To the Editor:
Cazzola et al1 recently reported two kindreds with hereditary hyperferritinemia cataract syndrome (HHCS) associated with novel point mutations within a regulatory stem-loop motif in the L-ferritin mRNA termed the iron-responsive element (IRE). Affected individuals showed a characteristic clinical phenotype of elevated serum ferritin concentration and cataract developing early in life. The proposed pathogenesis of this disorder is that nucleotide substitutions within the IRE disrupt its specific interaction with the cytoplasmic iron regulatory protein (IRP). Failure of optimal IRP-IRE binding in turn leads to failure of suppression of L-ferritin translation.
There are now increasing numbers of reports that describe the genotype-phenotype relationship in kindreds with naturally occurring IRE mutations, and as Cazzola et al1 report, the phenotype varies with the position of the mutation in the IRE. These descriptions now provide clinical data that support the structural model of the IRE-IRP interaction deduced from in vitro binding studies using artificially created IRE mutants.2-4
We have identified two further kindreds with HHCS and novel mutations in the L-ferritin IRE that further support this model.
Kindred I.
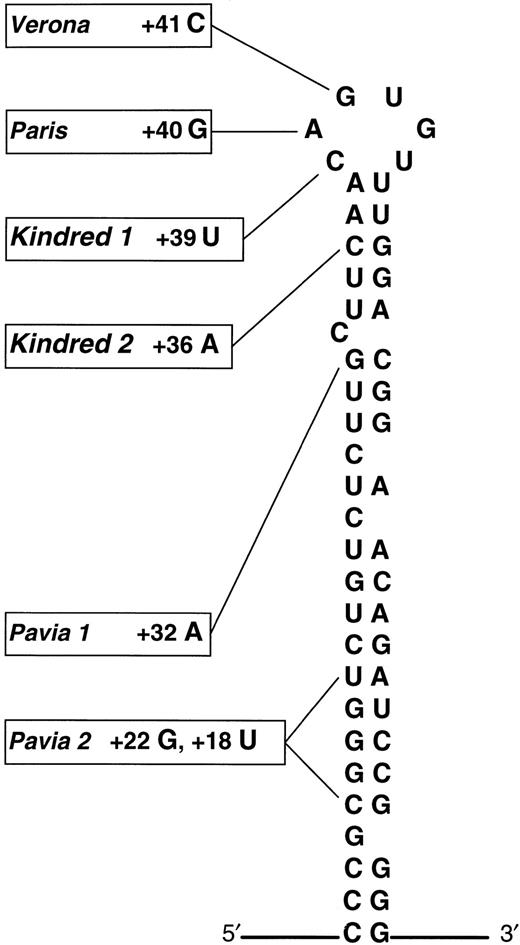
The 51-year-old male proband of English origin developed visual symptoms in his mid-thirties from cataracts, but was otherwise asymptomatic. Investigations revealed a serum ferritin of 1,389 μg/L but normal transferrin saturation. Similar abnormalities were noted in the proband's sister, and liver biopsy specimens from both these individuals showed no iron overload. Sequencing of genomic DNA from the proband showed a heterozygous point mutation that corresponded to a +39 C → U substitution in the L-ferritin mRNA.
Kindred 2.
The 42-year-old female proband of English origin was investigated for anemia detected at one of her regular blood transfusion sessions. Although her red cell indices and transferrin saturation were consistent with mild iron deficiency, her serum ferritin was elevated at 1,020 μg/L. The proband herself had had previous surgical extraction of cataracts, and there were premature cataracts in 8 other family members. The son of the proband required cataract extraction at 5 years old. Hyperferritinemia was confirmed only in family members with cataract. Analysis of genomic DNA also showed a heterozygous point mutation, this time corresponding to a +36 C → A substitution in the L-ferritin mRNA. This substitution created an Mse I restriction site within the amplified sequence, and restriction digests from additional family members confirmed that the substitution segregated with the hyperferritinemia-cataract phenotype.
The nucleotide substitutions detected in kindreds 1 and 2 lie in the apical loop and upper stem of the IRE, respectively (Fig1). We note that in both kindreds individuals display a severe phenotype, and this is consistent with the observations of Cazzola et al that mutations near the apex of the IRE result in higher serum ferritin concentrations and denser cataracts. These results also comply with data from in vitro binding studies; nucleotide substitutions in the apical loop of the IRE dramatically reduce IRP affinity, consistent with its putative role as the IRP binding site.2,3 Individuals from kindred 1 with a naturally occurring mutation at this site are therefore expected to have a severe defect in L-ferritin regulation. In the case of kindred 2, artificially created nucleotide substitutions in the IRE upper stem exert a profound effect on IRP binding in vitro, but only if complementary base pairing in the stem is disrupted.4Pairing of nucleotides may facilitate IRE-IRP binding by maintaining an optimum secondary structure of the IRE. The severe phenotype of kindred 2, who have a naturally occurring noncomplementary nucleotide substitution close to the IRP binding site, may therefore reflect a broader structural derangement of the IRE.
Schematic representation of the L-ferritin IRE adapted from Cazzola et al showing the updated distribution of genotypic abnormalities in HHCS. Substitutions +39 C → U in kindred 1 and +36 C → A lie within the apical loop and upper stem, respectively. (Adapted and reprinted with permission.1)
Schematic representation of the L-ferritin IRE adapted from Cazzola et al showing the updated distribution of genotypic abnormalities in HHCS. Substitutions +39 C → U in kindred 1 and +36 C → A lie within the apical loop and upper stem, respectively. (Adapted and reprinted with permission.1)
Our kindreds help clarify the relationship between genotype and phenotype in HHCS, and the description of two novel mutations illustrates the increasing genotypic diversity of this disorder. The severity of the phenotype of our patients and the position of the nucleotide substitution support the existing models of IRE-IRP interaction.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal