Abstract
The 160-170 sequence of human immunodeficiency virus (HIV)-1 gp120 mimics a nicotinic receptor-binding motif of rabies virus glycoprotein and snake neurotoxins. This sequence has been proposed to be involved in the binding of HIV-1 gp120 to the acetylcholine binding sites of nicotinic receptors. By using biomolecular interaction analysis (BIA) technology we have found that HIV-1 gp120 can bind to detergent-extracted nicotinic receptor from fetal calf muscle. The binding is inhibited by nicotine and by a synthetic peptide reproducing the gp120 160-170 sequence. The molecular mimicry between gp120 and rabies virus glycoprotein is confirmed by cross-reacting antibodies. We have found that vaccination against rabies can induce the production of anti–HIV-1 gp120 antibodies in humans. The cross-reacting antibodies are directed to the gp120 sequence involved in the mimicry with the rabies virus glycoprotein. The cross-reactivity between the rabies virus and HIV-1 has important implications in transfusion medicine. Moreover, the presence of cross-reacting antibodies between the nicotinic receptor binding site of rabies virus glycoprotein and a fragment of HIV-1 gp120 strengthens the hypothesis about the possible role of nicotinic receptors as potential receptors for HIV-1 in the central nervous system.
THE SEQUENCE 160-170 of human immunodeficiency virus (HIV)-1 envelope protein gp120 is highly homologous to a sequence shared by snake curare-mimetic neurotoxins and the rabies virus glycoprotein.1 This sequence is involved in the specific binding of rabies virus glycoprotein and snake neurotoxins to the nicotinic acetylcholine receptor (AChR) at neuromuscular junctions.2-5
In previous reports,1,6 we proposed that the mimicry of a nicotinic receptor binding motif by HIV-1 gp120 can be important in HIV-1 infectivity by enabling HIV-1 binding to the nicotinic receptor acetylcholine binding sites. This hypothesis was supported by experimental data indicating that recombinant gp120 from HIV-1 IIIB can inhibit the binding of the snake neurotoxin α-bungarotoxin (α-bgt) to the nicotinic receptor in a human rhabdomyosarcoma cell line.6 Moreover, we showed that immunization of mice with a synthetic peptide reproducing the sequence 160-170 of HIV-1 gp120 induces the production of antibodies cross-reacting with rabies virus glycoprotein and α-bgt.6 Taken together, these data indicate that a region of HIV-1 gp120 is structurally and functionally related to an important functional site of rabies virus glycoprotein.
Apart from the possible relevance in HIV-1 infectivity, it is reasonable to expect that this similarity can cause the production of cross-reacting antibodies. Our hypothesis is supported by a report7 describing a false-positive HIV-1 screening related to rabies vaccination. The investigators reported a case of a woman who had been vaccinated against rabies and soon after the last boost gave a positive result in an enzyme immunoassay (EIA) for anti–HIV-1 antibodies. The woman was a blood donor, and she had been monitored for the presence of anti–HIV-1 antibodies and she tested negative in the EIA until a few months before the antirabies vaccination. Fifteen days after the last injection of the rabies vaccine, a repeatedly positive EIA occurred. At the same time a polymerase chain reaction for proviral DNA was negative. The EIA for anti–HIV-1 antibodies was negative again 6 weeks after the first positive result.
We have tested different serum samples of the described patient in enzyme-linked immunosorbent assay (ELISA), and we have found a clear cross-reactivity with HIV-1 recombinant gp120 and with a synthetic peptide reproducing the rabies virus glycoprotein-homologous sequence of HIV-1 gp120. Moreover, we have analyzed the binding of HIV-1 gp120 to detergent-extracted nicotinic receptor from fetal calf muscle membranes by surface plasmon resonance (SPR). We have found that a component from fetal calf muscle membranes binds to HIV-1 gp120. The binding is inhibited by nicotine and by the synthetic peptide, which reproduces the snake neurotoxin and rabies virus glycoprotein mimicking sequence of HIV-1 gp120.
MATERIALS AND METHODS
Peptide synthesis. Peptide B2 (C-S-F-N-I-S-T-S-I-R-G-K-V-Q-K-E) was synthesized by solid phase synthesis using a MultiSynTech model “Syro” multiple peptide synthesizer (Bochum, Germany) employing fluorenylmethoxycarbonyl (Fmoc) chemistry. Fmoc amino acid derivatives were coupled by using N-hydroxybenzotriazole-esters and each coupling step was monitored by the bromophenol blue acid-base indicator. Peptides were cleaved from the resin and simultaneously deprotected using a 93% trifluoroacetic acid/2% anisole/3% ethandithiol/2% water mixture. The crude products were gel-filtrated on Sephadex G10 and purified by reverse-phase high performance liquid chromatography (HPLC). Purified peptides were controlled by capillary electrophoresis (Biofocus 3000; Bio-Rad Laboratories, Hercules, CA) and by amino acid composition and sequence analysis.
ELISA on recombinant gp120. EIA plates (96-well) were coated with 2 μg/mL of recombinant gp120 (IIIB, Intracel, London, UK) in 50 mmol/L carbonate buffer, pH 9.6, overnight at 4°C. Plates were washed after each step with phosphate-buffered saline (PBS), pH 7.4, containing 0.05% Tween 20 (PBS-T) and saturated with 3% bovine serum albumin (BSA) in PBS-T for 2 hours at 37°C. Serial dilutions of sera in PBS-T–1% BSA were added to the wells for 2 hours at 37°C. Horseradish peroxidase-conjugated goat antihuman IgG (Jackson Immunoresearch Laboratories, West Grove, PA) was added at a suitable dilution for 1 hour at 37°C followed by the addition of 3,3′,5,5′-tetramethy benzidine in the presence of H2O2 to reveal the binding. The reaction was stopped after 15 minutes by the addition of 1 N HCl and optical density (OD) values were measured at 450 nm. Nonspecific binding of each serum dilution was evaluated in noncoated, but saturated, wells.
ELISA on synthetic peptide B2. ELISA on peptide B2 was essentially as described above on plates coated with 4 μmol/L peptide in 50 mmol/L carbonate buffer, pH 9.6.
ELISA on rabies virus glycoprotein. Different samples of the patient's serum (RI, RII, RIII) collected at different times were tested on rabies virus glycoprotein using an EIA kit for the determination of antirabies virus glycoprotein antibodies (Platelia Rage; Diagnostics Pasteur, Marnes-la-Coquette, France). Specific antibody titer was calculated following manufacturer's instructions.
Competition ELISA. Different concentrations of the synthetic peptide B2 diluted in PBS-T–1% BSA were mixed with a 1:10 dilution of the RI sample of the patient's serum and then added to the gp120-coated plate as described above. Horseradish peroxidase-conjugated goat antihuman IgG and substrate was used in the same condition described for the ELISA on gp120.
Extraction of fetal calf muscle nicotinic receptor. The nicotinic receptor was solubilized from fetal calf muscle membranes with Triton X-100 following the procedure described by Gotti et al.8 The nicotinic receptor concentration in the detergent extracts was measured by a radioimmunoassay as described previously.4 Briefly, serial dilutions were incubated with 105 cpm of 125I-labelled α-bungarotoxin. Toxin-receptor complexes were precipitated by adding 18% polyethylene glycol (PEG) 6000, and the supernatants containing unbound 125I-α–bungarotoxin were discarded. The resulting receptor concentration was expressed as moles of 125I-α–bgt bound per liter. Alternatively, unbound 125I-labelled α-bgt was separated from toxin-receptor complexes by absorbtion on diethyl aminoethyl (DEAE) paper discs such as described in Schmidt and Raftery.9
SPR. The detection principle of BIAcore (Biacore, Uppsala, Sweden) relies on the optical phenomenon of SPR and has been described in detail elsewere.10 11 Recombinant HIV-1 gp 120 (from IIIB strain, expressed in Baculovirus and purchased from Intracel) (20 μg/mL in 10 mmol/L sodium acetate, pH 4.75) was covalently immobilized via primary amino groups on the carboxymethylated surface of a CM5 BIAcore sensor chip previously activated with a 1:1 mixture of 100 mmol/L N-hydroxysuccinimide (NHS) and 400 mmol/L N-ethyl-N′(dimethylaminopropyl) carbodiimide (EDC). Unreacted activated groups were deactivated by injection of 1 mol/L ethanolamine hydrocloride, pH 8.5. Detergent-solubilized AChR from fetal calf muscle membranes was diluted in 10 mmol/L acetate buffer, 150 mmol/L NaCl, pH 6.5, and injected over the matrix. Regeneration of the matrix was obtained by injection of 10 mmol/L NaOH.
RESULTS
Several experimental data described previously by our group indicate the presence of a molecular mimicry between HIV-1 gp120 and the rabies virus glycoprotein.1,6 The sequence 160-170 of HIV-1 gp120 is highly homologous to the sequence 189-199 of the rabies virus glycoprotein and to the sequence 30-40 of snake venom neurotoxins1 (Fig 1). We found that immunization of mice with a synthetic peptide reproducing the sequence 160-173 of HIV-1 gp120 induces the production of anti-gp120 antibodies efficiently cross-reacting with both rabies virus glycoprotein and α-bgt.6
Sequence homology of HIV-1 gp120 with rabies virus glycoprotein and snake venom neurotoxins. Boldface letters indicate amino acid identities between gp120 and the rabies virus glycoprotein, italic letters indicate identities between gp120 and snake neurotoxins, italic bold letters are used for amino acids that are identical in gp120, rabies virus glycoprotein, and snake neurotoxins.
Sequence homology of HIV-1 gp120 with rabies virus glycoprotein and snake venom neurotoxins. Boldface letters indicate amino acid identities between gp120 and the rabies virus glycoprotein, italic letters indicate identities between gp120 and snake neurotoxins, italic bold letters are used for amino acids that are identical in gp120, rabies virus glycoprotein, and snake neurotoxins.
Consistent with our hypothesis, a possible case of antibody cross-reactivity was described in a woman who gave a false-positive result in an ELISA test for anti–HIV-1 antibody after antirabies vaccination.7 12
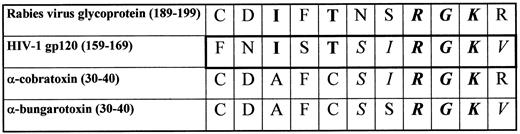
We have tested the serum of the described patient on recombinant HIV-1 gp120 in ELISA. A panel of 11 HIV-negative sera from blood donors was tested in parallel at the same dilutions. Three samples of the rabies vaccinated patient's serum were collected at different times after the rabies vaccination and tested in identical experimental conditions. The first sample (RI) was collected at the time of the positive HIV-1 screening (August, 1992) and the second (RII) and third (RIII) samples were collected some years later when the EIA for HIV-1 antibodies was negative again (January, 1995 and November, 1995, respectively). We found that antibodies from the first sample of the rabies vaccinated patient's serum clearly recognize recombinant HIV-1 gp120 in ELISA. In the same assay, the serum samples collected later when the anti-HIV test was negative again, gave a much lower response on gp120, with the RIII sample giving OD values superimposable to those obtained with reference sera from HIV-1–negative blood donors at the same dilution (Fig 2).
ELISA on recombinant HIV-1 gp120 of the serum from the rabies vaccinated patient. Serial dilutions of three samples of serum (RI [•], RII [▪], and RIII [▴]), which had been collected at different times, were tested on EIA plates coated with recombinant gp120. Each point in the RI, RII, and RIII lines is the average of triplicate samples for each dilution of serum in two different experiments, while each point of the normal human sera (NHS [○]) line is the average of triplicate samples of 11 different HIV-negative sera at the same dilutions in two different experiments.
ELISA on recombinant HIV-1 gp120 of the serum from the rabies vaccinated patient. Serial dilutions of three samples of serum (RI [•], RII [▪], and RIII [▴]), which had been collected at different times, were tested on EIA plates coated with recombinant gp120. Each point in the RI, RII, and RIII lines is the average of triplicate samples for each dilution of serum in two different experiments, while each point of the normal human sera (NHS [○]) line is the average of triplicate samples of 11 different HIV-negative sera at the same dilutions in two different experiments.
The three samples of serum were tested in an EIA kit for the determination of antirabies virus glycoprotein antibodies. The titer of antirabies glycoprotein specific antibodies was lower in the second and third samples compared with the first specimen where anti–HIV-1 antibodies were detected (Fig 3 and Table 1).
ELISA on rabies virus glycoprotein of the serum from the rabies vaccinated patient. Serial dilutions of three samples of the patient's serum (RI, RII, and RIII) collected at different times were tested on rabies virus glycoprotein using an EIA kit for the determination of antirabies virus glycoprotein antibodies.
ELISA on rabies virus glycoprotein of the serum from the rabies vaccinated patient. Serial dilutions of three samples of the patient's serum (RI, RII, and RIII) collected at different times were tested on rabies virus glycoprotein using an EIA kit for the determination of antirabies virus glycoprotein antibodies.
Antirabies Virus Glycoprotein Titer of Serum Samples From the Rabies Vaccinated Patient
| Serum Specimen . | Date of Collection . | Antirabies Virus Glycoprotein Titer . |
|---|---|---|
| RI | 8/92 | 2.0 UI/mL |
| RII | 1/95 | 1.2 UI/mL |
| RIII | 11/95 | 1.0 UI/mL |
| Serum Specimen . | Date of Collection . | Antirabies Virus Glycoprotein Titer . |
|---|---|---|
| RI | 8/92 | 2.0 UI/mL |
| RII | 1/95 | 1.2 UI/mL |
| RIII | 11/95 | 1.0 UI/mL |
The three serum samples described above were tested in ELISA also on a synthetic peptide (peptide B2) reproducing the HIV-1 gp120 sequence 157-172, which contains the rabies virus glycoprotein-homologous motif. A reactivity on the synthetic peptide B2 is evident in the three serum specimens. The response seems to be decreasing with time. Nevertheless, the OD values remain superior to the OD values we get with control HIV-1–negative sera tested with the same peptide (Fig 4).
ELISA on the synthetic peptide B2 of the serum from the rabies vaccinated patient. Experimental conditions were the same as described in the Fig 2 legend. (RI [•], RII [▪], RIII [▴], NHS [○]).
ELISA on the synthetic peptide B2 of the serum from the rabies vaccinated patient. Experimental conditions were the same as described in the Fig 2 legend. (RI [•], RII [▪], RIII [▴], NHS [○]).
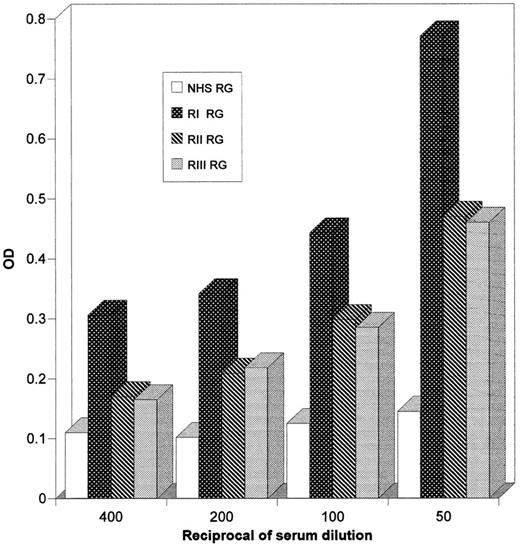
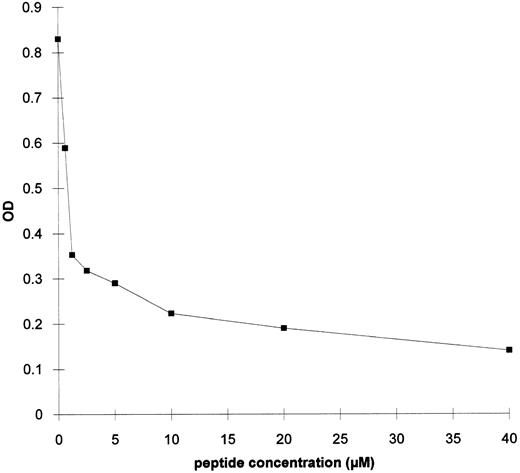
Experiments were performed to test the ability of the synthetic peptide B2 to inhibit the binding of serum antibodies from the rabies vaccinated patient to gp120 in ELISA. The synthetic peptide was able to inhibit up to 85% of the antibody binding to gp120. Fifty percent of maximum binding was obtained with 1 μmol/L peptide (Fig 5).
ELISA on gp120 of the serum from the rabies vaccinated patient in the presence of peptide B2. A 1:10 dilution of RI sample from the rabies vaccinated serum was mixed with increasing concentrations of peptide B2 and added to a gp120-coated EIA plate.
ELISA on gp120 of the serum from the rabies vaccinated patient in the presence of peptide B2. A 1:10 dilution of RI sample from the rabies vaccinated serum was mixed with increasing concentrations of peptide B2 and added to a gp120-coated EIA plate.
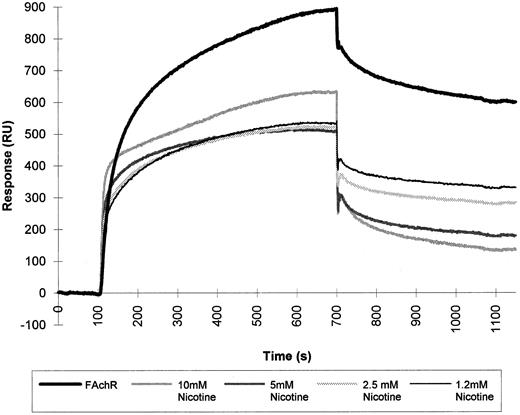
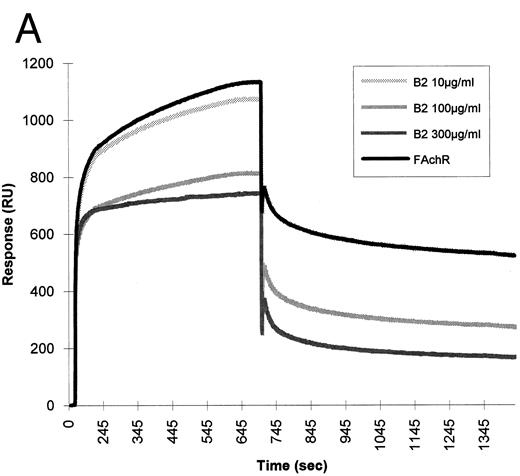
We used the BIAcore to study the interaction between HIV-1 gp120 and mammalian AChR solubilized from fetal calf muscle membranes. HIV-1 gp120 was covalently immobilized via primary amino groups on the dextran matrix, and detergent-solubilized nicotinic receptor prepared as described in Gotti et al8 was subsequently injected over the matrix. This experimental approach enabled us to detect the binding of gp120 to a component from the detergent-solubilized fetal calf muscle membranes. Competition experiments were done to test the specificity of the binding. The detergent-extracted fetal calf muscle membranes were injected over the matrix-coupled HIV-1 gp120 in the presence of nicotine. Using increasing concentrations of nicotine, we obtained up to 80% inhibition of the binding (Fig 6).
SPR of fetal calf muscle nicotinic receptor binding to HIV-1 gp120. Triton-extracted membranes from fetal calf muscle (FAchR) were diluted in 10 mmol/L acetate buffer, pH 6.5, 150 mmol/L NaCl to a concentration of 0.5 nmol of 125I-α–bgt bound/liter and then injected at a flow rate of 5 μL/minute over a matrix where HIV-1 gp120 had been covalently immobilized. The same sample was injected in the presence of increasing concentrations of nicotine. Regeneration of the matrix was obtained by injection of 10 mmol/L NaOH at the end of each cycle.
SPR of fetal calf muscle nicotinic receptor binding to HIV-1 gp120. Triton-extracted membranes from fetal calf muscle (FAchR) were diluted in 10 mmol/L acetate buffer, pH 6.5, 150 mmol/L NaCl to a concentration of 0.5 nmol of 125I-α–bgt bound/liter and then injected at a flow rate of 5 μL/minute over a matrix where HIV-1 gp120 had been covalently immobilized. The same sample was injected in the presence of increasing concentrations of nicotine. Regeneration of the matrix was obtained by injection of 10 mmol/L NaOH at the end of each cycle.
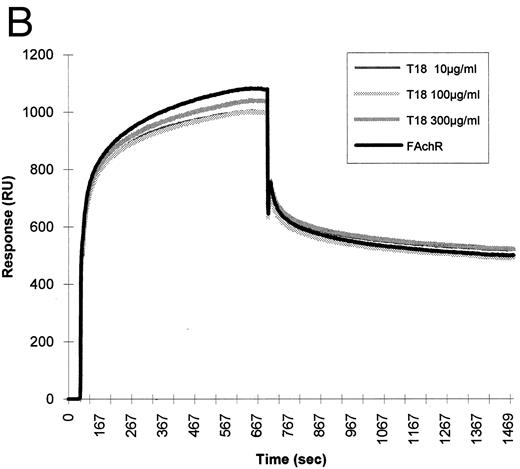
To test whether the binding of HIV-1 gp120 to the nicotinic receptor could be mediated by the gp120 sequence, which mimics the nicotinic receptor-binding motif shared by snake neurotoxins and rabies virus glycoprotein, the synthetic peptide B2 was tested for its ability to interfere with the binding of the nicotinic receptor to gp120 in BIAcore. This peptide was able to inhibit the binding of fetal calf nicotinic receptor to immobilized gp120. A synthetic peptide of the same length, but with a random sequence, had no effect on the binding (Fig 7).
Inhibition of fetal calf muscle nicotinic receptor binding to HIV-1 gp120 by the B2 synthetic peptide. (A) Increasing amounts (10, 100, and 300 μg/mL) of the synthetic peptide B2 were mixed with the diluted detergent-solubilized receptor (0.5 nmol of 125I-α–bgt bound/L). Aliquots of each mixture were injected at a flow rate of 5 μL/min in a flow cell where gp120 had been previously covalently immobilized. Regeneration of the matrix was obtained by injection of 10 mmol/L NaOH at the end of each cycle. (B) Identical concentrations of a peptide (T18) of the same lenght, but with a random sequence, were injected in identical experimental conditions.
Inhibition of fetal calf muscle nicotinic receptor binding to HIV-1 gp120 by the B2 synthetic peptide. (A) Increasing amounts (10, 100, and 300 μg/mL) of the synthetic peptide B2 were mixed with the diluted detergent-solubilized receptor (0.5 nmol of 125I-α–bgt bound/L). Aliquots of each mixture were injected at a flow rate of 5 μL/min in a flow cell where gp120 had been previously covalently immobilized. Regeneration of the matrix was obtained by injection of 10 mmol/L NaOH at the end of each cycle. (B) Identical concentrations of a peptide (T18) of the same lenght, but with a random sequence, were injected in identical experimental conditions.
DISCUSSION
The results reported seem to indicate that rabies vaccination can induce the production of antibodies cross-reacting with HIV-1 gp120. These cross-reacting antibodies decrease with time and can probably be detected only when the antirabies virus antibody titer is relatively high. Because a synthetic peptide reproducing the 157-172 sequence of HIV-1 gp120 is clearly recognized, both when used in solution and when immobilized on a ELISA plate, by serum antibodies from the rabies vaccinated patient, it seems that at least some of the cross-reacting antibodies are directed to this gp120 sequence, which is highly homologous to the nicotinic receptor-binding motif of rabies virus glycoprotein.
The reactivity of serum antibodies with the synthetic peptide B2 is decreasing with time. Nevertheless, an OD response higher than that we have with control HIV-1 negative sera is present even in the RIII sample of serum, which is negative in the ELISA on recombinant gp120. This can be explained by the high homology between the gp120 sequence reproduced in the B2 peptide and the corresponding rabies virus glycoprotein sequence. Due to this similarity, peptide B2 is probably recognized by a higher percentage of antirabies antibodies compared with the same sequence in the native gp120 conformation.
The antibody cross-reactivity between the rabies virus and HIV-1, which can be induced by rabies vaccination, has important implications in transfusion medicine. We are not aware of other cases of false-positive HIV tests related to rabies vaccination. This is rather understandable considering the relatively low incidence of rabies vaccination in humans. Nevertheless, it would be interesting to test different sera from individuals who have been vaccinated against rabies, to see whether the cross-reaction of antirabies antibodies with HIV-1 gp120 is a common event.
The cross-reactivity of antirabies virus glycoprotein antibodies with HIV-1 gp120 confirms the data previously obtained through immunization of mice with the gp120 peptide B2: antipeptide antibodies were reacting with both gp120 and rabies virus glycoprotein.6 Analogous cross-reacting antibodies seem to be induced by vaccination with rabies virus, thus confirming the existence of a similar structural motif between the rabies virus glycoprotein and HIV-1 gp120. This common structural motif is probably related to a common functional feature that is binding to nicotinic receptors.
By using SPR, we have found that a component from detergent solubilized fetal calf membranes binds to HIV-1 gp120. The binding is inhibited by nicotine, which is a selective ligand of nicotinic acetylcholine receptor. Moreover, the binding is inhibited also by the synthetic peptide B2, which reproduces the putative nicotinic receptor binding motif of gp120.
These data, together with our previously described finding that gp120 is able to inhibit the binding of the snake neurotoxin α-bgt to nicotinic receptor in the human rhabdomyosarcoma cell line TE671,6 strongly indicate that HIV-1 gp120 can actually bind to muscle nicotinic receptors.
In conclusion, our results indicate that a region of HIV-1 gp120, such as rabies virus glycoprotein, can strucurally and functionally mimic a snake neurotoxin specific receptor binding motif. The ability of HIV-1 to bind to nicotinic receptors could be of primary importance in virus neuropathogenesis. Nicotinic receptors are widely distributed in the central nervous system.13-15 Even if the function of neuronal nicotinic receptor is not fully understood, some data indicate that they might be involved in the regulation of excitatory transmission in the central nervous system and in the release of transmitters such as glutammate16,17 and dopamine.18 Interestingly, stimulation of the α7 subunit of neuronal nicotinic receptors can induce an increase in presynaptic calcium influx, which is specifically blocked by α-bgt.16 17
The neurotoxic effect of HIV-1 and gp120 has been reported in several studies19-22 despite the fact that neurons seem not to be infected in vivo.23 24 The binding of HIV-1 gp120 to nicotinic receptors in the brain could produce several dysfunctions in synaptic excitability, transmitters relase, and calcium influx, even in the absence of the direct infection of neurones.
Supported by grants from the Italian Ministry of Health, VIII AIDS Project, Grant No. 9304-94.
Address reprint requests to Luisa Bracci, PhD, Dipartimento di Biologia Molecolare, Policlinico Le Scotte, V.le M.Bracci, 53100 Siena, Italy.

![Fig. 2. ELISA on recombinant HIV-1 gp120 of the serum from the rabies vaccinated patient. Serial dilutions of three samples of serum (RI [•], RII [▪], and RIII [▴]), which had been collected at different times, were tested on EIA plates coated with recombinant gp120. Each point in the RI, RII, and RIII lines is the average of triplicate samples for each dilution of serum in two different experiments, while each point of the normal human sera (NHS [○]) line is the average of triplicate samples of 11 different HIV-negative sera at the same dilutions in two different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3623/4/m_bl_0002f2.jpeg?Expires=1767814097&Signature=yD7MhVZZx3i4Av~5UWlNUsnkXLehAsUc~B-wuggzSw~dw0~fvMCFMUOz4shTdL9KVYUza-YwkQ62ZMEKKYkUXl-7vx-ZwGp1l2TDpuN9QBttTrgxTW3YBlfQP~8tSQ-6FcSVfmq9c9rjKG5xdYdnpzsVRPY7wt5zZoIr2yx6i2t40cKWHifX2OVHyGd~AG-kW4~OxXh3jRfoarTmIyEGZ9HwMVwXl4v1FoiAohzu82vPNgqkI9Q~PATgf4py-Tq~YV7Drtm0Acp1oak5DcH70FihBa~6DZeMIPwmvdsqLsHrC-HHMArpHaigZNf-9tgm5CgS8O7JMN2fNXEPug~M5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. ELISA on the synthetic peptide B2 of the serum from the rabies vaccinated patient. Experimental conditions were the same as described in the Fig 2 legend. (RI [•], RII [▪], RIII [▴], NHS [○]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3623/4/m_bl_0002f4.jpeg?Expires=1767814097&Signature=l6LNFOiwrtUnq8NqvlCMsZ5loOCPm8wTXl~MbgKSJF2p3cGe3x4zv6nJKp2hU-QqxI0LM54y9EOm72t6bzmW3cNUwVf~eFye-Dm9h-FGzpQC1TIa8J~KWCOYwQ6ns6LowwRj-~BUTSzR5p04iHt031srZ490Niy9CI1XIqiVgKzlQ4XMK-lCJdcpugciBHhlv05iHTOq2tEdx2TAcOokaGn9xYZl~AA6mv6KW4p0AwGGlnBKwFwJHpQlZYI0aUeBucyB1-HZpRgmA57LIFof7ytFJt6g46IhpJabqJstcr~hE5sDa7B-mkCU0DYEs~YaCuebMvJhDc64Xj3gYsqSuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal