Abstract
Anaplastic, CD30+, large-cell lymphoma is now a well-recognized pathologic entity that accounts for 2% to 8% of all lymphomas. Recent progress has been made in the understanding of certain biologic features found in anaplastic large-cell lymphoma, but information about its clinical behavior, in comparison to other large-cell lymphomas, is limited. The pathologic review of a large multicenter study of the treatment of aggressive lymphoma identified 146 cases of anaplastic large-cell lymphoma (ALCL) on the basis of morphology and CD30 expression. We compared initial presentation, immunophenotype, and clinical outcome of these cases with those of the 1,695 nonanaplastic diffuse large-cell lymphomas (non-ALCL) included in the same trial. Patients with ALCL were more likely to be male (P = .018) and were younger (P < .0001) than those with non-ALCL. B symptoms were more frequent in ALCL (P = .006). Skin (P < .0001) and lung (P < .05) involvement was also more frequent in ALCL, but frequency of bone marrow involvement was identical (P = .5). Tumor cell phenotype was B in 56 cases (38%), T in 49 cases (34%), and null in 33 cases (22%). Response to chemotherapy (P = .001), event-free survival (P = .006), and overall survival (P = .0004) were better for ALCL than for non-ALCL. Multivariate analyses identified anaplastic character as an independent factor that predicted a longer survival. Tumor cell phenotype did not influence event-free survival (P = .72) or overall survival (P = .83). ALCL in adults is a clinicopathologic entity which, independent of its phenotypic characteristics, has a better outcome than other diffuse large-cell lymphomas.
ANAPLASTIC large cell lymphoma (ALCL), also called Ki-1 lymphoma, is a morphologically and immunologically distinct subset of non-Hodgkin's lymphoma (NHL) originally described by Stein et al,1 which accounts for 2% to 8% of all lymphomas. This disease is characterized by the proliferation of pleomorphic large neoplastic lymphoid cells, which strongly express the CD30 antigen (Ki-1 antigen), usually growing in a cohesive pattern and preferentially spreading in the lymph node sinuses.1-4
Recent studies have shown that ALCL is an heterogeneous group of diseases, differing in their histology, phenotype, cytogenetics, and clinical course.5 Morphologic heterogeneity of ALCL explains why many cases were formerly diagnosed as malignant histiocytosis, Hodgkin's disease, or nonhematopoietic tumors.4-6 Although a majority of ALCL express antigens of T- or B-cell lineage, some cases may lack lymphoid antigens and very rare cases express both T- and B-cell markers.6 The recently proposed Revised European-American Lymphoma (REAL) classification of lymphoid neoplasms4 included the B-cell type of ALCL among the morphologic variants of diffuse large B-cell lymphoma, limiting the term of anaplastic large cell lymphoma to T- and null-cell types. The translocation t(2; 5)(p23; q35), initially described in T-cell ALCL,7 has now been cloned.8 The specificity of this translocation in the spectrum of ALCL and Hodgkin's disease is currently under investigation.9 10
Although rare cases of ALCL occur in patients with a history of previous lymphoma, most cases are qualified as primary. Two distinct clinical forms of primary ALCL are now recognized: limited to the skin and systemic.4 The purely cutaneous ALCL may be indistinguishable from lymphomatoid papulomatosis and regressing atypical histiocytosis and, like these two entities, may undergo spontaneous regression.11 Conversely, systemic ALCL has an aggressive clinical course and patients frequently present with systemic symptoms, advanced-stage disease, and extranodal localizations.5,12 Response to treatment and overall survival of systemic ALCL in children is good. In adults, however, whether or not prognosis of ALCL is different from other diffuse large cell lymphomas is still controversial.12-27
The purpose of this study was to compare clinical, immunopathologic, and evolutive features of 146 adult patients with primary ALCL to 1,695 cases of nonanaplastic diffuse large-cell lymphoma (non-ALCL). All of these patients were enrolled in a prospective multicenter study and were uniformly staged and treated.
MATERIALS AND METHODS
Patients. In October 1987, the Groupe d'Etudes des Lymphomes de l'Adulte (GELA) initiated a prospective multicenter study, which was designated as LNH-87 study, for patients with aggressive NHL, in which patients were stratified in four treatment groups according to age and prognostic factors. Newly diagnosed patients older than 16 years with intermediate- or high-grade lymphoma were considered eligible for the study. Patients were not included if they had a positive serology to human immunodeficiency virus, a concomitant or previous cancer (except in situ cervix carcinoma or skin epithelioma), congestive heart failure, recent myocardial infarction or conduction abnormalities, uncontrolled diabetes mellitus, and liver or kidney failure.
A review of histologic documents by three independent pathologists from the GELA was planned in the protocol and performed in 88% of the 3,232 patients included. Lymphomas were classified according to the Working Formulation,28 but ALCL, as defined by the updated Kiel classification,29 was added to the categories of this classification. Only patients with a confirmed diagnosis of ALCL were selected for the present study.
Histopathologic and phenotypic analysis. Slides from routinely paraffin-embedded tissues were stained with hematoxylin-eosin, Giemsa, and Gordon-Sweet. For all cases with anaplastic morphology, slides were available for phenotypic study on paraffin sections using immunoperoxidase or alkaline-phosphatase antialkaline phosphatase (APAAP) labeling and a panel of paraffin-resistant antibodies were applied, which included CD45/LC (leukocyte common antigen, MO701; Dako, Glostrup, Denmark), CD30/Ber-H2 (MO751; Dako), CD20/L26 (MO786), CD45R0/UCHL1 (MO742; Dako), CD3 (AO452; Dako), CD68/KP1 (MO814; Dako), CD15 (MO733; Dako), EMA/E29 (epithelial membrane antigen, MO613; Dako), and BNH9 (MO743; Immunotech, Lumigny, France). Additional phenotyping could be obtained on frozen sections in 49 cases.
The histologic and phenotypic criteria used for the definition of ALCL were those defined by Stein et al.1 Briefly, the diagnosis of ALCL was based both on the histopathologic presence of large cells with anaplastic morphology (pleomorphic nuclei, proeminent nucleoli, and abundant cytoplasm) frequently involving preferentially lymph node sinuses and on the expression of CD30 antigen by the tumor cells. Hodgkin's related30 or Hodgkin's-like variants4 of ALCL were excluded from this study.
Lymphomas were considered of B-cell lineage when tumor cells expressed CD20. They were considered of T-cell origin when tumor cells expressed CD3 or, when CD3 negative, expressed CD45RO, but not CD20 and CD68. On frozen sections, cases were considered as T-cell lymphoma when they expressed CD3 or at least two other T-cell antigens (CD2, CD4, CD5, or CD7). Histiocytic type was suspected when only CD45RO and CD68 were expressed on paraffin sections and CD4 was the only T-cell marker expressed on frozen sections.
The group of patients used for comparison consisted of the nonanaplastic diffuse large-cell lymphomas (diffuse large-cell and immunoblastic types of the Working Formulation).27 These cases were only studied on paraffin sections.
Staging. The extent of the disease was studied by physical examination, computerized tomographic scan of chest and abdomen, cerebrospinal fluid examination, and bone marrow biopsy. The number of extranodal sites and the diameter of the largest tumor mass was determined. Patients were staged according to the Ann Arbor classification.31 Performance status was assessed according to the Eastern Cooperative Oncology Group (ECOG) scale (0 to 4).32 Serum lactate dehydrogenase (LDH) level was expressed as the ratio over the maximum normal value.
Treatment and assessment of response. Patients in the LNH-87 study were enrolled in four different randomized trials according to age and to the presence of any of the following adverse prognostic factors: ECOG performance status of 2 to 4, two or more extranodal sites, tumor burden ≥10 cm in largest dimension, bone marrow or central nervous system involvement, and Burkitt's or lymphoblastic subtypes.
Patients in group 1 were younger than 70 years and had no adverse prognostic factor. Treatment in this group randomly compared the LNH-84 regimen33 consisting of three cycles of ACVBP (doxorubicine, cyclophosphamide, vindesine, bleomycin, prednisone) chemotherapy followed by a consolidation phase with high-dose methotrexate, ifosfamide plus etoposide, asparaginase and cytosine-arabinoside, versus eight cycles of m-BACOD (methotrexate, bleomycin, cyclophosphamide, vincristine, dexamethasone) as described by Shipp et al.34
Patients in group 2 were younger than 55 years and had at least one of the adverse prognostic factors.35 The induction treatment was randomized between ACVBP and NCVBP (mitoxantrone, cyclophosphamide, vindesine, bleomycin, prednisone) where mitoxantrone replaced doxorubicin. Patients in complete remission were then randomly assigned to receive either the LNH-84 consolidation chemotherapy or intensive consolidation and autologous bone marrow support.35
Patients in group 3 were aged between 55 and 70 years and had at least one adverse prognostic factor. Patients in this group were randomly assigned to receive either the LNH-84 regimen or an alternating chemotherapy VIMMM (VM26, ifosfamide, mitoxantrone, methyl-gag, methotrexate)/ACVBP.36
Patients in group 4 were patients over 70 years of age. This study prospectively investigated the effect of the addition of tetrahydropyranyl (THP)-doxorubicin to the CVP regimen (cyclophosphamide, vincristine, prednisone.37
Criteria for assessment of response to treatment have been previously described.35 At the time of this analysis, the median follow-up period was 55 months, and the maximal follow-up period was 8 years.
Statistical methods. Patient characteristics and complete remission rates were compared by χ2 tests. The event-free survival was measured from the date of randomization to disease progression, relapse, or death. The overall survival was measured from the date of randomization to death from any cause or date of last follow-up evaluation. The rates of survival were estimated by the method of Kaplan and Meier38 and compared by log-rank tests. Stepwise Cox proportional-hazards regression analysis with the overall survival as the dependent variable was used to adjust the effect of anaplastic character for potential independent prognostic factors.39 Tests for comparison were regarded as significant if the two-sided P value was less than .05.
RESULTS
Among the 1,841 patients with diffuse large-cell lymphoma included in the LNH-87 trial who had a histologic review, 146 (8%) were classified as having ALCL.
Clinical presentation. Main clinical characteristics of patients with ALCL as compared with those with non-ALCL are listed in Table 1. The male/female ratio was 1.81:1 in ALCL, the male predominance being significantly more important than in non-ALCL. Patients with ALCL were significantly younger than those with non-ALCL (P < .0001). B symptoms were more frequent in ALCL. As in non-ALCL, a majority of patients with ALCL had a disseminated disease (Ann Arbor stage III or IV) and a limited number of extranodal sites. However, skin and lung lesions were more frequent in ALCL, occurring respectively in 15% (P < .0001) and 16% (P < .05) of these patients. Conversely, lesions of the digestive tract were less frequently found in ALCL (P < .002). Bone marrow involvement was detected in 15% of the patients with ALCL as compared with 17% of the patients with non-ALCL (P = .5). ALCL and non-ALCL had a similar distribution in the different risk groups of the lymphoma international prognostic index.32
Characteristics of Patients With Either ALCL or Non-ALCL
| Characteristic . | Anaplastic . | Nonanaplastic . | P . |
|---|---|---|---|
| No. | 146 | 1,695 | |
| n (%) | n (%) | ||
| Sex | |||
| M | 94 (64) | 918 (54) | |
| F | 52 (36) | 777 (46) | .018 |
| Age (yr) | |||
| ≤60 | 107 (73) | 988 (58) | |
| >60 | 39 (27) | 707 (42) | <.0001 |
| Performance status* | |||
| 0-1 | 103 (72) | 1,221 (74) | |
| >1 | 40 (28) | 420 (26) | .5 |
| B symptoms* | |||
| Absent | 67 (47) | 939 (58) | |
| Present | 77 (53) | 673 (42) | .006 |
| Mediastinal involvement | 55 (38) | 539 (32) | .2 |
| Extranodal sites | |||
| Bone marrow | 21 (15) | 268 (17) | .5 |
| Liver | 17 (12) | 188 (11) | .8 |
| Spleen | 26 (18) | 333 (20) | .6 |
| Central nervous system | 11 (8) | 129 (8) | 1 |
| Skin | 22 (15) | 93 (5) | <.0001 |
| Lung | 23 (16) | 210 (12) | .05 |
| Gut | 10 (7) | 277 (16) | .002 |
| Bone | 12 (8) | 138 (8) | 1 |
| Waldeyer's ring | 11 (8) | 187 (11) | .2 |
| No. of extranodal sites | |||
| 0-1 site | 112 (77) | 1,290 (76) | |
| >1 sites | 34 (23) | 405 (24) | .9 |
| Ann Arbor stage | |||
| I-II | 59 (40) | 683 (41) | |
| III-IV | 87 (60) | 969 (59) | .8 |
| LDH level* | |||
| ≤1 × normal | 69 (50) | 659 (43) | |
| >1 × normal | 68 (50) | 877 (57) | .09 |
| Albumin level* | |||
| ≤30 g/L | 34 (24) | 271 (17) | |
| >30 g/L | 106 (76) | 1,294 (83) | .04 |
| International prognostic index31* | |||
| Low | 63 (45) | 564 (36) | |
| Low-intermediate | 28 (20) | 405 (26) | |
| High-intermediate | 23 (17) | 349 (22) | |
| High | 25 (18) | 253 (16) | .08 |
| Treatment received | |||
| ACVBP | 69 (47) | 714 (42) | |
| m-BACOD | 22 (15) | 187 (11) | |
| NCVBP | 22 (15) | 251 (15) | |
| VIMMM | 18 (12) | 241 (14) | |
| CVP/CTVP | 15 (10) | 302 (18) | .2 |
| Characteristic . | Anaplastic . | Nonanaplastic . | P . |
|---|---|---|---|
| No. | 146 | 1,695 | |
| n (%) | n (%) | ||
| Sex | |||
| M | 94 (64) | 918 (54) | |
| F | 52 (36) | 777 (46) | .018 |
| Age (yr) | |||
| ≤60 | 107 (73) | 988 (58) | |
| >60 | 39 (27) | 707 (42) | <.0001 |
| Performance status* | |||
| 0-1 | 103 (72) | 1,221 (74) | |
| >1 | 40 (28) | 420 (26) | .5 |
| B symptoms* | |||
| Absent | 67 (47) | 939 (58) | |
| Present | 77 (53) | 673 (42) | .006 |
| Mediastinal involvement | 55 (38) | 539 (32) | .2 |
| Extranodal sites | |||
| Bone marrow | 21 (15) | 268 (17) | .5 |
| Liver | 17 (12) | 188 (11) | .8 |
| Spleen | 26 (18) | 333 (20) | .6 |
| Central nervous system | 11 (8) | 129 (8) | 1 |
| Skin | 22 (15) | 93 (5) | <.0001 |
| Lung | 23 (16) | 210 (12) | .05 |
| Gut | 10 (7) | 277 (16) | .002 |
| Bone | 12 (8) | 138 (8) | 1 |
| Waldeyer's ring | 11 (8) | 187 (11) | .2 |
| No. of extranodal sites | |||
| 0-1 site | 112 (77) | 1,290 (76) | |
| >1 sites | 34 (23) | 405 (24) | .9 |
| Ann Arbor stage | |||
| I-II | 59 (40) | 683 (41) | |
| III-IV | 87 (60) | 969 (59) | .8 |
| LDH level* | |||
| ≤1 × normal | 69 (50) | 659 (43) | |
| >1 × normal | 68 (50) | 877 (57) | .09 |
| Albumin level* | |||
| ≤30 g/L | 34 (24) | 271 (17) | |
| >30 g/L | 106 (76) | 1,294 (83) | .04 |
| International prognostic index31* | |||
| Low | 63 (45) | 564 (36) | |
| Low-intermediate | 28 (20) | 405 (26) | |
| High-intermediate | 23 (17) | 349 (22) | |
| High | 25 (18) | 253 (16) | .08 |
| Treatment received | |||
| ACVBP | 69 (47) | 714 (42) | |
| m-BACOD | 22 (15) | 187 (11) | |
| NCVBP | 22 (15) | 251 (15) | |
| VIMMM | 18 (12) | 241 (14) | |
| CVP/CTVP | 15 (10) | 302 (18) | .2 |
Data were unavailable for some patients.
Histopathology and immunophenotype. Most cases (129 of 146) were consistent with the common type of ALCL.6 Nine cases were considered as small-cell variant,40 five cases as giant cell rich variant, and the remaining three as lymphohistiocytic type.6 We were unable to delineate the pleomorphic and the monomorphic types as described by Chott et al15 or the pale cell and basophilic cell types as described by Chan et al.13 Partial involvement with pseudo-metastatic pattern was found in 33 patients. Additional morphologic features were: important necrosis in 6 cases, erythrophagocytosis in 3, numerous eosinophils in 3, and angiocentrism in 1 case.
By definition, all cases of ALCL had evidence of expression of CD30 by tumor cells. In each specimen, 75% to 100% of the tumor cells reacted with the anti-CD30 antibodies as a dot in the Golgi zone and/or a membrane staining. Seventy-eight percent of the cases expressed CD45, 42% EMA, 23.5% BNH9, and 16.5% CD15. In the eight CD45 negative cases with a null phenotype, the lack of cytokeratin and melanoma markers was assessed.
As shown in Table 2, the phenotype was considered as B in 56 cases (38%). T-lineage antigens were detected in 49 cases (34%), confirmed on frozen material in 19 cases: 31 cases reacted with CD3 antibodies (with or without CD45RO coexpression), 12 cases with CD45RO only (in the absence of B-cell and CD68 markers) and six cases disclosed T-cell antigens on frozen sections only. B- and T-cell markers were coexpressed in four cases (3%) studied on both paraffin and frozen sections. Four other cases (3%), studied both on paraffin and frozen sections, were considered to have a histiocytic origin. The remaining 33 cases (23%) lacked B- and T-cell antigens expression using our limited panel. In the non-ALCL group, 1,368 cases (81%) expressed B-cell antigens, 144 T-cell antigens (8.5%), and 183 cases (11%) did not show detectable lineage antigens on paraffin-embedded sections.
Characteristics of Patients With Anaplastic Large-Cell Lymphoma According to the Phenotype of Tumor Cells
| Characteristic . | B . | T . | Null . | P . |
|---|---|---|---|---|
| No.* | 56 | 49 | 33 | |
| Sex | ||||
| M | 30 | 38 | 19 | |
| F | 26 | 11 | 14 | .03 |
| Age (yr) | ||||
| ≤60 | 36 | 41 | 23 | |
| >60 | 20 | 8 | 10 | .08 |
| Performance status† | ||||
| 0-1 | 41 | 36 | 21 | |
| >1 | 14 | 11 | 12 | .4 |
| B symptoms† | ||||
| Absent | 28 | 24 | 13 | |
| Present | 27 | 24 | 20 | .5 |
| Mediastinal involvement | 26 | 12 | 13 | .07 |
| Extranodal sites | ||||
| Bone marrow | 7 | 7 | 6 | .7 |
| Liver | 4 | 8 | 3 | .3 |
| Spleen | 14 | 6 | 5 | .2 |
| Central nervous system | 5 | 3 | 3 | .8 |
| Skin | 3 | 13 | 5 | .01 |
| Lung | 10 | 6 | 7 | .5 |
| Gut | 3 | 3 | 3 | .8 |
| Bone | 4 | 3 | 4 | .6 |
| Waldeyer's ring | 5 | 2 | 4 | .8 |
| No. of extranodal sites | ||||
| 0-1 site | 41 | 38 | 27 | |
| >1 sites | 15 | 11 | 6 | .6 |
| Ann Arbor stage | ||||
| I-II | 20 | 27 | 10 | |
| III-IV | 36 | 22 | 23 | .05 |
| LDH level† | ||||
| ≤1 × normal | 19 | 32 | 13 | |
| >1 × normal | 31 | 16 | 18 | .01 |
| Albumin level* | ||||
| ≤30 g/L | 10 | 15 | 7 | |
| >30 g/L | 43 | 33 | 25 | .3 |
| International prognostic index31† | ||||
| Low | 16 | 31 | 12 | |
| Low-intermediate | 16 | 4 | 6 | |
| High-intermediate | 9 | 5 | 8 | |
| High | 10 | 9 | 5 | .01 |
| Treatment received | ||||
| ACVBP | 28 | 20 | 17 | |
| m-BACOD | 6 | 13 | 2 | |
| NCVBP | 7 | 7 | 6 | |
| VIM3/ACVBP | 8 | 6 | 3 | |
| CVP/CTVP | 7 | 3 | 5 | .3 |
| Characteristic . | B . | T . | Null . | P . |
|---|---|---|---|---|
| No.* | 56 | 49 | 33 | |
| Sex | ||||
| M | 30 | 38 | 19 | |
| F | 26 | 11 | 14 | .03 |
| Age (yr) | ||||
| ≤60 | 36 | 41 | 23 | |
| >60 | 20 | 8 | 10 | .08 |
| Performance status† | ||||
| 0-1 | 41 | 36 | 21 | |
| >1 | 14 | 11 | 12 | .4 |
| B symptoms† | ||||
| Absent | 28 | 24 | 13 | |
| Present | 27 | 24 | 20 | .5 |
| Mediastinal involvement | 26 | 12 | 13 | .07 |
| Extranodal sites | ||||
| Bone marrow | 7 | 7 | 6 | .7 |
| Liver | 4 | 8 | 3 | .3 |
| Spleen | 14 | 6 | 5 | .2 |
| Central nervous system | 5 | 3 | 3 | .8 |
| Skin | 3 | 13 | 5 | .01 |
| Lung | 10 | 6 | 7 | .5 |
| Gut | 3 | 3 | 3 | .8 |
| Bone | 4 | 3 | 4 | .6 |
| Waldeyer's ring | 5 | 2 | 4 | .8 |
| No. of extranodal sites | ||||
| 0-1 site | 41 | 38 | 27 | |
| >1 sites | 15 | 11 | 6 | .6 |
| Ann Arbor stage | ||||
| I-II | 20 | 27 | 10 | |
| III-IV | 36 | 22 | 23 | .05 |
| LDH level† | ||||
| ≤1 × normal | 19 | 32 | 13 | |
| >1 × normal | 31 | 16 | 18 | .01 |
| Albumin level* | ||||
| ≤30 g/L | 10 | 15 | 7 | |
| >30 g/L | 43 | 33 | 25 | .3 |
| International prognostic index31† | ||||
| Low | 16 | 31 | 12 | |
| Low-intermediate | 16 | 4 | 6 | |
| High-intermediate | 9 | 5 | 8 | |
| High | 10 | 9 | 5 | .01 |
| Treatment received | ||||
| ACVBP | 28 | 20 | 17 | |
| m-BACOD | 6 | 13 | 2 | |
| NCVBP | 7 | 7 | 6 | |
| VIM3/ACVBP | 8 | 6 | 3 | |
| CVP/CTVP | 7 | 3 | 5 | .3 |
Four other patients had both B- and T-cell markers and four patients had histiocytic type.
Data were unavailable for some patients.
ALCL with skin involvement had preferentially a T-cell phenotype (P < .01). T-cell ALCL, as compared with B-cell and null ALCL, had a less disseminated disease (P < .05) and a lower LDH level (P < .01) at diagnosis. Consequently, ALCL of T-cell phenotype was more frequently classified in the low risk category of the international lymphoma prognostic index (P < .01).
Response to treatment and survival. The rate of patients who obtained a complete remission was 75% in the ALCL group and 61% in the non-ALCL group (P < .001). As shown in Fig 1, survival of patients with ALCL (estimated survival at 5 years, 66%; 95% confidence interval, 58 to 74) was significantly longer than those with non-ALCL (estimated survival at 5 years, 48.3%; 95% confidence interval, 45.8 to 50.8) (P = .0004). A significant difference was also observed in event-free survival (P = .006). Other factors were found to be related to survival in univariate analysis, namely: age, performance status, presence of B symptoms, LDH level, serum albumin level, Ann Arbor stage, bone marrow involvement, and number of extranodal sites of the disease. A multivariate analysis using these factors and histology (anaplastic v nonanaplastic) identified the anaplastic histology as an independent factor related to survival (P = .0028) (Table 3).
Overall survival from the time of inclusion in the LNH-87 trial. Comparison of patients with ALCL (n = 146) and patients with nonanaplastic diffuse large-cell lymphoma (n = 1,695); P = .0004.
Overall survival from the time of inclusion in the LNH-87 trial. Comparison of patients with ALCL (n = 146) and patients with nonanaplastic diffuse large-cell lymphoma (n = 1,695); P = .0004.
Factors Independently Related to Overall Survival in 1,710 Cases of Diffuse Large-Cell Lymphoma
| Factor . | Relative Risk . | P . |
|---|---|---|
| Age (≤60 v >60) | 1.72 | <.0001 |
| Ann Arbor stage (I-II v III-IV) | 1.60 | <.0001 |
| LDH level (≤1 × normal v >1 × normal) | 1.59 | <.0001 |
| Performance status (0-1 v 2-4) | 1.59 | <.0001 |
| Histology (anaplastic v nonanaplastic) | 1.60 | .0028 |
| B symptoms (no v yes) | 1.07 | .0097 |
| Serum albumin level (≤30 g/L v <30 g/L) | 1.21 | .01 |
| Bone marrow involvement (no v yes) | 1.04 | .036 |
| Extranodal involvement (≤1 site v >1 site) | 1.18 | .05 |
| Factor . | Relative Risk . | P . |
|---|---|---|
| Age (≤60 v >60) | 1.72 | <.0001 |
| Ann Arbor stage (I-II v III-IV) | 1.60 | <.0001 |
| LDH level (≤1 × normal v >1 × normal) | 1.59 | <.0001 |
| Performance status (0-1 v 2-4) | 1.59 | <.0001 |
| Histology (anaplastic v nonanaplastic) | 1.60 | .0028 |
| B symptoms (no v yes) | 1.07 | .0097 |
| Serum albumin level (≤30 g/L v <30 g/L) | 1.21 | .01 |
| Bone marrow involvement (no v yes) | 1.04 | .036 |
| Extranodal involvement (≤1 site v >1 site) | 1.18 | .05 |
The International Lymphoma Prognostic Index,32 developed for aggressive lymphoma in general, predicted survival in patients with ALCL. Overall 5-year survival was, respectively, 82% for the low risk group (95% confidence interval, 72.7 to 92.5), 78.3% for the low-intermediate risk group (95% confidence interval, 62.9 to 93.7), 50.2% for the high-intermediate risk group (95% confidence interval, 28.8 to 71.6), and 25.7% for the high risk group (95% confidence interval, 6.5 to 44.9) (P < .0001). In the non-ALCL patients, overall 5-year survival was, respectively, 69.3% for the low risk group (95% confidence interval, 65.3 to 73.3), 47.1% for the low-intermediate risk group (95% confidence interval, 41.8 to 52.4), 34.5% for the high-intermediate risk group (95% confidence interval, 28.7 to 40.2), and 22% for the high risk group (95% confidence interval, 16.6 to 27.4). A second multivariate analysis was made to evaluate the additional prognostic value of histology on the International Prognostic Index. In this analysis, putting only the Index risk groups into the model along with histology (Table 4), the anaplastic character was again independently prognostic (P < .0011).
Multivariate Analysis of Overall Survival in Large-Cell Lymphoma Using the International Prognostic Index32 Risk Groups and Histology as Variables
| Factor . | Relative Risk . | P . |
|---|---|---|
| International Prognostic Index (low v low intermediate v high intermediate v high risk group) | 1.70 | <.0001 |
| Histology (anaplastic v nonanaplastic) | 1.62 | <.0011 |
| Factor . | Relative Risk . | P . |
|---|---|---|
| International Prognostic Index (low v low intermediate v high intermediate v high risk group) | 1.70 | <.0001 |
| Histology (anaplastic v nonanaplastic) | 1.62 | <.0011 |
n = 1,710.
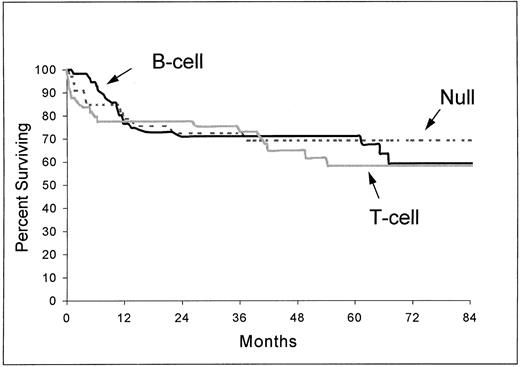
In the ALCL group, the complete remission rate was 80% for B-cell subtype, 69% for T-cell subtype, and 64% for null subtype (P = .09). The differences between subtypes in overall survival (Fig 2) and event-free survival were not significant (respectively, P = .83 and P = .72).
Overall survival from the time of inclusion in the LNH-87 trial of patients with ALCL according to immunophenotype: B-cell (n = 56), T-cell (n = 49), and null type (n = 33). P = .83.
Overall survival from the time of inclusion in the LNH-87 trial of patients with ALCL according to immunophenotype: B-cell (n = 56), T-cell (n = 49), and null type (n = 33). P = .83.
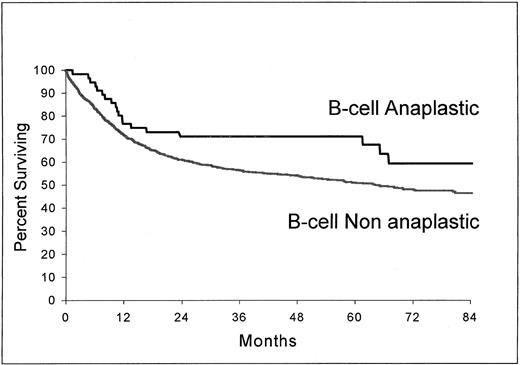
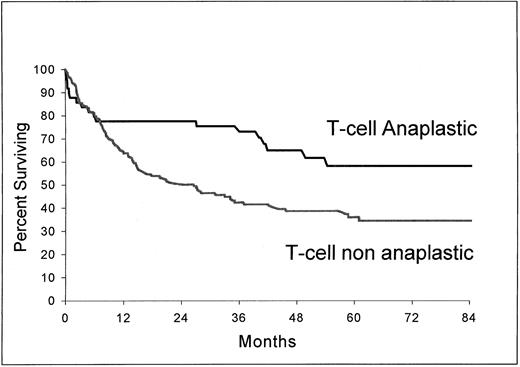
As shown in Fig 3 and 4, B-cell ALCL (estimated survival at 5 years, 71%; 95% confidence interval, 58.2 to 83.8) had a longer survival than non-ALCL of the same phenotype (estimated survival at 5 years, 51%; 95% confidence interval, 48.2 to 53.8) (P = .037) and T-cell ALCL (estimated survival at 5 years, 63.2%; 95% confidence interval, 47.8 to 78.6), a longer survival than T-cell non-ALCL (estimated survival at 5 years, 36%; 95% confidence interval, 27.5 to 44.5) (P = .0061).
Overall survival from the time of inclusion in the LNH-87 trial. Comparison of patients with ALCL of B-cell type (n = 56) and patients with nonanaplastic diffuse large-cell lymphoma of B-cell type (n = 1,368); P = .037.
Overall survival from the time of inclusion in the LNH-87 trial. Comparison of patients with ALCL of B-cell type (n = 56) and patients with nonanaplastic diffuse large-cell lymphoma of B-cell type (n = 1,368); P = .037.
Overall survival from the time of inclusion in the LNH-87 trial. Comparison of patients with ALCL of T-cell type (n = 49) and patients with non-ALCL of T-cell type (n = 144); P = .0061.
Overall survival from the time of inclusion in the LNH-87 trial. Comparison of patients with ALCL of T-cell type (n = 49) and patients with non-ALCL of T-cell type (n = 144); P = .0061.
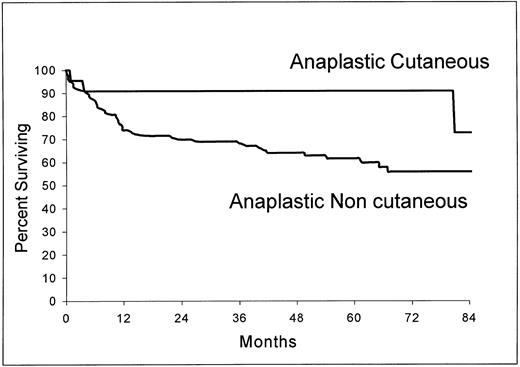
Of the 22 patients with cutaneous involvement, only five had a disease limited to the skin. Three of these patients had a bulky mass (>10 cm) and two had multifocal disease. As shown in Fig 5, the overall survival of the 22 patients with cutaneous ALCL (estimated survival at 5 years, 90.9%; 95% confidence interval, 78.9 to 100) was significantly longer than those of the 124 patients without this localization (estimated survival at 5 years, 61.6%; 95% confidence interval, 52.6 to 70.6) (P = .0258).
Overall survival from the time of inclusion in the LNH-87 trial of patients with ALCL according to the presence (n = 22) or absence (n = 124) of cutaneous involvement at the time of diagnosis. P = .0258.
Overall survival from the time of inclusion in the LNH-87 trial of patients with ALCL according to the presence (n = 22) or absence (n = 124) of cutaneous involvement at the time of diagnosis. P = .0258.
DISCUSSION
Since ALCL has been described as a morphologic entity, several clinicopathologic studies have been reported.13-27 However, the small number of patients in the series, the heterogeneity of the treatment received, and the lack of comparison with non-ALCL do not allow drawing firm conclusions in terms of clinical presentation and outcome. Moreover, the varying proportion of ALCL in reported series, ranging from 2% of all lymphomas18 to 22% of the large cell lymphomas22 reflects this difficulty.
The aim of this study was to individualize ALCL in a cohort of patients prospectively treated in a large multicenter trial and to compare their clinical characteristics and treatment outcome with those of patients with non-ALCL included in the same trial. An additional goal of this report was to study the influence of immunophenotype on these parameters.
Our study confirms that ALCL mostly affects young men,15,17 but male predominance appeared only for T-cell type. It is admitted that ALCL patients frequently present with advanced disease,5,17 but initial Ann Arbor stage and number of extranodal sites of the diseases were not different from those of non-ALCL. The higher frequency of B symptoms in ALCL is a common finding,15,22,24,25 which might be related to the elevated levels of the soluble form of CD30 molecule in this disease.41 In Hodgkin's disease, sCD30 levels have been found to be closely correlated with the presence of B symptoms.42,43 Circulating sCD30 in this disease might reflect the effects of cytokines produced by tumor cells.43
The high frequency of skin involvement is recognized as a characteristic of ALCL.5,21 The clinical distinction between primary cutaneous lymphoma and cutaneous involvement of a systemic disease form is not always easy to establish. Some phenotypic20 and genotypic44 features might help in this distinction. In this series, we confirm the predominance of T-cell phenotype among the cutaneous forms of ALCL. The presence of skin involvement was associated with a longer survival, which is in accordance with the rather favorable outcome reported for adults with primary cutaneous ALCL.11,20,45 However, skin involvement has also been described to be associated with an increased risk of treatment failure in children46 and adults23 with ALCL. As only 15% of the patients with ALCL had skin involvement, their better survival cannot explain the favorable outcome of the whole population observed in our series.
Bone marrow involvement has been initially considered as a rare event in ALCL.6,12 Our study, using conventional methods, did not show any difference in this involvement between ALCL and non-ALCL. When immunohistochemistry with anti-CD30 and anti-EMA is used to study bone marrow samples, malignant cells could be detected in as much as 43% of the cases.47 We confirm the previously reported low incidence of digestive tract involvement, which could be a typical feature of ALCL.19 27
As CD30 positivity appears more widespread in non-Hodgkin's lymphoma than originally thought, identification of ALCL requires the combination of an histologic and immunophenotypic approach.6,48 Immunohistochemical detection of CD30 expression is particularly useful for the diagnosis of the lymphohistiocytic variant,6 which was only observed in three patients. The development of antibodies directed against chimeric anaplastic lymphoma kinase might be useful in the diagnosis of ALCL, at least in those bearing the t(2; 5) translocation.9 49
Although no large comparative studies have been published, most investigators reported that response of ALCL to chemotherapy was good, ranging from 60% to 90%.13-27 The overall survival of localized disease is known to be good, especially in children.17,21,27,46 More advanced stages have a high relapse rate and their prognosis in comparison to that of other large-cell lymphomas is controversial. Although few studies have suggested that advanced-stage ALCL may have a short disease-free survival and may require more intensive therapy,21,25 most investigators consider that ALCL generally behaves as a usual intermediate to high grade lymphoma.17,22,24 However, comparative studies in diffuse large-cell lymphomas have shown an association between CD30 expression and a favorable outcome.16,23 A recent study of a large cohort of patients with lymphoma identified ALCL of T/null-cell type as a subgroup with a particularly good survival.50 In our study, ALCL had a longer event-free and overall survival than non-ALCL. In multivariate analyses, anaplastic histology was an independent prognostic factor outweighing some variables, as bone marrow involvement or number of extranodal sites involved, and remaining independent from the risk groups defined by the International Prognostic Index.32 These findings question the introduction of histology as a prognostic factor in future predictive models used for aggressive non-Hodgkin's lymphoma. Finally, the better outcome of ALCL does not support the use of intensive therapy with bone marrow transplant as a front-line treatment in this disease.25
The predominance of B-cell phenotype in our patients with ALCL is noteworthy. The same finding has only been reported in two recent series of ALCL.24,25 Although we cannot exclude that some ALCL classified as having a null phenotype could, in fact, bear T-cell antigens, only detectable on frozen sections, the proportion of 22% of null type lymphomas found in our study is in accordance with most published series.13-15,18,20-22,24,25,46 The explanation of the predominance of B-cell phenotype could be found in the population studied which excluded children, secondary forms of ALCL, and comprised only a few patients with purely cutaneous involvement, all conditions known to be predominantly of T-cell type.6,11,46 Phenotype was not found to influence the outcome of ALCL, B-, and T-cell ALCL lymphomas, each having a better outcome than their respective non-ALCL counterparts. The larger difference observed between the T-cell lymphomas could be attributed to the poor prognosis of T-cell non-ALCL.51 In contrast to the recent proposition of the REAL classification4 to consider B-cell ALCL as a variant of diffuse large B-cell lymphoma, these results indicate the clinical importance to recognize ALCL, whatever its phenotype could be.
In conclusion, ALCL, which is recognized as a pathologic entity with heterogeneous phenotypic and genotypic features, represents a homogeneous group of patients with an independent prognostic expression. Within the ALCL, phenotype does not influence prognosis, whereas the clinical variables of the International Prognostic Index predict survival. Whether ALCL requires a specific therapeutic approach remains to be determined.
ACKNOWLEDGMENT
Dr Marie-Françoise d'Agay initiated this work. This report is dedicated to her memory. We thank Catherine Balmale who did the computer programming for this analysis, Catherine Bellorgey for excellent techical assistance, Richard Medeiros for help in the preparation of the manuscript, and Drs Bertrand Coiffier, Christian Gisselbrecht, Félix Reyes, and Philippe Solal-Céligny for their comments on the manuscript.
APPENDIX
The following clinicians and pathologists participated in the LNH-87 study:
A. Abdalsamad, C. Allard, R. Angonin, J. d'Anjou, B. Audhuy, J. Audouin, G. Auzanneau, A.C. Baglin, C. Bailly, Y. Bastion, E. Baumelou, P. Bensimon, F. Berger, P. Biron, A.M. Blaise, M. Blanc, F. Boman-Ferrand, A. Boehn, J. Boniver, M. Bordes, D. Bordessoule, A. Bosly, R. Bouabdallah, S. Boucheron, J. Bouvier, P. Brice, N. Brousse, P. Brousset, P.A. Bryon, D. Caillot, J.P. Carbillet, R.O. Casasnovas, T. Caulet, D. Cazals, F. Charlotte, L. Charvillat, A.M. Chesneau, B. Christian, B. Coiffier, T. Conroy, J.F. Cordier, C. Cordonnier, J.P. Clauvel, E. Deconinck, M. Delage, A. Delannoy, G. Delsol, M. Delos, A. Devidas, M. Diviné, H. Dombret, C. Doyen, H. Duplay, B. Dupriez, C. Duval, J.C. Eisenmann, J.M. Emberger, B. Epardeau, B. Fabiani, P. Felman, J.P. Fermand, A. Ferrant, C. Fermé, A. Ferrand, M. French, M. Fievez, Y. Fonck, N. Froment, J. Gabarre, P. Galian, O. Gasser, C. Gisselbrecht, B. Gosselin, H. Guy, D. Guyotat, C. Haioun, J. Hamels, R. Herbrecht, C. Hopfner, N. Horschowski, F. Huguet, P. Jacomy, J. Jaubert, R. Jeandel, Y. Kerneis, J.P. Knopf, M. Kuentz, E. Labouyrie, B. Lancien, G. Laurent, A. Lavergne, C. Lavignac, V. Leblond, M. Lecomte-Houke, F. Lejeune, M.B. Leger-Ravet, R. Loire, R. Marcellin, J.P. Marolleau, G. Marit, C. Martin, C. Marty-Double, A. de Mascarel, S. Méhaut, J.P. Merlio, C. Merignargues, J.M. Micléa, J.L. Michaux, V. de Montpreville, M. Monconduit, P. Morel, F. Morvan, J.F. Mosnier, G. Nédellec, G. Netter-Pinon, H. Noel, C. Nouvel, M. Patey, P.Y. Peaud, G. Perie, M. Peuchmaur, B. Pignon, C. Platini, M. Pluot, J.P. Pollet, E. Pujade-Lauraine, M. Raphael, M.C. Raymond-Gelle, J. Reiffers, F. Reyes, M. Rochet, J.F. Rossi, A.M. Roucayrol, A. Rozenbaum, G. Salles, H. Schill, C. Sebban, M. Simon, Ph. Solal-Céligny, P. Straub, E. Suc, L. Sutton, G. Tertian, S. Thiebaut, A. Thyss, Ph. Travade, V. Trillet, J.P. Vernant, L. Xerri.
Supported in part by grants from the Délégation à la Recherche Clinique de l'Assistance Publique-Hôpitaux de Paris, Paris, France; the Fondation contre la Leucémie, Paris, France; and the Caisse Nationale d'Assurance Maladie des Travailleurs Salariés, Paris, France.
Address reprint requests to Hervé Tilly, MD, Centre Henri Becquerel, Rue d'Amiens, 76038 Rouen, France.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal