophils <0.2 × 109/L.
Months since presentation.
Blood count at time of study.
Evidence for PNH and interval in months between presentation of AA and development of PNH positivity.
Patient with acquired cytogenetic abnormality (see also Table 2).
¶Duration of familial AA time in months since first detection of AA.
Prevalence of late clonal complications.
Thirteen patients who had been PNH-negative at presentation had acquired a secondary PNH clone, as shown by a positive Ham test, or by demonstration of GPI-anchored protein deficiency. Only 1 developed frank hemolysis, and in 2 cases evidence for PNH was transient, with expression of GPI-anchored proteins subsequently reverting to normal. Only 1 patient, an 8-year-old child with FA, had developed overt MDS at the time of the study, in association with abnormal bone marrow cytogenetics. However, a further 3 patients with normal cytogenetics at presentation had developed an abnormal karyotype (2 acquired AA and 1 familial AA). The abnormality was transient in 1 case.
Normal Controls
Peripheral blood samples were collected from 60 individuals, 1 to 90 years of age with normal blood counts, including mean cell volume (MCV) within the normal range.
Leukocyte Separation and DNA Extraction
Total leukocyte DNA was extracted from unseparated blood samples using a genomic DNA purification kit (Promega, Madison, WI). In some cases, DNA was extracted from purified populations of granulocytes and mononuclear cells (lymphocytes and monocytes), separated by Polymorphprep density gradient centrifugation (Nycomed Pharma AS, Oslo, Norway). Residual contaminating erythrocytes were lysed with EPICS lysis solution (Coulter Electronics, Ltd, Luton, UK). Ninety-six percent purity was achieved in both cell fractions, as shown by FACS analysis (data not shown). Integrity of extracted DNA was confirmed by agarose gel electrophoresis.
Mean Terminal Restriction Fragment Length (TRF) Measurement
Five-microgram aliquots of genomic DNA were digested to completion with RsaI (Promega) and resolved on 0.7% agarose gels, alongside35S-labeled DNA size markers (Amersham UK, Little Chalfont, UK). Completeness of digestion was confirmed by ethidium bromide staining. Gels were blotted onto Hybond N+ filters (Amersham) and alkali-fixed according to the manufacturer's instructions. Filters were prehybridized in 5× SSC/4× Denhardt's/0.5% sodium dodecyl sulfate (SDS)/100 mg/mL herring sperm DNA at 48°C and then hybridized with γ32P end-labeled (TTAGGG)4telomere repeat probe in 5× SSC overnight at 48°C. Filters were rinsed in 4× SSC at room temperature and then washed twice for 10 minutes in 4× SSC/0.1% SDS at 48°C. Filters were exposed to x-ray film (Hyperfilm MP; Amersham) for 24 to 72 hours. The mean TRF was determined by densitometric analysis of autoradiographs using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).25 Analysis was performed without knowledge of the identity of the samples, and normals were included on each gel to minimize the risk of bias and to confirm that results were comparable between different experiments, because there was usually insufficient sample to allow replicate TRF measurement.
Statistical Analysis
Linear regression analysis was performed using Fig.P software (Biosoft, Cambridge, UK). The Mann-Whitney U test was used in the comparison of telomere loss and MCV between subgroups, and the Wilcoxon matched-pairs test was used to compare results from different cell fractions (Stat-100 software; Biosoft).
RESULTS
Telomere Shortening With Age in Normals and Patients
As expected, there was progressive shortening of total leukocyte TRF with age in normals (Fig 1), equivalent to a loss of 36 bp per year (r = −.838, P < .0001), consistent with the rate of loss reported by others.24 31 TRF lengths in the patient group, although also related to age, were significantly shorter than normals (Fig 2), with 27 of 79 (34%) below the normal 95% confidence interval for age (P < .0001). Results from different subgroups of patients were then analyzed separately to identify any factors that might predispose to telomere loss in AA. TRF loss was expressed as the residual value between the observed TRF and the regression estimate of TRF for age in normals (TRFO-E), representing the difference in kilobases between the measured TRF and the age-appropriate point on the normal regression line (Fig 2). This facilitated comparison between normals and subgroups of patients of different ages by allowing for the normal decline of TRF length with age.
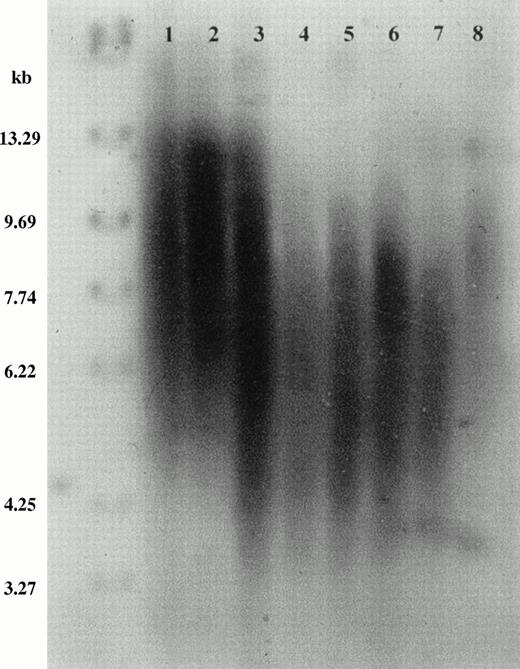
Telomere shortening with age and in AA. Southern blot ofRsa I-digested genomic DNA from unfractionated peripheral blood leukocytes, probed with (TTAGGG)4. Positions of molecular weight markers are shown on the left. Tracks 1 through 5, normal donors: 1, age 29 years; 2, age 28 years; 3, age 90 years; 4, age 73 years; 5, age 66 years. Tracks 6 through 8, patients with acquired AA: 6, age 30 years, 99 months from diagnosis; 7, 43 years, 53 months from diagnosis; 8, 37 years, 4 months from diagnosis.
Telomere shortening with age and in AA. Southern blot ofRsa I-digested genomic DNA from unfractionated peripheral blood leukocytes, probed with (TTAGGG)4. Positions of molecular weight markers are shown on the left. Tracks 1 through 5, normal donors: 1, age 29 years; 2, age 28 years; 3, age 90 years; 4, age 73 years; 5, age 66 years. Tracks 6 through 8, patients with acquired AA: 6, age 30 years, 99 months from diagnosis; 7, 43 years, 53 months from diagnosis; 8, 37 years, 4 months from diagnosis.
Scatter of TRF with age in normals and patients. TRF values from unfractionated peripheral blood leukocytes plotted against age in years at last birthday. Results from normal donors (n = 60; ▵) show a progressive shortening of TRF with age. The regression line for normals is shown as a solid line, and the 95% confidence limits are shown as broken lines (r = −.838, P < .0001). The slope equates to a telomere loss of 36 bp per year. (⧫) TRF values for patients (n = 79).
Scatter of TRF with age in normals and patients. TRF values from unfractionated peripheral blood leukocytes plotted against age in years at last birthday. Results from normal donors (n = 60; ▵) show a progressive shortening of TRF with age. The regression line for normals is shown as a solid line, and the 95% confidence limits are shown as broken lines (r = −.838, P < .0001). The slope equates to a telomere loss of 36 bp per year. (⧫) TRF values for patients (n = 79).
Acquired AA
This category excluded patients with FA or familial AA or those with de novo PNH without evidence for AA at presentation. Five patients who had successful allografts for AA were also considered separately, because such patients do not appear to be at the same risk of late clonal complications.17 23 However, this analysis did include 3 patients in whom allogeneic bone marrow transplantation (BMT) had resulted in graft rejection followed by autologous marrow recovery, as demonstrated by analysis of DNA polymorphisms (data not shown). Patients for whom hematological and clinical data were incomplete were also excluded from this analysis. There was a tight scatter of TRFO-E for normals (n = 60; mean, 0.069 kb; Fig 3). The TRFO-E values for the acquired aplastic anemia group showed a wider scatter, but were significantly lower than normals (n = 60; mean, −0.780 kb; P < .0001). There was no apparent correlation between TRFO-Eand age, whether at presentation or at time of testing, or with severity at presentation and treatment received (data not shown).
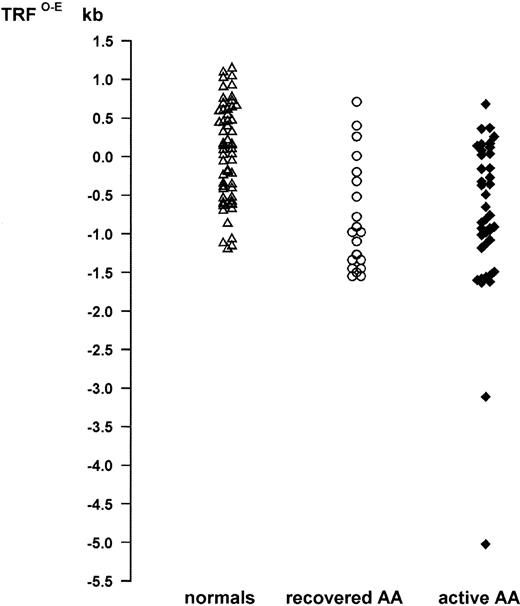
Telomere loss in AA. Telomere loss is expressed as distance in kilobases (TRFO-E) from the age-appropriate point on the normal regression line of TRF against age. Normals: (▵; n = 60), mean TRFO-E = 0.069 kb. Patients with acquired AA: active AA, persistent cytopenias (⧫; n = 40), mean TRFO-E = −0.776 kb; recovered AA, normal blood count (○; n = 20), mean TRFO-E = −0.789 kb.
Telomere loss in AA. Telomere loss is expressed as distance in kilobases (TRFO-E) from the age-appropriate point on the normal regression line of TRF against age. Normals: (▵; n = 60), mean TRFO-E = 0.069 kb. Patients with acquired AA: active AA, persistent cytopenias (⧫; n = 40), mean TRFO-E = −0.776 kb; recovered AA, normal blood count (○; n = 20), mean TRFO-E = −0.789 kb.
Hematological status at time of TRF measurement.
Patients with acquired AA were subdivided into those who had achieved complete remission (recovered AA; n = 20) and those who still had active AA, as shown by persistent cytopenia (active AA; n = 40). In this study, complete remission was defined by transfusion independence, with neutrophils greater than 1.8 × 109/L and platelets greater than 100 × 109/L.41 The recovered AA and active AA groups differed significantly with respect to MCV, endorsing the selection of these criteria. Median MCV for patients with active AA who were not red blood cell transfusion-dependent was 108 fL (mean, 106.5 fL; n = 25), whereas the median MCV for the recovered AA group was 96 fL (mean, 95.5 fL; n = 20; P < .01). The mean TRFO-E was equivalent for both groups (−0.776 kb active AA; −0.789 kb recovered AA; Fig 3). Thus, both groups had significant TRF loss when compared with normals (P < .0001), but there was no difference when active AA patients were compared with recovered AA (P = .546).
Evidence for progressive TRF loss.
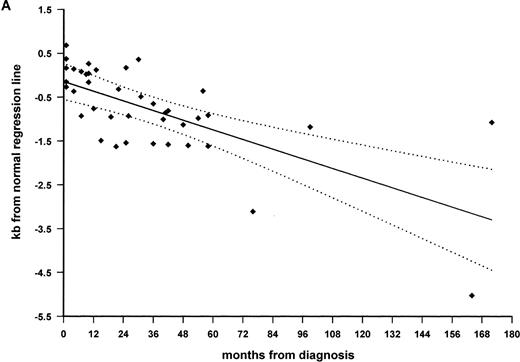
TRFO-E was plotted against time since diagnosis to investigate at what stage TRF loss had occurred during the clinical course. There was some evidence for progressive loss of TRF length when the whole acquired AA group was included (r = −.311;P < .02). However, when the subgroups of recovered AA and active AA were considered separately, a significant difference between the two groups became apparent (Fig 4A and B). There was strong correlation between TRF loss and months since diagnosis (r = −.685; P < .0001) in the active AA group of 40 patients with persistent cytopenia, studied at a median interval of 25 months after diagnosis (range, 1 to 172 months; Fig 4A). Although the 2 patients in this group with the greatest telomere loss had acquired cytogenetic abnormalities, which might influence telomere length regulation, there was still significant correlation with time when they were excluded from analysis (r = −.476;P < .005). The regression line intersects the y-axis close to zero (at TRFO-E = −0.145 kb), indicating that the TRF length is likely to be normal at presentation and that TRF loss occurs subsequently. The slope of the regression line equates to telomere shortening at 216 bp per year, in addition to the normal age-related annual loss of 36 bp. By contrast, the TRF loss in patients with recovered AA (n = 20) was stable over time, with no correlation between TRFO-E and interval since diagnosis (r = .156;P = .51; Fig 4B). These patients had been studied at a median interval of 59 months since diagnosis (range, 6 to 372 months). There was also no correlation between TRF loss and the interval between presentation and recovery of counts (data not shown).
Progressive telomeric shortening in acquired AA. Telomere loss, expressed as distance in kilobases (TRFO-E) from the age-appropriate point on the normal regression line of TRF against age, is plotted against months since presentation with idiopathic AA. (A) Patients with active AA, with persistent cytopenia (n = 40; ⧫), showing significant correlation between TRF loss and disease duration (r = −.685; P < .0001). The slope of the regression line, shown as a solid line (95% confidence band shown as dotted lines), predicts a loss of 216 bp per year, in addition to the normal age-related loss. (B) Patients with fully recovered blood counts (n = 20; ○), showing lack of correlation between telomere loss and interval since diagnosis.
Progressive telomeric shortening in acquired AA. Telomere loss, expressed as distance in kilobases (TRFO-E) from the age-appropriate point on the normal regression line of TRF against age, is plotted against months since presentation with idiopathic AA. (A) Patients with active AA, with persistent cytopenia (n = 40; ⧫), showing significant correlation between TRF loss and disease duration (r = −.685; P < .0001). The slope of the regression line, shown as a solid line (95% confidence band shown as dotted lines), predicts a loss of 216 bp per year, in addition to the normal age-related loss. (B) Patients with fully recovered blood counts (n = 20; ○), showing lack of correlation between telomere loss and interval since diagnosis.
TRF Loss After BMT
Eight patients in the total group had been treated by BMT 4 to 174 months before this study. Three had failed to engraft and subsequently showed evidence of autologous marrow recovery. These are included in the acquired AA group considered above. Five patients had successfully engrafted with donor marrow after matched sibling donor BMT. For these 5 patients, the mean TRFO-E calculated on the basis of recipient age was −1.07 kb (range, −2.28 to 0.14 kb) and −1.11 kb on the basis of donor age. There was no apparent correlation with time since BMT, suggesting stable loss, but the numbers are small.
TRF Loss and PNH
PNH at presentation.
Three patients had hemolytic PNH at presentation, without evidence for AA. TRFO-E ranged from −0.63 to −0.19 kb, with no apparent correlation with time. Four patients with AA who had been PNH-positive at presentation showed correlation of TRF loss with time (r = −.981; P < .02). These 4 patients are included in the above analysis of the acquired AA group, 1 in the recovered AA subgroup and 3 with persisting cytopenia.
Secondary PNH.
Thirteen patients within the study had become PNH-positive during the course of their illness, having been negative at presentation. In all but 1 case this represented the finding of a deficiency in GPI-anchored proteins on FACS analysis, rather than hemolytic PNH. The median interval between diagnosis and development of PNH was 34 months. Seven patients with secondary PNH were still cytopenic at the time of TRF measurement, whereas 6 had fully recovered blood counts at the time of study. All 6 had achieved normal counts before the PNH clone was detectable, including 1 patient who had autologous bone marrow recovery after a failed allogeneic BMT. PNH did not develop in any of the 5 patients who had successfully engrafted after BMT. There was no apparent association between the development of PNH positivity and age or severity at diagnosis or previous therapy (data not shown). The 13 patients with late onset PNH are included in the recovered AA and active AA subgroups analyzed above, but were also analyzed separately to determine whether TRF loss might be influenced by the development of a PNH clone. AA patients with secondary PNH showed a similar pattern of telomere loss to the whole acquired AA group. TRFO-E for the 6 with recovered AA and secondary PNH ranged from −1.52 to 0.71 kb, with no correlation between telomere loss and time since diagnosis (r = −.359; P = .48). The mean TRFO-E for the active AA/secondary PNH group was lower, at −1.14 kb (range, −5.02 to 0.17 kb), and there was evidence for progressive loss with time (r = −.954; P < .001). However, when the patient in this group with a cytogenetic abnormality was excluded from analysis, on the grounds that this might influence telomere loss, the correlation with time no longer achieved statistical significance (P = .09), but the numbers are small.
FA
The study included 6 patients with FA from 4 families, including 2 sibling pairs. Both sibling pairs showed telomere loss that was more pronounced in the older child, consistent with progressive TRF loss with age. One pair had TRFO-E values of −1.50 and −1.21 kb aged 15 and 5 years, respectively, and the other had TRFO-E of −2.24 and −1.95 kb aged 6 and 4 years, respectively. However, the other 2 FA patients had TRF values within the normal range. TRFO-E was 0.55 kb in a girl 8 years of age and 0.18 kb in another 18 years of age, despite both having progressed to end-stage bone marrow failure and the 8-year-old having subsequently developed MDS.
Non-Fanconi Familial AA
Significant telomere loss was apparent in 2 patients who gave a history of AA in a first-degree relative, but who tested negative for FA on induced breakage studies. One had TRFO-E of −4.13 kb (15 years of age) and the other had TRFO-E of −3.11 kb (36 years of age).
TRF Loss and Risk of MDS
An association between TRF loss and MDS in AA is suggested by the finding that, of the 5 patients in this study with TRF length less than 5.0 kb, 3 (2 with acquired AA and 1 with familial AA) had acquired an abnormal karyotype, although none had developed morphological evidence of MDS at the time of testing. The cytogenetic abnormality was transient in 1 patient and was not subsequently detected. The 2 patients with acquired, nonfamilial AA were both in the group with residual cytopenia (active AA), in which there was evidence for progressive telomere loss. Serial cytogenetic data were not available for every patient, reflecting the difficulties inherent in marrow chromosome studies in the presence of profound marrow hypoplasia. However, cytogenetic abnormalities were detected in a further 2 cases, neither of whom had excessive telomere loss (Table 2). One patient had pericentric inv(9), detected at presentation, which probably represents a normal polymorphic variant, seen in up to 1% of white caucasians.42 The other patient, who had underlying FA, did have frank MDS, with a hypercellular marrow and trilineage dysplasia, but no excess of blasts.
Cytogenetic Abnormalities
| Diagnosis . | Age (yrs) . | TRF (kb) . | Months Since Diagnosis . | Karyotype . |
|---|---|---|---|---|
| AA | 30 | 7.13 | 0 | Pericentric inv (9) |
| AA | 49 | 4.51 | 65 | 46 XY,add(10)(q22) |
| AA | 22 | 3.58 | 115 | del 2(q) (transient) |
| Familial AA | 36 | 4.98 | 73 | 47 XXX |
| FA | 8 | 9.65 | Aged 8 yrs | 48 XXXX |
| Diagnosis . | Age (yrs) . | TRF (kb) . | Months Since Diagnosis . | Karyotype . |
|---|---|---|---|---|
| AA | 30 | 7.13 | 0 | Pericentric inv (9) |
| AA | 49 | 4.51 | 65 | 46 XY,add(10)(q22) |
| AA | 22 | 3.58 | 115 | del 2(q) (transient) |
| Familial AA | 36 | 4.98 | 73 | 47 XXX |
| FA | 8 | 9.65 | Aged 8 yrs | 48 XXXX |
Details of cytogenetic abnormalities detected in the patient group, showing TRF length and the interval between diagnosis and the abnormality first being detected. The pericentric inversion of chromosome 9 was detected at presentation and probably reflects a normal variant.42
TRF Loss at the Level of the Hematopoietic Stem Cell
The above-reported data refer to TRF length measurement in total leukocyte DNA, extracted from blood samples containing differing proportions of granulocytes and lymphocytes. In some cases, the granulocytes represented less than 10% of the samples studied. TRF loss is therefore likely to have occurred in both myeloid and lymphoid cells, consistent with loss at the level of the hematopoietic stem cell. To investigate this further, DNA was extracted from peripheral blood mononuclear cells (MNC) and granulocytes separated on density gradients. In normals (n = 6), there was no significant difference between the TRF lengths (P = .345) measured in granulocyte and mononuclear cell fractions (Fig 5), consistent with previous reports.43
Telomere length in separated granulocyte and mononuclear cell fractions. Southern blot of Rsa I-digested genomic DNA extracted from density gradient separated peripheral blood granulocytes or mononuclear (MN) cells, probed with (TTAGGG)4, showing equivalent results for both cell fractions. Positions of molecular weight markers are shown on the left. Tracks 1 and 2: MN (1) and granulocytes (2) from normal donor age 45 years; tracks 3 and 4: MN (3) and granulocytes (4) from normal donor age 54 years; tracks 5 and 6: MN (5) and granulocytes (6) from normal donor age 57 years.
Telomere length in separated granulocyte and mononuclear cell fractions. Southern blot of Rsa I-digested genomic DNA extracted from density gradient separated peripheral blood granulocytes or mononuclear (MN) cells, probed with (TTAGGG)4, showing equivalent results for both cell fractions. Positions of molecular weight markers are shown on the left. Tracks 1 and 2: MN (1) and granulocytes (2) from normal donor age 45 years; tracks 3 and 4: MN (3) and granulocytes (4) from normal donor age 54 years; tracks 5 and 6: MN (5) and granulocytes (6) from normal donor age 57 years.
One patient with acquired AA was studied shortly after presentation, before undergoing BMT from her identical twin. There was a 1.8-kb difference between the patient and her twin with respect to TRF from granulocytes (TRFgran), although their values for MNC TRF (TRFMN) were comparable and within the normal range for age (Table 3). Because there is a strong genetic influence on TRF length,44 the difference affecting the granulocyte fraction is likely to be AA related.
Telomere Loss Affecting Granulocytes But Not Mononuclear Cells in a Patient Studied Shortly After Diagnosis in Comparison With Her Identical Twin
| . | Patient . | Twin . |
|---|---|---|
| TRFgran (kb) | 7.10 | 8.97 |
| TRFMN (kb) | 9.13 | 8.82 |
| . | Patient . | Twin . |
|---|---|---|
| TRFgran (kb) | 7.10 | 8.97 |
| TRFMN (kb) | 9.13 | 8.82 |
Separated cell fractions were then studied from a total of 17 patients with nonfamilial acquired AA (9 active AA; 8 recovered AA). TRF values were again found to be significantly lower in the granulocytes than in the mononuclear cell fraction (P < .001). This difference was significant (P < .05) in both active AA and recovered AA groups. Although there was no significant difference between TRFO-E from normals and MNC from the 17 AA patients, there was evidence for progressive TRFMN loss in AA, in that there was correlation between MNC TRFO-E and months since diagnosis (r = −.555, P < .05). In addition, when the 2 patients who had been studied within 1 month of diagnosis were excluded from analysis, there was significant TRF loss in AA MNC in comparison with normals (AA MNC mean TRFO-E = −0.314; P = .044).
DISCUSSION
We have found evidence for significant telomere shortening in a wide cross-section of patients with AA of varying disease severity and duration. Moreover, we have shown that there is evidence for progressive telomere erosion in patients with ongoing disease. Our results suggest that a proportion of the excessive telomere loss occurs at the level of the hematopoietic stem cell, in that there was evidence for telomere shortening affecting both granulocyte and mononuclear cell fractions, although it was more pronounced in granulocytes. This discrepancy between the different cell fractions could be in part attributable to the longer half life of lymphocytes or to differing regulation of telomere length between cell types.
Telomeres are specialized protein-DNA complex structures at the ends of eukaryotic chromosomes, with a probable role in preventing degradation and aberrant recombination of the chromosome ends.45Because the ends of linear chromosomes cannot be replicated by normal DNA polymerases,46,47 the amount of telomeric DNA decreases by 50 to 100 bp with each cell division,25,27,38 unless replaced by telomerase, a specialized polymerase with an integral RNA component containing a template element directing the addition of telomeric repeats to chromosome ends48 (reviewed by Greider49). There is a relative loss of telomere DNA in hematopoietic progenitor cells from adult bone marrow compared with fetal liver or umbilical cord cells, correlating with ontogeny-related differences in proliferative potential.32,33 The length of telomeric repeats has been shown to decrease with cell division both in vivo and in vitro, as evidenced by age-related decreases in mean telomere length of blood and marrow cells24,31 and by telomere loss during in vitro hematopoietic cell proliferation.32 34
The simplest explanation for our findings is that, in AA, hematopoietic progenitor cells undergo a higher than normal number of cell divisions to achieve the generation of mature end cells. The rate of telomere loss in the subgroup of patients with active AA would predict a sevenfold increase in the number of cell divisions in comparison with normal. One potential mechanism could be excessive wastage of stem cells and committed progenitor cells during the process of hematopoiesis in AA, whether due to increased apoptosis, as described in AA CD34+ cells,50,51 or to a more direct process of immune destruction.52,53 In addition, with increased loss of primitive progenitor cells, a compensatory increase in cycling of more mature progenitor cells might result in further telomere loss. Alternatively, disruption of the normal cycling of hematopoietic stem cells could perturb the normal mechanisms for telomere maintenance. Although telomerase activity can be detected in normal hematopoietic progenitor cells,34,39,54-57 the factors that determine telomere loss and replenishment in hematopoiesis are still unclear. Ex vivo expansion of normal CD34+ cells has been shown to result in loss of telomeric repeat sequence,32,34 but there is some evidence for active maintenance of telomere length, as suggested by the higher rate of loss during the weeks of culture in which telomerase activity was lowest.34
We found that the rate of telomere shortening reverted to the normal age-related loss in patients who had apparently made a full hematological recovery from aplastic anemia. However, the extent of telomere loss in this group was greater than would be predicted simply from an increased rate of loss during the period of active disease. TRF length was on average nearly 800 bp shorter than expected for age, a loss that would take more than 3 years at a rate of 216 bp per year, whereas the median time to achieve normal blood counts in this group was 18 months. In addition, there was no apparent correlation between telomere loss and the time to remission (data not shown). It is possible that the replicative stress associated with the process of marrow recovery, with reconstitution of normal cellularity from a reduced number of hematopoietic stem cells, is the cause of irreversible telomere loss. This would be consistent with the results from patients in our study who had achieved apparently normal hematopoiesis of fully donor origin after successful allogeneic BMT. This group of 5 patients had a mean posttransplant TRF length of greater than 1 kb shorter than predicted for donor age. Equivalent findings have been reported in patients undergoing stem cell transplant for disorders other than AA.58-60 In one report of 11 donor-recipient pairs studied posttransplant, the recipient group showed significant TRF loss in comparison with their donors studied at the same time.58 Unlike our study, which relies on a calculated difference between TRF length and the predicted value for age, Notaro et al58 showed a clear difference between hematopoietic cells derived from the same stem cell pool. In addition, they made the important observation that the extent of TRF reduction was inversely correlated with the number of nucleated cells transplanted, thus providing supporting evidence for irreversible telomere loss occurring as a consequence of the expansion of normal hematopoietic stem cells in vivo under conditions of replicative stress.
A report of the EBMT severe AA (SAA) working group showed a cumulative incidence of MDS of 9.6% at 10 years, excluding those diagnosed within 6 months of presentation.23 All cases of MDS occurred in patients who had been treated with immune suppressive therapy (IS) rather than BMT. The risk increased with age, age being a continuous variable, and with multiple courses of IS, splenectomy, and the addition of androgens, but there were no data as to whether the patients had ever achieved normal blood counts. However, in another study, significant prognostic variables for the development of MDS included neutropenia and macrocytosis,7 which would be analogous to our active AA subgroup, characterized by persistent cytopenias and macrocytosis. Similarly, multiple courses of IS and other therapies are more likely in patients who fail to achieve remission. Thus, on the basis of our results, we would predict that these prognostic variables for MDS in AA would also be associated with progressive telomere loss, consistent with telomere erosion being a factor in the pathogenesis of MDS occurring as a late clonal complication of AA. The normal telomere loss with age could explain why age acts as a continuous risk variable; a short TRF at onset of the aplastic anemia would reduce the degree of additional erosion required to approach a critical level.
Telomeres are believed to play an integral role in the maintenance of chromosome integrity.45 It is of interest in this context that the cytogenetic abnormalities acquired during the course of aplastic anemia are generally characterised by aneuploidy,19,61 rather than balanced translocations, including the patients in our own study. MDS arising on a background of aplastic anemia thus resembles the class of therapy-related MDS, which also increases in frequency with age,62 and is characterized by numerical chromosomal abnormalities, such as monosomy of chromosomes 5 and 763 or trisomy 8.62,64Telomere loss is a plausible factor in the pathogenesis of these categories of MDS, and certainly the patients within our study with cytogenetic abnormalities acquired on a background of idiopathic AA all had TRF less than 5.0 kb. However, none had monosomy 7 or monosomy 5, and none had developed overt MDS. The observed cytogenetic abnormalities are more likely to be a reflection of the genetic instability caused by telomere erosion, rather than signifying the onset of MDS. In addition, the del 2(q) abnormality detected in 1 patient in our study was a transient finding. According to the telomere model, the transient nature of such clones could be explained by replicative cell senescence in the presence of critical telomere shortening, if the abnormality did not allow the cells to bypass the Hayfick limit.37-39
Telomere loss was also prominent in the 2 patients in this study with familial AA, but without evidence for FA. By contrast, of the 6 patients with FA, 2 had normal TRF for age, despite having end-stage marrow failure, including 1 who had developed a cytogenetically abnormal clone. However, 2 pairs of siblings with FA in our study did show evidence for telomere loss, more pronounced in the older child in each case, which would be consistent with progressive telomere erosion. In addition, telomere shortening has recently been reported in 12 patients with FA.65 Further studies on a larger group of patients are warranted; in particular, it would be very interesting to determine whether different FA complementation groups show differing patterns of telomere loss.
Thirteen (20%) of the patients in our study with acquired AA developed secondary PNH during the course of their aplastic anemia, although in only 1 case was there active hemolysis. These patients were represented in both the active AA and recovered AA groups, and there was no apparent association between telomere loss and the acquisition of a PNH clone, in keeping with the previous impression that secondary PNH and MDS in AA have different pathogenetic mechanisms, as shown by different prognostic variables for their development.6,7 The apparent advantage of a PNH clone in an aplastic marrow66 is therefore unlikely to be directly related to telomere loss. However, this question should be further addressed by the study of telomere lengths in different populations of cells sorted on the basis of normal or defective expression of GPI-anchored proteins.
Further studies of telomeres in AA are thus clearly warranted, both to elucidate the mechanism of telomere loss in aplastic anemia, and its possible association with the development of MDS. It will be important to follow individual patients sequentially to analyze the events that occur during active disease and at the time of full hematological recovery. If telomere loss is confirmed as an important risk factor in MDS arising as a late clonal complication of AA, then this patient group provides an important in vivo model20 in which to study the earliest events of leukemogenesis in relation to telomere loss, including the timing of the acquisition of mutations, the emergence of clonal hematopoiesis, and the development of overt MDS and progression to leukemia.
ACKNOWLEDGMENT
The authors thank Drs Rose Ann Padua and Tim Rutherford for helpful discussion.
Supported by the Leukaemia Research Fund.
Address reprint requests to Sarah E. Ball, DM, Division of Haematology, Department of Cellular and Molecular Sciences, St George's Hospital Medical School, Cranmer Terrace, London SW17 0RE, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal