Abstract
Recent studies in our laboratory, as well as others, have suggested that fibrin can regulate cell function in vitro and likely control inflammation in vivo by acting as a potent cell activator. This has led us to hypothesize that during tissue and vascular injury, fibrin can enhance leukocyte recruitment by inducing vascular endothelial cell expression of leukocyte chemotactic factors. To begin to test this hypothesis, we developed an in vitro model of in situ fibrin polymerization on human umbilical vein endothelial cell culture (HUVEC) and determined the ability of fibrin to induce HUVEC expression of the potent leukocyte chemotactic factor interleukin-8 (IL-8). Our initial studies showed that fibrin induced IL-8 expression in a time- and dose-dependent fashion. Fibrin-induced IL-8 expression in HUVEC could be seen as early as 2 hours post-fibrin stimulation. Additionally, fibrin concentrations as low as 30 μg/mL stimulated a detectable level of IL-8 antigen expression from HUVEC. We also showed that this fibrin induced IL-8 had the identical molecular weight and similar antigenic identity as recombinant and monocyte derived IL-8. Northern blot analysis showed that the IL-8 antigen increase seen in fibrin treated HUVEC was due to fibrin induced elevation of steady state mRNA expression in HUVEC. These data clearly support our hypothesis that fibrin is a potent vascular endothelial cell (VEC) activator that can directly contribute to leukocyte recruitment and activation by inducing leukocyte chemotactic factor expression from VEC.
INTRAVASCULAR AND extravascular fibrin deposition on vascular endothelial cells is a consistent feature of a variety of pathologic processes including trauma, neoplasms, infection, and inflammation. Previously, fibrin was thought to function primarily as a hemostatic plug or temporary matrix. However, there is a growing body of information on the role of fibrin(ogen) metabolites as cell regulatory molecules, and thus suggests that fibrin actively participates in the regulation of the disease process. For example, studies from our laboratory as well as others showed that fibrin induces rapid disorganization of the endothelial cell monolayers1 and migration of the cells.2 Additionally, fibrin has been shown to induce release of von Willebrand factor from Weibel-Palade bodies of vascular endothelial cell (VEC),3 and to increase synthesis of prostacyclin and tissue plasminogen activator by VEC.4 Furthermore, fibrin degradation product D-dimer has been reported to induce the synthesis and release of biologically active interleukin-1β (IL-1β), IL-6, and plasminogen activator inhibitors from monocytes in vitro.5 These in vitro observations have led us to speculate that fibrin may be a key activator of VEC in vivo. Since the physical presence of fibrin on VEC is the common finding at the site of trauma and inflammation, it is possible that fibrin endothelial cell interaction may be involved in initiation and maintenance of inflammation. In fact, fibrin may be particularly important in the development of the proinflammatory endothelium that controls the movement of cells and fluids during tissue trauma and inflammation.6 The key role of VEC in the development of the proinflammatory endothelium has been shown by expression of various proinflammatory factors such as leukocyte chemotactic factors and adhesion molecules.7 These observations have led us to hypothesize that in vitro fibrin directly activates vascular endothelial cells to express proinflammatory factors such as chemotactic factors and adhesion molecules. We further speculate that in vivo this fibrin-mediated activation of VEC participates in the regulation of inflammatory process by induction of a proinflammatory vascular endothelium that controls recruitment and activation, eg, leukocyte chemotactic factors (LCFs) expression from VEC. In the present study, we have begun to test this hypothesis by determining the ability of fibrin to induce IL-8, a potent LCF, expression from VEC. Further studies are necessary to investigate the role of fibrin in the regulation of other inflammatory mediators in VEC. These studies may be important in not only developing a better understanding of the role of fibrin in tissue injury and inflammation, but possibly providing insights for the development of therapeutic agents that may be useful in regulating inflammation.
MATERIALS AND METHODS
Human umbilical vein endothelial cells (HUVEC). HUVEC were purchased from Clonetics (San Diego, CA) at passage 1 and grown to confluence on plastic culture flasks (Falcon Labware, Oxnard, CA) coated with 0.5% of gelatin (Sigma, St Louis, MO) in EGM media (Clonetics, San Diego, CA), which contain 10% fetal calf serum (FCS; HyClone, Logan, UT), 1.0 mg hydrocortisone, 10 μg epidermal growth factor, 2 mL bovine brain extract, and 0.5 mL gentamycin in 500 mL media. Cells were grown under 5% CO2 , 6% O2 and humidified conditions at 37°C. Routine subcultures were done at 1:3 split ratios with 0.05% trypsin/0.02% EDTA (GIBCO, Grand Island, NY). HUVEC were used at passage 3 to 6 and 1 to 3 days post-confluence.
Coculture of HUVEC with fibrin. Fibrin was polymerized in situ using physiologic relevant concentrations of fibrinogen, and low concentrations of thrombin, to simulate in vivo conditions. Briefly, post-confluent HUVEC (approximately 1 × 106 VEC per T25 flask) were incubated with culture media containing 10% FCS (absence of heparin) overnight at 37°C. The media were aspirated the next day before fresh heparin- free media containing 10% serum was added to each flask. Fibrinogen and thrombin (Sigma) were added directly to HUVEC flasks to achieve final concentrations of fibrin (0.003 to 1.0 mg/mL) and thrombin (0.08 U/mL). Polymerization was complete within 15 minutes at 37°C and produced a three-dimensional gel of fibrin and entrapped culture medium. Culture flasks were handled carefully to avoid disrupting HUVEC-fibrin associations. Control HUVEC received the following: (1) heparin-free media or (2) heparin-free media supplemented with thrombin (0.08 U/mL). Lipopolysaccharide (LPS) 1 μg/mL was used as positive control for IL-8 induction from HUVEC. In selected experiments, HUVEC were cultured in serum-free media for a short period of time (ie, 4 hours). The 4-hour time point was selected because incubation of HUVEC in serum-free media for greater than 4 hours consistently caused HUVEC injury and cell death. The serum-free conditions were required to test fibrinogen effects on HUVEC as a control, because the presence of serum in the media caused spontaneous clotting of fibrinogen to fibrin, presumably due to the presence of thrombin in serum. The short-term culturing under serum-free conditions was used to show that fibrinogen alone or any other contaminants in the fibrinogen preparation were not responsible for IL-8 expression by HUVEC.
Harvest of fibrin-treated HUVEC. Control and fibrin-treated HUVEC were procured after 0.5 to 24 hours. At the time of harvest, media control cells retained a cobblestone appearance. Fibrin-treated HUVEC appeared retracted from each other and had an elongated cell morphology. Flasks containing HUVEC-fibrin cocultures were tapped gently to loosen the fibrin clot from HUVEC, then inverted to allow the fibrin gel to be poured out. HUVEC monolayers remained intact as evidenced by phase-contrast microscopy. The conditioned media were separated from the fibrin matrix by centrifugation (15 minutes at 10,000g ).
Anti-IL-8 antibody preparation. Chicken antihuman IL-8 antibody was prepared by intramuscular injection of 100 mg recombinant human IL-8 (77 amino acids, PeproTech Inc, Rocky Hill, NJ) prepared in Hunter's Titer Max (CytRx Corp, Norcross, GA), and boosted 1 month later after primary immunization. Egg yolks containing antibody were processed as previously described.8 Antibody titer and specificity were assessed by double-immunodiffusion, immunoelectrophoresis, and Western blot analysis.
IL-8 radioimmunoassay (RIA). HUVEC-conditioned medium was analyzed by an IL-8 specific RIA developed in our laboratory. Briefly, the sample (100 μL) was incubated (25°C, 2 hours) with chicken anti-IL-8 (100 μL) diluted (1:4,000) in phosphate-buffered saline (PBS) (containing 1% bovine serum albumin [BSA], 0.1% Triton X-100 [Sigma]). Human 125I-IL-8 (NEN products, Boston, MA) at 70,000 to 80,000 counts per minute/mL was added (100 μL) and the reaction mix was incubated overnight (4°C). Immune complexes were precipitated by adding (100 μL) affinity-purified goat antichicken IgG-coupled microspheres (Kirkegaard & Perry Lab Inc, Gaithersburg, MD) diluted in PBS (1:20). After incubation (25°C, 2 hours) beads were pelleted (2,500g, 15 minutes), blotted and radioactivity was counted. Samples were quantified by reference to a standard curve constructed using rIL-8 standards (0.2 to 10 ng/mL) and levels of IL-8 antigen were expressed ng/1 × 106 cells.
Gel filtration chromatography. HUVEC-conditioned medium was lyophilized, reconstituted (20×) and fractionated (0.5 mL) by gel filtration using 1.0 cm × 45 cm Biogel P-30 column (Bio-Rad, Melville, NY) which has a fractionation range between 2.5 to 40 kD. Columns were standardized with various protein standards of known molecular weight: thyroglobulin (669 kD), albumin (66 kD), carbonic anhydrase (29 kD), Cytochrome C (12.4 kD), aprotinin (6.5 kD), and phenol red (0.4 kD). Fractions (0.9 mL) were eluted (0.08 mL/min) with PBS, pH 7.4 and aliquots from each fraction were assayed for IL-8 antigen by RIA.
Immunoprecipitation. IL-8 antigen in the conditioned media was immunoprecipited with rabbit antihuman IL-8 antibody. Molecular weight and molecular identity of the fibrin induced IL-8 were determined by standard Western blot analysis. Briefly, 5 mL of conditioned media from fibrin treated or nontreated HUVEC were incubated with rabbit antihuman IL-8 antibody (PeproTech) (1:200 dilution) at 4°C in the presence of 0.2% NP40. After overnight incubation, protein-A Sepharose CL-4B at 1:10 was added to the reaction mixture and incubated for 2 hours. The immune complexes were precipitated with protein-A Sepharose, washed, and resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer with reducing agent and assayed by Western blot analysis.
Western blot analysis. The samples from immunoprecipitation by antihuman IL-8 antibody were separated by 15% SDS-PAGE reduced gel and proteins were transferred to Immobilon-P (Millipore, Boston, MA). After the electroblotting was completed, the membrane were blocked with 5% BSA-Tris buffer for 30 minutes at 37°C. The resulting blots were reacted with rabbit anti-IL-8 primary antibody at 1:100 dilution overnight at 4°C, followed by reaction with the secondary antibody, biotinylated goat antirabbit antibody (1:250) for 2 hours at room temperature. The blots were then incubated with HRP-steptavidin (Vector Lab Inc, Burlingame, CA) at 1:250 dilution, washed, and developed with chloro-naphthol. The rabbit anti-IL-8 antibody used in this assay did not cross-react with other cytokines tested by Western blot.
Collagen on HUVEC. The effect of matrix on IL-8 induction was tested by polymerization of collagen on HUVEC monolayers. Collagen gels were prepared from acid-dissolved bovine type 1 collagen (Vitrogen 100) as described by Delvos et al.9 Briefly, the collagen gels were polymerized in situ on HUVEC monolayers at concentrations of 0.1 to 1.0 mg/mL. The type 1 collagen at these concentrations had a consistency similar to the fibrin clots used. After 24 hours incubation, the conditioned media were collected and assayed for IL-8 expression.
Isolation of total RNA and Northern blot analysis. The total RNA were isolated from HUVEC by the method described previously.10 Briefly, HUVEC treated with fibrin, control factors, or control media were lysed with guanidine thiocyanate lysing solution after removal of supernatants from culture flasks. The total RNA were extracted with 0.1 vol of 2 mol/L sodium acetate, 1 vol phenol (water saturated), and 0.2 vol of chloroform. This mixture was vortexed and kept in ice for 15 minutes. The aqueous phase was recovered after centrifugation and resulting RNA was precipitated overnight at −20°C by addition of equal volume isopropanol. Fifteen micrograms of total RNA from each sample were then electrophoresed in a 1.2% agarose gel containing 0.2 mol/L MOPS, 80 mmol/L sodium acetate, 10 mmol/L EDTA, and 6% formaldehyde. The RNA was then transferred to Genescreen (NEN Products, Boston, MA) in 10× SSC (1.5 mol/L sodium chloride and 150 mmol/L sodium citrate) using capillary blotting overnight. The blot was baked and prehybridized at 40°C in a sealed bag containing 5× Denhardt's, 7× sodium saline citrate (SSC), and 0.1 mg/mL boiled salmon sperm DNA (Sigma). The bolt was then probed with IL-8 oligo probe (R&D Systems, Minneapolis, MN) radiolabled by 5′ end labeling with γ32P ATP > 6,000 Ci/mmol (Amersham, Arlington Heights, IL) at 50°C for overnight. The blots were washed at 50°C in 5× SSC with 0.1% SDS. They were then autoradiographed with Kodak XAR film (Eastman Kodak, Rochester, NY) at −70°C with an intensifying screen.
Data analysis. All data were expressed as mean ± SD. Statistically significant differences were determined by using the two-tailed Student's t-test considering P < .05 to be significant.
RESULTS
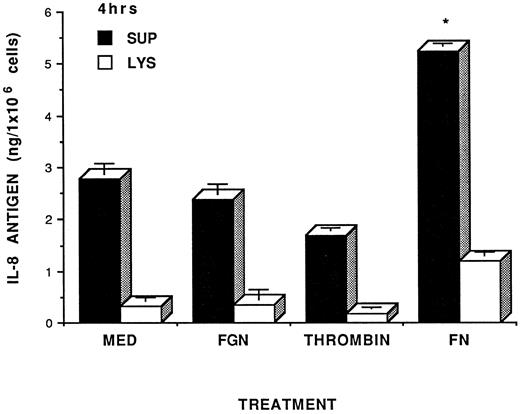
IL-8 expression in fibrin stimulated HUVEC. The ability of fibrin to induce IL-8 expression in HUVEC was initially evaluated by analyzing fibrin HUVEC cocultures (supernatants and cell lysates) for IL-8 using an IL-8 specific RIA. As seen in Fig 1, the increases in both extracellular and cell-associated IL-8 antigen could be seen after HUVEC were in contact with fibrin for 4 hours, ie, there was a twofold increase of extracellular IL-8 antigen in fibrin-treated cells compared with fibrinogen, thrombin or culture medium treated cells (P < .05). It should be noted that this experiment was run in serum-free condition. We used serum-free conditions to test the effects of fibrinogen on HUVEC as a control because the presence of serum in the media caused spontaneous clotting of fibrinogen to fibrin, presumably due to the presence of thrombin in serum. Additionally, the fibrinogen control study could only be run in short period of time (ie, 4 hours), since HUVEC do not survive longer culturing in the absence of serum. It should be noted that there was no change of IL-8 antigen in either supernatants or cell lysates when HUVEC were incubated with fibrinogen, thrombin, or culture medium alone. Other studies have also shown that IL-8 induction was significant at 4 hours coculturing of HUVEC with LPS or cytokines.11 Interestingly, if HUVEC were plated on top of the fibrin matrix, we did not see any significant increase in IL-8 expression (data not shown).
Fibrin induction of IL-8 in HUVEC. HUVEC monolayers were incubated either with control factors such as control media (MED), fibrinogen (FGN, 1.0 mg/mL) thrombin (0.08 U/mL), or with fibrin (FN, 1.0 mg/mL) for 4 hours in the absence of serum. Since the presence of serum causes spontaneous fibrinogen clotting, serum-free culturing studies were undertaken. Previous studies in our laboratory indicated that serum-free culturing of HUVEC for more than 4 hours resulted in significant cell injury and death. Therefore, we were limited to short-term cell culture studies (ie, 4 hours) for this specific study. After incubation, the conditioned media were collected and cells were lysed with 0.1% Triton/PBS. The conditioned media (▪) and cell lysates (□) from HUVEC monolayer treated with fibrin or other control factors were tested for IL-8 induction by specific IL-8 RIA. Level of IL-8 antigen were expressed as nanogram per 1 × 106 cells. Results were expressed as the mean of IL-8 ± SD and were representative of two separate experiments with similar results. Asterisk indicates statistical significance of P ≤ .05 compared to media control.
Fibrin induction of IL-8 in HUVEC. HUVEC monolayers were incubated either with control factors such as control media (MED), fibrinogen (FGN, 1.0 mg/mL) thrombin (0.08 U/mL), or with fibrin (FN, 1.0 mg/mL) for 4 hours in the absence of serum. Since the presence of serum causes spontaneous fibrinogen clotting, serum-free culturing studies were undertaken. Previous studies in our laboratory indicated that serum-free culturing of HUVEC for more than 4 hours resulted in significant cell injury and death. Therefore, we were limited to short-term cell culture studies (ie, 4 hours) for this specific study. After incubation, the conditioned media were collected and cells were lysed with 0.1% Triton/PBS. The conditioned media (▪) and cell lysates (□) from HUVEC monolayer treated with fibrin or other control factors were tested for IL-8 induction by specific IL-8 RIA. Level of IL-8 antigen were expressed as nanogram per 1 × 106 cells. Results were expressed as the mean of IL-8 ± SD and were representative of two separate experiments with similar results. Asterisk indicates statistical significance of P ≤ .05 compared to media control.
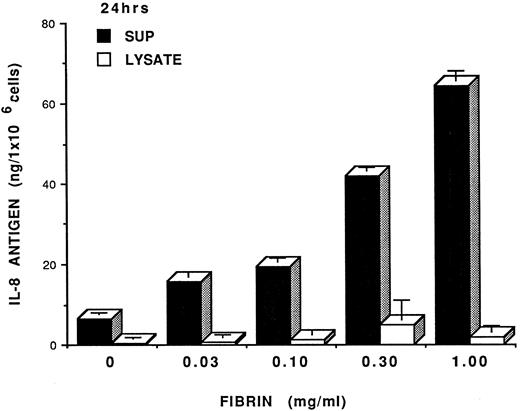
Fibrin concentration dependent IL-8 induction. In our initial studies, we showed that fibrin endothelial cell coculture induced endothelial cell activation as evidenced by IL-8 expression. To extend our observation, we next determined a dose response of fibrin in IL-8 induction in HUVEC. For this study, HUVEC were incubated with various concentrations of fibrin (0.03 to 1.0 mg/mL) or control factors (eg, thrombin or culture media) for 24 hours. The conditioned media and cell lysates were then harvested and analyzed for IL-8 antigen. Fibrin induced IL-8 expression from HUVEC in a dose-dependent fashion (Fig 2). The maximal IL-8 induction could be seen when HUVEC were treated with fibrin at 1.0 mg/mL. When a concentration of fibrin higher than 1 mg/mL was used (ie, 2 mg/mL), there was no increase in IL-8 expression when compared to fibrin concentration 1 mg/mL. Interestingly, fibrin, at a concentration as low as 0.03 mg/mL induced an increased IL-8 expression relative to control HUVEC. Thrombin (0.08 μ/mL) had no effect on IL-8 induction (not shown). Furthermore, IL-8 antigen expression induced by fibrin was dependent on protein synthesis, since HUVEC treated with fibrin and cycloheximide resulted in significant inhibition of IL-8 expression (data not shown). It should be noted that the fibrin obtained from the in situ studies was likely cross-linked due to the fact that we were unable to solubilize the fibrin clot with either 8 mol/L urea or 10% SDS. This is not surprising since the culture media contains 10% serum, thus, providing the factors needed to cross-link the fibrin.
Fibrin dose-dependent IL-8 induction in HUVEC. Endothelial cell monolayers were incubated with fibrin at different concentrations for 24 hours IL-8 antigen were determined by specific IL-8 RIA. Results were expressed as mean of IL-8 ng/ 1 × 106 cells ± SD. The results were representative of 3 separate experiments with similar results.
Fibrin dose-dependent IL-8 induction in HUVEC. Endothelial cell monolayers were incubated with fibrin at different concentrations for 24 hours IL-8 antigen were determined by specific IL-8 RIA. Results were expressed as mean of IL-8 ng/ 1 × 106 cells ± SD. The results were representative of 3 separate experiments with similar results.
Time-dependent IL-8 expression. To determine the IL-8 time response of HUVEC to fibrin, kinetic expression of IL-8 from fibrin treated HUVEC was studied by in situ incubation of HUVEC with fibrin 1 mg/mL for various times. As shown in Fig 3, extracellular IL-8 antigen appeared as early as 2 hours, increased at 8 hours and remained at a high level at 24 hours. IL-8 antigen levels in the conditioned media at all time points tested here were significantly different from the supernatants of nonfibrin treated cells (P < .005). The level of cell associated IL-8 antigen was not significantly different from untreated cells. IL-8 antigen was not detected in the control cultures of HUVEC treated with thrombin, fibrinogen, or media.
Time course of expression of fibrin-induced IL-8 by HUVEC. HUVEC monolayers were treated with fibrin (1 mg/mL) or with control media at various times. The supernatants from control cell (c sup) or fibrin treated cells (FN sup) and cell lysates from control cells (c lys) or fibrin treated cells (FN lys) were collected and measured for IL-8 antigen. Each data point represents the mean of IL-8 ng/ 1 × 106 cells ± SD of duplicate samples. The results were representative of two separate experiments.
Time course of expression of fibrin-induced IL-8 by HUVEC. HUVEC monolayers were treated with fibrin (1 mg/mL) or with control media at various times. The supernatants from control cell (c sup) or fibrin treated cells (FN sup) and cell lysates from control cells (c lys) or fibrin treated cells (FN lys) were collected and measured for IL-8 antigen. Each data point represents the mean of IL-8 ng/ 1 × 106 cells ± SD of duplicate samples. The results were representative of two separate experiments.
Molecular weight estimate of fibrin induced IL-8 by gel filtration chromatography. The 24-hour fibrin conditioned media were concentrated 20× by lyophilization, and fractionated by applying to a gel filtration P-30 column which has a fractionation range between 2.5 to 40 kD. The column was previously calibrated with protein standards. Samples were eluted under physiologic salt and pH conditions. Protein concentration was determined by spectrophotometer at 280 nm and IL-8 antigen was assayed by RIA from each fraction. The result was expressed as mean of IL-8 ng/mL ± SD of a duplicate sample and was representative of 2 separate experiments. One major IL-8 peak appeared with molecular weight range between 6,500 to 29,000 d.
Molecular weight estimate of fibrin induced IL-8 by gel filtration chromatography. The 24-hour fibrin conditioned media were concentrated 20× by lyophilization, and fractionated by applying to a gel filtration P-30 column which has a fractionation range between 2.5 to 40 kD. The column was previously calibrated with protein standards. Samples were eluted under physiologic salt and pH conditions. Protein concentration was determined by spectrophotometer at 280 nm and IL-8 antigen was assayed by RIA from each fraction. The result was expressed as mean of IL-8 ng/mL ± SD of a duplicate sample and was representative of 2 separate experiments. One major IL-8 peak appeared with molecular weight range between 6,500 to 29,000 d.
IL-8 molecular weight estimation by gel filtration chromatography. To confirm the molecular identity of the IL-8 antigen detected in the fibrin-VEC coculture, the molecular weight of fibrin induced IL-8 was determined by gel filtration chromatography. For these studies, fibrin conditioned media from the HUVEC were concentrated 20× and applied to a P30 gel filtration column, which has a fractionation range of 2.5 to 40 kD. Fractions from the P-30 column were measured for protein concentration by spectrophotometer at 280 nm and assayed for IL-8 by RIA. The major protein peak appeared with a molecular weight greater than 29 kD. The peak IL-8 antigen occurred in the molecular weight range between 6.5 and 29 kD, whereas other fractions did not contain IL-8 antigen (Fig 4).
Immunoprecipitation and Western blot analysis. To confirm and extend our initial molecular characterization of the fibrin induced IL-8, the antigenic identity and molecular weight of the fibrin induced IL-8 was studied by Western blot analysis. For these studies, supernatants from fibrin treated or untreated VEC culture for 24 hours were incubated with rabbit antihuman IL-8 antibody and immune complexes were precipitated with protein A beads. The resulting samples were applied to 15% SDS-PAGE gel and analyzed by standard Western blot using rabbit antihuman IL-8 antibody. As seen in Fig 5, there was a weak band in nonfibrin treated supernatant which may represent the basal level of IL-8 antigen (lane 3). In contrast, the significant increase in the band corresponding to molecular weight of approximately 10 kD could be seen in the fibrin-treated conditioned media lane (lane 4). This fibrin induced band migrated to the same position as recombinant IL-8 (lane 2), supporting the molecular identity of fibrin induced IL-8 as similar to that of recombinant IL-8. Conditioned media without specific IL-8 antibody mediated immunoprecipitation were also run in parallel (lanes 5 and 6). The anti-IL-8 antibody reacting band could only be observed in fibrin conditioned supernatant (lane 6), not in untreated supernatant (lane 5). There was no visible band seen from thrombin treated supernatants (lane 7) or from fibrinogen itself (lane 8). The Western blot analysis showed the antigenic identity of fibrin induced IL-8 and the molecular weight of fibrin induced IL-8 is similar to recombinant IL-8.
Western blot analysis of fibrin induced IL-8 expression in HUVEC. Twenty-four hour control or fibrin treated HUVEC supernatants were either immunoprecipitated with anti-IL-8 antibody before being applied to SDS-PAGE or added to SDS-PAGE directly. The resulting blot was analyzed by standard Western blot assay. The molecular weight marker showed on lane 1. Human recombinant IL-8 (50 ng) as positive control was on lane 2. The control or fibrin treated HUVEC samples with anti-IL-8 antibody immunoprecipitation were on lane 3 and lane 4. The samples without immunoprecipitation were on lane 5 or lane 6, respectively. Lane 7 and lane 8 were samples from thrombin treated cells or fibrinogen itself (1 mg/mL), respectively.
Western blot analysis of fibrin induced IL-8 expression in HUVEC. Twenty-four hour control or fibrin treated HUVEC supernatants were either immunoprecipitated with anti-IL-8 antibody before being applied to SDS-PAGE or added to SDS-PAGE directly. The resulting blot was analyzed by standard Western blot assay. The molecular weight marker showed on lane 1. Human recombinant IL-8 (50 ng) as positive control was on lane 2. The control or fibrin treated HUVEC samples with anti-IL-8 antibody immunoprecipitation were on lane 3 and lane 4. The samples without immunoprecipitation were on lane 5 or lane 6, respectively. Lane 7 and lane 8 were samples from thrombin treated cells or fibrinogen itself (1 mg/mL), respectively.
Effect of matrix on IL-8 induction. As a control for possible nonspecific “matrix”-associated effects on IL-8 induction from HUVEC, we tested the ability of alternative insoluble matrix in IL-8 induction from HUVEC. Native type I collagen gels (Invitrogen) were polymerized in situ on cultures of HUVEC and collagen had a consistency similar to the fibrin clots used. The concentrations of collagen gels were prepared using the same concentrations as that of the fibrin used. Figure 6 shows that HUVEC incubation with collagen at concentrations of 0.1 to 1.0 mg/mL did not increase the basic level of IL-8 expression. Other matrices, such as agarose which had also been tested for the matrix effect, did not show increased IL-8 expression from HUVEC either (data not shown). These results showed that IL-8 induction from HUVEC is not due to nonspecific polymerization of insoluble matrix on the cells.
Effect of matrix on IL-8 induction. The conditioned media were collected after 24-hour incubation of HUVEC monolayers with control media, or various concentrations of fibrin or collagen (0.1 to 1.0 mg/mL) and analyzed by specific IL-8 RIA. Results are expressed as mean ± SD of duplicate samples and are representative of 2 individual studies.
Effect of matrix on IL-8 induction. The conditioned media were collected after 24-hour incubation of HUVEC monolayers with control media, or various concentrations of fibrin or collagen (0.1 to 1.0 mg/mL) and analyzed by specific IL-8 RIA. Results are expressed as mean ± SD of duplicate samples and are representative of 2 individual studies.
Fibrin induction of IL-8 mRNA expression. To determine whether the IL-8 antigen increase in fibrin treated HUVEC was due to elevated steady state mRNA expression, we next examined the expression of steady-state IL-8 mRNA in HUVEC. The total RNA from 4 hour fibrin-treated or nontreated HUVEC were isolated and analyzed by standard Northern blot. As could be seen in Fig 7, fibrin increased steady state levels of IL-8 in a dose-dependent fashion. Fibrin at 0.01 mg/mL (lane 3) showed an apparent increase in IL-8 mRNA. The peak effect of fibrin on IL-8 mRNA induction was seen with 1 mg/mL of fibrin (lane 7). IL-8 mRNA expression induced by 1 mg/mL of fibrin on HUVEC were comparable to the effect by LPS (5 μg/mL) (lane 8). In contrast, untreated (lane 1) and thrombin (0.08 u/mL) treated endothelial cells (lane 9) showed the basal level of IL-8 mRNA expression.
Fibrin dose-dependent IL-8 mRNA expression. IL-8 mRNA expression in response to various doses of fibrin (top) and 28S and 18S ribosomal RNA (bottom). Incubation of HUVEC with fibrin was performed for 4 hours. Fibrin concentrations 0, 0.003, 0.01, 0.03, 0.1, 0.3, and 1.0 mg/mL were on lanes 1, 2, 3, 4, 5, 6, and 7, respectively. A sample from LPS-treated HUVEC was used as a positive control (lane 8). RNA from thrombin treated cells was run as control (lane 9). The RNA blot data in this figure are representative of two individual studies.
Fibrin dose-dependent IL-8 mRNA expression. IL-8 mRNA expression in response to various doses of fibrin (top) and 28S and 18S ribosomal RNA (bottom). Incubation of HUVEC with fibrin was performed for 4 hours. Fibrin concentrations 0, 0.003, 0.01, 0.03, 0.1, 0.3, and 1.0 mg/mL were on lanes 1, 2, 3, 4, 5, 6, and 7, respectively. A sample from LPS-treated HUVEC was used as a positive control (lane 8). RNA from thrombin treated cells was run as control (lane 9). The RNA blot data in this figure are representative of two individual studies.
Kinetics of IL-8 mRNA expression. To characterize the temporal induction of IL-8 mRNA in HUVEC by fibrin, the kinetics of IL-8 mRNA expression in fibrin treated endothelial cells were examined. As seen in Fig 8, HUVEC showed a rapid increase in IL-8 mRNA steady state levels in response to fibrin (1 mg/mL). Steady-state IL-8 mRNA levels increased in a time-dependent fashion, with increased amounts of IL-8 mRNA seen within the first 1 hour (lane 7), with peak expression occurring at 6 hours post-fibrin (1 mg/mL) stimulation. Levels of IL-8 mRNA in 6 hours fibrin-treated HUVEC were comparable with that in 4 hours LPS (5 μg/mL) treated HUVEC (lane 3). IL-8 mRNA in fibrin-treated HUVEC migrated to the same position as monocyte IL-8 mRNA (1.8 kb) (lane 1). Thrombin-treated HUVEC did not increase IL-8 mRNA expression (lane 2).
Time kinetic expression of IL-8 mRNA from fibrin treated HUVEC. Northern blot analysis of IL-8 mRNA expression (top), and 28S and 18S ribosomal RNA (bottom). Lanes 4, 6, 8, 10, and 12 were RNA from HUVEC with control media for 0.5, 1.0, 2.0, 4.0, and 6.0 hours, respectively. Samples from fibrin (1 mg/mL) treated cells for 0.5, 1, 2, 4, and 6 hours were on lanes 5, 7, 9, 11, and 13, respectively. Endothelial cells treated either with thrombin (lane 2), or with LPS 5 μg/mL (lane 3) for 4 hours, and LPS treated human monocytes (lane 1) were run as controls. The RNA blot analyses are representative of two individual studies.
Time kinetic expression of IL-8 mRNA from fibrin treated HUVEC. Northern blot analysis of IL-8 mRNA expression (top), and 28S and 18S ribosomal RNA (bottom). Lanes 4, 6, 8, 10, and 12 were RNA from HUVEC with control media for 0.5, 1.0, 2.0, 4.0, and 6.0 hours, respectively. Samples from fibrin (1 mg/mL) treated cells for 0.5, 1, 2, 4, and 6 hours were on lanes 5, 7, 9, 11, and 13, respectively. Endothelial cells treated either with thrombin (lane 2), or with LPS 5 μg/mL (lane 3) for 4 hours, and LPS treated human monocytes (lane 1) were run as controls. The RNA blot analyses are representative of two individual studies.
DISCUSSION
In these studies, we found that fibrin induced human endothelial cell retraction, the same morphologic changes observed in bovine pulmonary artery endothelial cells.12 This result supported our speculation that although our VEC are clearly from distinct origins they would respond in a similar way to fibrin since the vascular endothelial cells and fibrin would function similarly in both species. In vivo, this fibrin induced endothelial cell retraction may have clinical significance since this retraction of VEC would promote the inflammation process by expediting the movement of both fluid and cellular components between the retracted VEC. In fact, previous studies have implicated various fibrin(ogen) metabolites in the increased vasopermeability, seen in various diseases and experimental models of inflammation.13 Far less is known about the role of fibrin-endothelial cell interactions in leukocyte recruitment and activation seen in inflammatory reactions. It should be noted that although fibrin could induce IL-8 expression in the HUVEC, fibrin(ogen) (1 mg/mL), Fibrinopeptide A (10−5 mol/L), Fibrinopeptide B (10−5 mol/L) or Fibrinopeptide A + Fibrinopeptide B could not induce IL-8 expression in the HUVEC.
The movement of leukocytes from blood into tissues mediated by chemoattractants is a characteristic feature of inflammation. Activated endothelial cells can express a number of chemotactic molecules including IL-8, in response to cytokine and LPS stimulation.14 In our present study, we found that fibrin is a potent endothelial cell activator, as reflected by its ability to induce IL-8 antigen expression in the endothelial cells. The molecular identity of fibrin induced IL-8 has identical molecular weight and antigenic identity as monocyte derived IL-8.11
Additionally, our studies showed that fibrin activity in induction of IL-8 from HUVEC is not due to LPS contamination of the fibrin(ogen) or thrombin because: (1) in the control experiments, neither fibrinogen nor thrombin increased IL-8 expression in either antigen or mRNA level; and (2) LPS inhibitor, Polymyxin B significantly inhibited LPS induced IL-8 induction, but did not have significant effect on fibrin induced IL-8 expression from HUVEC (data not shown). This result suggested the specific effect of fibrin on IL-8 expression in HUVEC. The other important feature of fibrin induced IL-8 expression is that fibrin at a concentration of 30 μg/mL, 100 times lower than that in serum, could induce a detectable level of IL-8. This result indicated that only a small amount of fibrin formation at the local area could initiate the IL-8 expression and inflammatory process.
In our kinetic studies, we found that fibrin induced a rapid IL-8 antigen expression, observed as early as 2 hour post-fibrin exposure, and maintained high levels at 24 hours. This early expression of fibrin induced IL-8 may be responsible for leukocyte movement in the early phase of inflammation process in vivo. Studies from several laboratories have implicated IL-8 as a proinflammatory mediator in vitro, eg, IL-8 stimulates neutrophil chemotaxis and activation15 and in vivo, IL-8 causes neutrophil infiltration at the site of subcutaneous injection.16 Recent studies by Rot17 showed that the presence of IL-8 binding sites on vascular endothelial cells in a variety of species including human. This increased local IL-8 concentration by IL-8 binding to endothelial cells is critical in inducing leukocyte migration. Fibrin which polymerized on endothelial cells induced inflammatory mediator IL-8 expression. On the other hand, fibrin meshwork may also maintain the high concentration of this chemoattractant factor by specific or nonspecific binding of IL-8 to fibrin. This may suggest that fibrin induced IL-8 expression may be involved in not only initiating but also mediating more prolonged inflammatory cell influx during the inflammatory process.
Fibrin as a stimulus is different from other factors, since fibrin forms an insoluble matrix. Thus, the question could be raised whether the IL-8 induction by fibrin is specific for fibrin or will any matrix induce the same effect? To answer this question, we studied the effect of other matrices on IL-8 expression in HUVEC. In our studies, we found neither collagen nor agarose induced IL-8 expression in HUVEC. Thus, we feel that fibrin induced IL-8 expression is not due to (1) the mechanical pressure formed by a matrix; (2) altered gas exchange; or (3) nonspecific interaction between protein polymer and vascular endothelial cells. Alternatively, we hypothesize that the fibrin induced IL-8 is a result of the specific binding of fibrin to HUVEC.
Our studies showed that IL-8 antigen induction by fibrin is due to increasing steady state level of IL-8 message in HUVEC. Clearly, this increase in steady-state mRNA level could be the result of increased synthesis and/or reduced mRNA degradation. IL-8 mRNA induction is time and dose dependent. As we see in the antigen expression, fibrin at 10 μg/mL induced increased amount of IL-8 mRNA. In vitro activation of HUVEC by fibrin results in the rapid induction of IL-8 message. IL-8 mRNA is apparent at 1 hour, maintaining high levels up to 6 hours and returning to baseline at 8 hours. IL-8 mRNA induction in endothelial cells by IL-1β, LPS, and tumor necrosis factor-α (TNF-α) have been shown to have maximal steady-state levels at 4 to 8 hours and persistence at 24 hours. In contrast, neutrophil-derived steady-state levels of IL-8 mRNA were maximal at 1 hour post-stimulation with LPS, TNF, or IL-1β, but then rapidly declined to essentially unmeasurable levels by 24 hours, even though the maximal levels of antigenic IL-8 were seen 24 hours post-stimulation.18 In our studies, this rapid regulation of IL-8 mRNA by fibrin in HUVEC is unknown. However, it may reflect the ability of vascular endothelial cells to respond rapidly to an activation signal delivered by fibrin. Less stability or rapid turnover of IL-8 mRNA in response to fibrin stimulation may also account for the rapid decline of IL-8 message. This rapid regulation of IL-8 mRNA by fibrin may be also due to the relative long-life and resistance to proteolytic enzymatic cleavage nature of IL-8 antigen as compared to other chemotactic factors.
It must be emphasized that this study supports our current hypothesis that fibrin activation of the VEC directly participates in inflammation by contributing to leukocyte activation and recruitment via activation of VEC that results in the release of proinflammatory cytokines from the fibrin-activated VEC. This study indicated that the fibrin induced increases of IL-8 expression from vascular endothelial cells may be responsible for recruitment of neutrophils to the site of inflammation in vivo since fibrin deposition is a common finding in a variety of disease processes. Fibrin deposition is universally seen in the inflammation process. The possibility of fibrin involvement in other cellular processes would provide insights into multifunctions of fibrin. Further studies will be needed to dissect the existence and nature of other inflammatory mediators that induce leukocyte migration and adhesion such as leukocyte adhesion molecules from vascular endothelial cells.
Supported in part by grants from The National Institutes of Health (Bethesda, MD), The Connecticut Lions Research Foundation (Hartford, CT), and Connecticut Eye Research Foundation (New Britain, CT).
Address reprint requests to Donald L. Kreutzer, PhD, Departments of Pathology and Surgery, University of Connecticut School of Medicine, Farmington, CT 06030-3105.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal