Abstract
We have recently identified a novel ligand of the vascular endothelial growth factor (VEGF) family termed VEGF-related protein (VRP), which specifically binds to the FLT4 receptor. To characterize the signaling events after VRP engagement of its cognate receptor in hematopoietic cells, a population of human erythroleukemia (HEL) cells, termed HEL-JW, expressing high levels of FLT4 receptor was isolated. Stimulation of HEL-JW cells with VRP alone and in combination with the c-kit ligand/stem cell factor increased cell growth. VRP induced tyrosine phosphorylation of various proteins, including the FLT4 receptor. Further characterization of these tyrosine phosphorylated molecules revealed that Shc, Grb2, and SOS form a complex with the activated FLT4 receptor. HEL-JW cells also expressed RAFTK, a recently identified member of the focal adhesion kinase family. RAFTK was phosphorylated and activated upon VRP treatment, and there was an enhanced association of this kinase with the adaptor protein Grb2. Furthermore, the c-Jun NH2-terminal kinase (JNK), involved in growth activation and shown to mediate RAFTK signaling in other cell types, was activated by VRP stimulation. We also observed that VRP treatment of HEL-JW cells resulted in the phosphorylation of the cytoskeletal protein paxillin. This treatment resulted in an increased association of paxillin with RAFTK, which was mediated by the C-terminal region of RAFTK. These studies indicate that VRP stimulation induced the formation of a signaling complex at its activated receptor as well as activation of RAFTK. VRP-mediated activation of RAFTK may facilitate signal transduction to the cytoskeleton and downstream to the JNK pathway in FLT4-expressing blood cells.
THE FLT4 receptor belongs to a family of protein tyrosine kinases that mediates signal transduction after engagement of their cognate ligands through a cascade of phosphorylation events.1-3 Along with FLT1 and FLK1 (or KDR), the two vascular endothelial growth factor (VEGF) receptors,4-7 FLT4 is a member of a subfamily of class III receptor tyrosine kinases characterized by seven extracellular Ig-like domains and a split, intracellular domain containing kinase activity.1,2,8-11 These three receptors have 31% to 36% amino acid identity in their extracellular ligand binding domains. The human FLT4 locus encodes two isoforms. The long form, FLT4L, differs from the short form, FLT4S, as it contains an additional 65 amino acids in the C-terminal region. Current data indicate that FLT4L may possess transforming activity whereas FLT4S does not.10,12-15 These long and short variants migrate as bands of 170 to 190 kD that appear to be partially cleaved proteolytically in the extracellular domain to produce a form of ∼125 kD.10 15
Tissue expression of the FLT4 receptor is relatively restricted, being found predominantly in lymphatic and microvascular endothelium.16,17 Some leukemia cell lines also have been shown to express both types of the FLT4 receptor,13 15 indicating that this receptor may be expressed on hematopoietic cells and play a role in the regulation of hematopoiesis.
Recently, we and others have identified a novel ligand for the FLT4 receptor that has homology with the VEGF protein family. This novel ligand was therefore termed VEGF-related protein (VRP)18 or VEGF-C.19 VRP or VEGF-C was found, in vitro, to stimulate the proliferation of human lung endothelial cells18 and the migration of bovine capillary endothelial cells.19 Using transfected Rat2 or NIH 3T3 fibroblasts13-15 containing a chimeric receptor made of the extracellular region of the c-Fms receptor and of the transmembrane and intracellular regions of FLT4, the signaling pathways of FLT4 have been preliminarily investigated. In these studies, it was shown that the FLT4 signaling pathway involved the interaction of the Shc and Grb2 adaptor molecules with the cytoplasmic domain of the chimeric receptor.13-15
After the discovery of VRP, we were then able to study the signaling events mediated by the native FLT4 receptor in hematopoietic cells using its cognate ligand. To that end, from a heterogeneous culture of human erythroleukemia (HEL) cells, we have isolated by fluorescence cell sorting a permanent hematopoietic cell line that robustly expresses the FLT4 receptor. Of particular note are our observations that the FLT4 receptor pathway in these blood cells involves, in addition to Shc and Grb2, a recently identified signaling molecule called related adhesion focal tyrosine kinase RAFTK/Pyk2/CAK-β20-22 and that FLT4 receptor stimulation is associated with enhanced JNK activation. These changes in signal transduction may be related to the observed mitogenic effects of VRP on these hematopoietic cells.
MATERIALS AND METHODS
Reagents and antibodies. Recombinant VRP was cloned and expressed as previously described.18 Antibodies to RAFTK were generated using Glutathione-S-transferase (GST)-fusion proteins to various domains of the molecule and by immunizing New Zealand rabbits as previously described.20 23 Serum R-4250, which did not cross-react with FAK and has previously been shown to be specific for RAFTK, was used for further studies. Rabbit polyclonal antibody to FLT4 was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). The mouse antiphosphotyrosine monoclonal antibody (MoAb) 4G10 was a generous gift of Dr Brian Druker (University of Oregon), and rabbit anti-Shc and anti-SOS polyclonal antibodies were purchased from Upstate Biotechnology, Inc (Lake Placid, NY). Anti-Grb2 and antipaxillin mouse MoAb were purchased from Transduction Laboratories (Lexington, KY). Normal rabbit serum and purified normal rabbit IgG were purchased from Organon Teknika Corp (West Chester, PA). Electrophoresis reagents and nitrocellulose membrane were obtained from Bio-Rad Laboratories (Hercules, CA). The protease inhibitors leupeptin, aprotinin, and alpha 1 antitrypsin and all other reagents were obtained from Sigma Chemical Co (St Louis, MO). Protein A-Sepharose CL-4B and Glutathione Sepharose 4B were obtained from Pharmacia Biotech (Alameda, CA).
Cells and cell culture. HEL cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and shown to be mycoplasma-free before their expansion in culture. The cells expressed markers of human megakaryocytes including the surface integrin GpIIb/IIIa and the FLT4 receptor.13 15 Using fluorescence cell sorting, a subpopulation of HEL cells, termed HEL-JW, were selected using polyclonal anti-FLT4 antibodies. These cells were expanded in RPMI-1640 medium containing 10% fetal calf serum (FCS), 2 mmol/L glutamine, 50 μg/mL penicillin, and 50 μg/mL streptomycin.
Ligand stimulation of cells. HEL-JW cells were starved in serum-free RPMI-1640 medium for 5 hours, washed twice with phosphate-buffered saline (PBS; GIBCO-BRL, Gaithersburg, MD) containing 0.1% bovine serum albumin (BSA), and then resuspended at 12 × 106/mL in PBS with 0.1% BSA. Cells were then stimulated in vitro with VRP at a range of concentrations for different time periods at 37°C. After stimulation, cell lysates were prepared by lysis in modified RIPA (50 mmol/L Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL of aprotinin, leupeptin and pepstatin, 10 mmol/L sodium vanadate, 10 mmol/L sodium fluoride, and 10 mmol/L sodium pyrophosphate). Total cell lysates (TCLs) were clarified by centrifugation at 10,000g for 10 minutes. Protein concentrations were determined by protein assay (Bio-Rad Laboratories).
Immunoprecipitation and Western blot analysis. For the immunoprecipitation studies, identical amounts of protein from each sample were clarified by incubation with protein A-Sepharose) for 1 hour at 4°C. Following the removal of protein A-Sepharose by brief centrifugation, the solution was incubated with different primary antibodies as detailed below for each experiment for 4 hours or overnight at 4°C. Immunoprecipitations of the antibody-antigen complexes were performed by incubation for 2 hours at 4°C with 75 μL of protein A-Sepharose (10% suspension). Nonspecific bound proteins were removed by washing the Sepharose beads three times with modified RIPA buffer and one time with PBS. Bound proteins were solubilized in 40 μL of 2× Laemmli sample buffer and further analyzed by immunoblotting. Samples were separated on 8% to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk protein and probed with primary antibody for 2 hours at room temperature (RT) or 4°C overnight. Immunoreactive bands were visualized using horseradish peroxidase (HRP)-conjugated secondary antibody and the enhanced chemiluminescent (ECL) system (Amersham Corp, Arlington Heights, IL). Immunoreactive bands were quantitated by scanning the blot under the Model GS-700 Imaging Densitometer (Bio-Rad). Control lanes were assigned a value of 1 and the quantitation of the immunoreactive bands was expressed as multiples of the control based on the densitometry values. Each experiment was repeated at least three times and the presented blots are representative of these experiments.
GST-fusion protein binding studies. A GST-fusion protein of the C-terminal domain of RAFTK was prepared as described.21 For the binding experiments, 7.5 μg of RAFTK-GST-fusion protein was added to 1.5 mg of cell lysate and the mixture was then incubated overnight at 4°C on a rotatory shaker. GST protein alone (Santa Cruz Biotechnology) was used as a negative control. Fifty microliters of the Glutathione Sepharose 4B beads were added to absorb the complex. After incubation for 4 hours at 4°C on a rotatory shaker, the beads were centrifuged, and washed three times with modified RIPA buffer. The bound proteins were eluted by boiling in Laemmli sample buffer and subjected to 8% SDS-PAGE and Western blot analysis.
JNK kinase assay. Cell lysates were immunoprecipitated with JNK antibody (Santa Cruz Biotechnology). The immune complex was washed twice with RIPA buffer and once in kinase buffer (50 mmol/L HEPES, pH 7.4, 10 mmol/L MgCl2 , 20 μmol/L adenosine triphosphate [ATP]). The complex was then incubated in a kinase buffer containing recombinant GST c-Jun 0.2 μg/μL (1-79 amino acids) (Santa Cruz Biotechnology) and 5 μCi γ32P-ATP for 30 minutes at RT. The reaction was terminated by adding 2× SDS sample buffer and boiling the sample for 5 minutes at 100°C. Proteins were separated on 12% SDS-PAGE and detected by autoradiography. Rabbit IgG was used as a negative control.
In vitro kinase assay. Cell lysates were immunoprecipitated with RAFTK antiserum. The immunoprecipitated complexes were washed twice with RIPA buffer and once in a kinase buffer (20 mmol/L HEPES, pH 7.4, 50 mmol/L NaCl, 5 mmol/L MgCl2 , 5 mmol/L MnCl2 , and 100 mmol/L Na3VO4 ). The immune complex was incubated in a kinase buffer containing 20 μg of poly ([Glu:Tyr] [4:1] 20 to 50 kD) (Sigma) and 5 μCi γ32P-ATP at RT for 15 minutes. The reaction was stopped by adding 2× SDS sample buffer and boiling the sample for 5 minutes at 100°C. Proteins were then separated on 7.5% SDS-PAGE and detected by autoradiography. Normal rabbit serum was used as a negative control.
Cell proliferation assay. Cell proliferation was measured using a nonradioactive cell proliferation assay system (MTT assay; Boehringer Mannheim, Indianapolis, IN). HEL-JW cells (2 × 104 ) were plated in 96-well plates in 100 μL RPMI-1640 medium with 1.0% FCS in the presence or absence of various concentrations of VRP with or without recombinant human stem cell factor (SCF; Amgen Inc, Thousand Oaks, CA) or interleukin-3 (IL-3; R & D Systems, Minneapolis, MN). After 48 hours of culture at 37°C, 10 μL of MTT (5 mg/mL) were added to each well. The reaction was stopped after 4 hours of incubation by adding 100 μL of 0.04 N HCl in isopropanol. The optical density (OD) value was obtained by measuring absorbance at the wavelength of 560 nm.
RESULTS
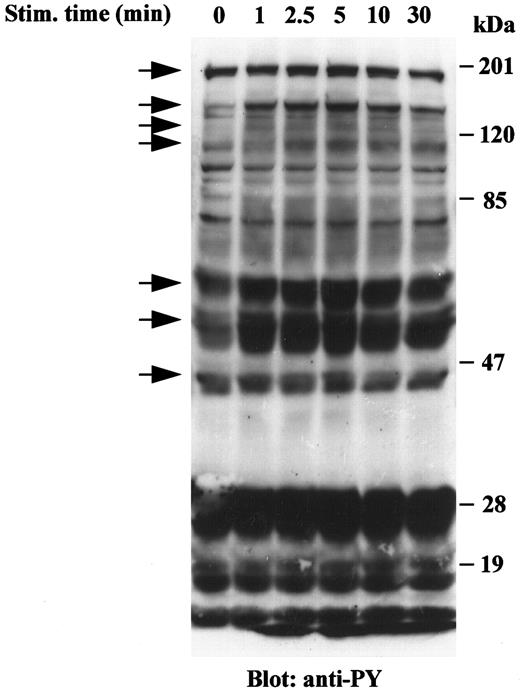
VRP induces tyrosine phosphorylation of cellular proteins in HEL-JW cells. Signal transduction by receptor tyrosine kinases involves the phosphorylation of an array of target proteins. Accordingly, we analyzed the spectrum of substrates phosphorylated after FLT4 receptor stimulation by its cognate ligand, VRP. Treatment of HEL-JW cells with a range of VRP concentrations was initially performed, and an optimal concentration of 125 ng/mL was chosen based on the maximum intensity of protein phosphorylation on SDS-PAGE. As shown in Fig 1, VRP stimulation resulted in the increased tyrosine phosphorylation of more than seven different proteins. These seven major bands had approximate molecular masses of 180, 145, 125, 115, 66, 52, and 46 kD. Tyrosine phosphorylation of these proteins was rapid and transient, observed within 1 minute, became maximal between 2.5 and 5 minutes and declined thereafter within 30 minutes. These results indicated that the FLT4 receptor on HEL-JW cells is functional and transduces signals by inducing the tyrosine phosphorylation of a number of proteins.
Tyrosine phosphorylation of cellular proteins in HEL-JW cells after VRP stimulation. Serum-starved HEL-JW cells (12 × 106/mL) were stimulated with VRP (125 ng/mL) for the indicated times. TCLs were prepared, fractionated on 10% SDS-PAGE and directly subjected to Western blot analysis with the antiphosphotyrosine antibody 4G10. The phosphorylated proteins present after VRP treatment are indicated by arrows.
Tyrosine phosphorylation of cellular proteins in HEL-JW cells after VRP stimulation. Serum-starved HEL-JW cells (12 × 106/mL) were stimulated with VRP (125 ng/mL) for the indicated times. TCLs were prepared, fractionated on 10% SDS-PAGE and directly subjected to Western blot analysis with the antiphosphotyrosine antibody 4G10. The phosphorylated proteins present after VRP treatment are indicated by arrows.
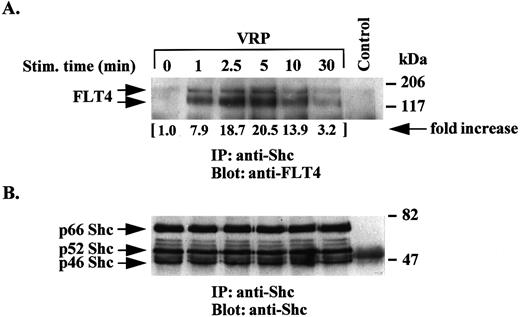
Time course of tyrosine phosphorylation of the FLT4 receptor by VRP. We next examined the time course of receptor phosphorylation following VRP treatment of HEL-JW cells. As shown in Fig 2A, VRP stimulation of the FLT4 receptor resulted in increased tyrosine phosphorylation within 1 minute with an apparent maximum reached between 2.5 to 5 minutes, and a gradual decline up to 30 minutes. The major species observed had a molecular weight of ∼125 kD, which has been previously reported as the proteolytically cleaved form of the FLT4 receptor.10 15 However, a faint band at a molecular weight of ∼180 kD corresponding to the minor noncleaved form of the receptor was also seen on overexposed autoradiographs (data not shown). Both forms of the receptor were observed in experiments where immunoprecipitation of cell lysates with anti-FLT4 antibody was followed by immunoblotting with the same antibody (Fig 2B). In these assays, we confirmed similar amounts of FLT4 receptor protein. Several coimmunoprecipitated proteins were also phosphorylated after stimulation with VRP. Besides the FLT4 receptor, we also observed other tyrosine phosphorylated proteins with molecular weights of approximately 66, 52, and 46 kD in these immunoprecipitations (Fig 2A). These results suggest that, upon VRP treatment, the FLT4 receptor is rapidly tyrosine phosphorylated and associates with other tyrosine phosphorylated proteins.
Time course of tyrosine phosphorylation of the FLT4 receptor after VRP treatment. HEL-JW cells were serum-starved and stimulated with VRP for the indicated times. TCLs were immunoprecipitated with anti-FLT4 antibody, and subjected to serial immunoblotting with antiphosphotyrosine antibody (A) and anti-FLT4 antibody (B). The phosphorylated proteins are indicated by arrows in (A). The increase in tyrosine phosphorylation of FLT4 is indicated (by fold increase) based on the densitometry values. Normal rabbit IgG was used as a negative control for the immunoprecipitations.
Time course of tyrosine phosphorylation of the FLT4 receptor after VRP treatment. HEL-JW cells were serum-starved and stimulated with VRP for the indicated times. TCLs were immunoprecipitated with anti-FLT4 antibody, and subjected to serial immunoblotting with antiphosphotyrosine antibody (A) and anti-FLT4 antibody (B). The phosphorylated proteins are indicated by arrows in (A). The increase in tyrosine phosphorylation of FLT4 is indicated (by fold increase) based on the densitometry values. Normal rabbit IgG was used as a negative control for the immunoprecipitations.
Phosphorylation of Shc by VRP and its association with FLT4, Grb2, and SOS. Analysis of the FLT4 signal transduction pathway in fibroblasts transfected with the chimeric c-Fms/FLT4 receptor and treated with macrophage colony-stimulating factor (M-CSF) has suggested that Shc may be a substrate of the FLT4 kinase.13-15 Shc interaction with the long form of the FLT4 receptor through its phosphotyrosine binding (PTB) domain is believed to be necessary for FLT4-mediated transforming activity.13 14 Therefore, we investigated whether Shc could be a substrate of the native FLT4 receptor in hematopoietic cells as was observed in transfected fibroblastic cells.
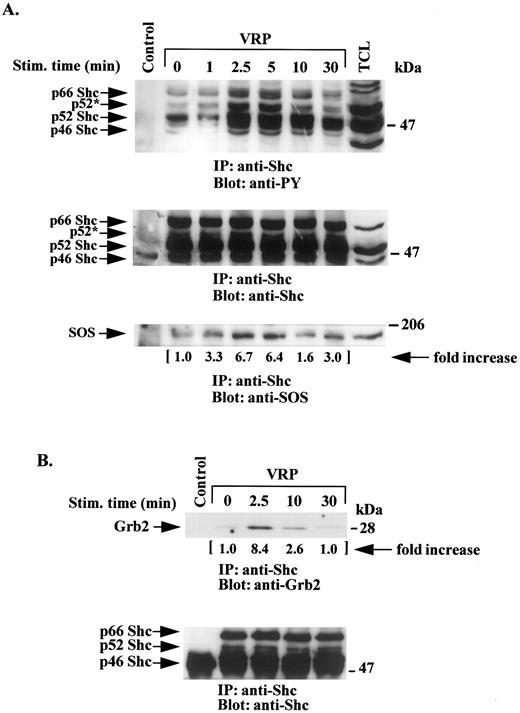
HEL-JW cells were treated with VRP as described above and the cell lysates were immunoprecipitated with anti-Shc antibodies and then immunoblotted with antiphosphotyrosine antibodies. As shown in Fig 3A, all three forms of the Shc protein (p66, p52, and p46) were significantly phosphorylated between 2.5 to 5 minutes after VRP stimulation, after which time the apparent phosphorylation of Shc then declined. The p52 Shc species appeared to be the most phosphorylated protein in these studies. A shift in the mobility of the highly phosphorylated p52 Shc protein (p52*) was observed. Similar amounts of Shc protein were present in each of the lysates as shown in Fig 3A (middle panel) by immunoblotting with anti-Shc antibody.
VRP stimulation induces tyrosine phosphorylation of Shc and its association with SOS and Grb2. HEL-JW cells unstimulated or stimulated with VRP for the indicated times were lysed in RIPA buffer and immunoprecipitated with anti-Shc antibody. (A) The immune complexes were resolved on 8% SDS-PAGE and immunoblotted with antiphosphotyrosine (upper panel), anti-Shc (middle panel), or anti-SOS (bottom panel) antibodies. (B) The immunocomplexes were resolved on 12% SDS-PAGE and immunoblotted with anti-Grb2 antibody (upper panel) or anti-Shc antibody (bottom panel). The changes in the association of SOS or Grb2 with Shc are indicated (by fold increase) based on the densitometry values. Normal rabbit IgG was used as a negative control for the immunoprecipitations.
VRP stimulation induces tyrosine phosphorylation of Shc and its association with SOS and Grb2. HEL-JW cells unstimulated or stimulated with VRP for the indicated times were lysed in RIPA buffer and immunoprecipitated with anti-Shc antibody. (A) The immune complexes were resolved on 8% SDS-PAGE and immunoblotted with antiphosphotyrosine (upper panel), anti-Shc (middle panel), or anti-SOS (bottom panel) antibodies. (B) The immunocomplexes were resolved on 12% SDS-PAGE and immunoblotted with anti-Grb2 antibody (upper panel) or anti-Shc antibody (bottom panel). The changes in the association of SOS or Grb2 with Shc are indicated (by fold increase) based on the densitometry values. Normal rabbit IgG was used as a negative control for the immunoprecipitations.
To further characterize the interacting molecules that might be associated with this complex, immunoprecipitation with anti-Shc antibody was performed followed by immunoblotting with anti-Grb2 antibody. A significant increase in the association between the adaptor protein Grb2 and Shc was observed 2.5 minutes after VRP treatment (Fig 3B).
The SOS molecule has been previously described as participating in Shc and Grb2 signaling pathways in several cell systems.1 24-26 Therefore, we examined HEL-JW cells after VRP treatment for an enhanced association between SOS and Shc. As shown in Fig 3A (bottom panel), VRP induced interaction of these two signaling molecules.
An association between Shc and the FLT4 receptor was also detected in VRP-stimulated cells (Fig 4). This association increased between 1 to 5 minutes and decreased to control levels after 10 to 30 minutes. These results indicate a minor constitutive association between the receptor and the Shc signaling moiety in hematopoietic cells, an association that significantly increased upon ligand stimulation.
Association of Shc with the FLT4 receptor upon VRP stimulation. TCLs from VRP-stimulated HEL-JW cells for the indicated times were immunoprecipitated with anti-Shc antibody or normal rabbit IgG as a negative control. The immune complexes were subjected to immunoblotting analysis with anti-FLT4 antibody (upper panel) or anti-Shc antibody (bottom panel). The changes in the association of Shc with the FLT4 receptor were quantitated based on the densitometry values as indicated (by fold increase).
Association of Shc with the FLT4 receptor upon VRP stimulation. TCLs from VRP-stimulated HEL-JW cells for the indicated times were immunoprecipitated with anti-Shc antibody or normal rabbit IgG as a negative control. The immune complexes were subjected to immunoblotting analysis with anti-FLT4 antibody (upper panel) or anti-Shc antibody (bottom panel). The changes in the association of Shc with the FLT4 receptor were quantitated based on the densitometry values as indicated (by fold increase).
VRP treatment of HEL-JW cells phosphorylates and activates the novel signal transduction molecule, RAFTK. RAFTK appears to link surface signaling from integrin and cytokine receptors to the cytoskeleton and downstream to the JNK pathway in certain cell types.23,27 28 VRP treatment of HEL-JW cells resulted in a phosphorylation of RAFTK within 1 minute, which was sustained for 30 minutes (Fig 5A). These studies revealed that several tyrosine phosphorylated proteins were coimmunoprecipitated with RAFTK. In these studies, there were equal amounts of RAFTK protein present as seen by immunoprecipitation followed by immunoblotting with anti-RAFTK antibodies (Fig 5A, bottom panel).
Tyrosine phosphorylation and activation of RAFTK is induced by VRP stimulation. Serum-starved HEL-JW cells were stimulated with VRP for the indicated times. TCLs were prepared and immunoprecipitated with anti-RAFTK antibody. (A) The immunoprecipitates were subjected to serial immunoblot analysis with antiphosphotyrosine antibody (upper panel) and anti-RAFTK antibody (bottom panel). The increase in tyrosine phosphorylation of RAFTK is indicated (by fold increase). (B) The immune complex was incubated with a kinase buffer containing 20 μg of poly (Glu:Tyr) (4:1) and 5 μCi γ32P-ATP at RT for 15 minutes. The 32P-incorporated proteins were resolved on 7.5% SDS-PAGE followed by autoradiography. Normal rabbit serum (NRS) was used as a negative control for the immunoprecipitations.
Tyrosine phosphorylation and activation of RAFTK is induced by VRP stimulation. Serum-starved HEL-JW cells were stimulated with VRP for the indicated times. TCLs were prepared and immunoprecipitated with anti-RAFTK antibody. (A) The immunoprecipitates were subjected to serial immunoblot analysis with antiphosphotyrosine antibody (upper panel) and anti-RAFTK antibody (bottom panel). The increase in tyrosine phosphorylation of RAFTK is indicated (by fold increase). (B) The immune complex was incubated with a kinase buffer containing 20 μg of poly (Glu:Tyr) (4:1) and 5 μCi γ32P-ATP at RT for 15 minutes. The 32P-incorporated proteins were resolved on 7.5% SDS-PAGE followed by autoradiography. Normal rabbit serum (NRS) was used as a negative control for the immunoprecipitations.
The tyrosine phosphorylation of protein tyrosine kinases can result in the activation of their kinase activity, which is essential for their role in signal transduction. Therefore, we performed an in vitro kinase assay in which poly (Glu:Tyr) (4:1) was used as an exogenous substrate to determine the intrinsic tyrosine kinase activity of RAFTK. As shown in Fig 5B, VRP stimulation of HEL-JW cells resulted in the activation of the intrinsic tyrosine kinase activity of RAFTK.
We then investigated if RAFTK might link the transduction of signals from the FLT4 receptor by interacting with the adaptor protein Grb2, as observed in PC12 neuronal cells.22 As shown in Fig 6, RAFTK associated with Grb2 in HEL-JW cells following VRP treatment. Interestingly, the kinetics of this association were different from that observed with Shc as the peak point of phosphorylation appeared to be at a later timepoint, at 10 minutes based on immunoprecipitation studies with anti-RAFTK antibody followed by immunoblotting with anti-Grb2 antibody. Similar results were obtained by reversing this procedure, ie, immunoprecipitation with anti-Grb2 antibody followed by immunoblotting with anti-RAFTK antibody (data not shown).
Association of RAFTK with Grb2 upon stimulation by VRP. HEL-JW cells were serum-starved, then stimulated with VRP for the indicated times. TCLs were prepared, immunoprecipitated with anti-RAFTK antiserum, and then subjected to serial immunoblot analysis with anti-Grb2 antibody (upper panel) and anti-RAFTK antiserum (bottom panel). Normal rabbit serum was used as a negative control for the immunoprecipitations. The changes in the association of RAFTK with Grb2 are indicated (by fold increase).
Association of RAFTK with Grb2 upon stimulation by VRP. HEL-JW cells were serum-starved, then stimulated with VRP for the indicated times. TCLs were prepared, immunoprecipitated with anti-RAFTK antiserum, and then subjected to serial immunoblot analysis with anti-Grb2 antibody (upper panel) and anti-RAFTK antiserum (bottom panel). Normal rabbit serum was used as a negative control for the immunoprecipitations. The changes in the association of RAFTK with Grb2 are indicated (by fold increase).
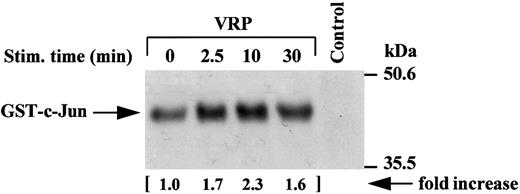
Activation of JNK activity by VRP treatment. JNK, a novel member of the mitogen-activated protein kinase (MAPK) family,29-31 has recently been shown to be a downstream mediator of the RAFTK/Pyk2 signaling pathway in PC12 neuronal cells.27 We sought to determine whether VRP, which stimulates RAFTK phosphorylation, also activated JNK in HEL-JW cells. As shown in Fig 7, VRP stimulation of HEL-JW cells resulted in the activation of JNK as determined by the phosphorylation of GST-c-Jun (1-79 amino acids).
Activation of JNK activity by VRP treatment. Cell lysates were immunoprecipitated with JNK antibody. The immune complexes were washed twice with RIPA buffer and once in kinase buffer. The complex was then incubated in a kinase buffer containing recombinant GST c-Jun 0.2 μg/μL (1-79 amino acids) and 5 μCi γ32P-ATP for 30 minutes at RT. The reaction was terminated by adding 2× SDS sample buffer and boiling the sample for 5 minutes at 100°C. Proteins were separated on 12% SDS-PAGE and detected by autoradiography. Normal rabbit IgG was used as a negative control for the immunoprecipitations. The increase in the activation of JNK is indicated (by fold increase).
Activation of JNK activity by VRP treatment. Cell lysates were immunoprecipitated with JNK antibody. The immune complexes were washed twice with RIPA buffer and once in kinase buffer. The complex was then incubated in a kinase buffer containing recombinant GST c-Jun 0.2 μg/μL (1-79 amino acids) and 5 μCi γ32P-ATP for 30 minutes at RT. The reaction was terminated by adding 2× SDS sample buffer and boiling the sample for 5 minutes at 100°C. Proteins were separated on 12% SDS-PAGE and detected by autoradiography. Normal rabbit IgG was used as a negative control for the immunoprecipitations. The increase in the activation of JNK is indicated (by fold increase).
VRP stimulates tyrosine phosphorylation of paxillin and its association with RAFTK. Focal adhesion kinases (FAKs) are known to associate with cytoskeletal proteins and thereby modulate migration and other cell functions related to adhesion.32-34 Thus, we addressed whether VRP treatment of HEL-JW cells resulted in the modulation of the phosphorylation state of paxillin, a major component of cytoskeletal complexes.35-37 As shown in Fig 8A, the tyrosine phosphorylation of paxillin was increased between 2.5 to 10 minutes after VRP treatment, and then declined by 30 minutes. An association of paxillin with RAFTK was also observed by immunoprecipitating lysates with antipaxillin antibodies and then immunoblotting with anti-RAFTK antibodies. As shown in Fig 8B, VRP treatment enhanced this association. Furthermore, using a GST-fusion protein of the C-terminal domain of RAFTK, we observed an association of RAFTK with paxillin after VRP stimulation (Fig 8C).
Tyrosine phosphorylation of paxillin and its association with RAFTK following VRP stimulation. Serum-starved HEL-JW cells were stimulated with VRP for the indicated times. TCLs were immunoprecipitated with antipaxillin antibody or normal mouse IgG as a negative control. (A) The immunoprecipitates were subjected to serial immunoblot analysis with antiphosphotyrosine (upper panel), and antipaxillin (bottom panel) antibody. (B) the immunoprecipitates were immunoblotted with either anti-RAFTK antiserum (upper panel) or antipaxillin antibody (bottom panel). (C) TCLs were also incubated with GST-RAFTK fusion protein or GST protein alone followed by incubation with Glutathione-Sepharose 4B beads. The precipitated complexes were then subjected to immunoblot analysis with antipaxillin antibody. The fold increases in the phosphorylation of paxillin and its association with RAFTK are indicated based on densitometry values.
Tyrosine phosphorylation of paxillin and its association with RAFTK following VRP stimulation. Serum-starved HEL-JW cells were stimulated with VRP for the indicated times. TCLs were immunoprecipitated with antipaxillin antibody or normal mouse IgG as a negative control. (A) The immunoprecipitates were subjected to serial immunoblot analysis with antiphosphotyrosine (upper panel), and antipaxillin (bottom panel) antibody. (B) the immunoprecipitates were immunoblotted with either anti-RAFTK antiserum (upper panel) or antipaxillin antibody (bottom panel). (C) TCLs were also incubated with GST-RAFTK fusion protein or GST protein alone followed by incubation with Glutathione-Sepharose 4B beads. The precipitated complexes were then subjected to immunoblot analysis with antipaxillin antibody. The fold increases in the phosphorylation of paxillin and its association with RAFTK are indicated based on densitometry values.
The effects of VRP on the proliferation of HEL-JW cells. The FLT4 receptor has been observed to transduce a mitogenic signal in both artificial chimeric receptor-transfected fibroblasts12,15 and in the native receptor expressed on endothelial cells.18 To test whether VRP has similar mitogenic activity in hematopoietic cells, HEL-JW cells were incubated with a range of concentrations of VRP in the presence or absence of SCF or IL-3. As shown in Table 1, VRP alone stimulated the proliferation of HEL-JW cells in a dose-dependent manner. A synergistic effect of VRP with SCF, but not with IL-3, was observed.
The Effect of VRP on the Proliferation of HEL-JW Cells
| Treatment (ng/mL) . | OD Value . |
|---|---|
| Control | 0.454 ± 0.025 |
| VRP (1.0) | 0.458 ± 0.030 |
| VRP (10.0) | 0.508 ± 0.029* |
| VRP (100.0) | 0.539 ± 0.053* |
| VRP (1000.0) | 0.540 ± 0.054* |
| SCF (50.0) | 0.632 ± 0.053 |
| SCF (50.0) + VRP (100.0) | 0.739 ± 0.109† |
| IL-3 (5.0) | 0.609 ± 0.041 |
| IL-3 (5.0) + VRP (100.0) | 0.628 ± 0.096 |
| Treatment (ng/mL) . | OD Value . |
|---|---|
| Control | 0.454 ± 0.025 |
| VRP (1.0) | 0.458 ± 0.030 |
| VRP (10.0) | 0.508 ± 0.029* |
| VRP (100.0) | 0.539 ± 0.053* |
| VRP (1000.0) | 0.540 ± 0.054* |
| SCF (50.0) | 0.632 ± 0.053 |
| SCF (50.0) + VRP (100.0) | 0.739 ± 0.109† |
| IL-3 (5.0) | 0.609 ± 0.041 |
| IL-3 (5.0) + VRP (100.0) | 0.628 ± 0.096 |
HEL-JW cells (2 × 104/well) were plated in a 96-well plate, cultured with or without different concentrations of VRP in the presence or absence of SCF or IL-3 as described in Materials and Methods. After 48 hours culture, cell growth was measured by MTT assay. The OD value was obtained by measuring the absorbance at 560 nm. The results represent the mean ± SD from four similar experiments performed in triplicate.
Statistically significant difference (P < .01) compared with control.
Statistically significant difference (P < .05) compared with treatment by SCF (50 ng/mL) or VRP (100 ng/mL) alone.
DISCUSSION
In this study, we have characterized in hematopoietic cells signal transduction pathways that are activated upon treatment with the recently identified VRP ligand for the FLT4 receptor. Our strategy was to isolate a subset of HEL cells that would provide a relatively enriched population expressing robust amounts of the FLT4 receptor. Treatment of these selected FLT4-expressing HEL-JW cells, with VRP alone and in combination with SCF, resulted in enhanced cell growth. There was prominent phosphorylation of a number of proteins under such conditions. We proceeded to characterize several of these proteins and their participation in VRP-mediated signaling pathways.
The FLT4 receptor was significantly phosphorylated in HEL-JW cells after stimulation by VRP. The time course we observed resembled that previously observed in chimeric c-Fms/FLT4 transfected Rat2 fibroblasts.14
It has been shown in early studies that FLT4L, but not FLT4S, interacts with the PTB domain of Shc proteins. This interaction and the phosphorylation of Shc are believed to be essential for FLT4 receptor-mediated mitogenic signaling.13,14 In the present studies, we have shown that, upon VRP stimulation, Shc proteins were phosphorylated in HEL-JW cells, which express both forms of the FLT4 receptor. Shc proteins have been shown to couple receptor and nonreceptor tyrosine kinases to the Ras/MAPK signaling pathway, which in turn, initiates signals for cell growth and differentiation.24-26,38,39 Tyrosine-phosphorylated Shc can activate the Ras signaling pathway by binding to the SH2 domain of the Grb2 adaptor protein that complexes to the guanine-nucleotide-releasing factor SOS by means of its SH3 domain.38 39 We observed the association of Shc with SOS (Fig 3A) as well as Grb2 (Fig 3B) in HEL-JW cells after VRP stimulation. These results indicate that the native FLT4 receptor pathway may signal to the Ras/MAPK pathway by interacting with and phosphorylating Shc. These changes in signal transduction may be related in part to the observed mitogenic effects of VRP on HEL-JW cells.
We also observed that VRP treatment of HEL-JW cells activated the novel signaling molecule RAFTK.20-22 RAFTK has been reported to participate in calcium-mediated signaling in PC12 neuronal cells,22 and recently in integrin-mediated signaling in megakaryocytes23 and B cells.28 RAFTK/Pyk2 was shown to activate the Ras/MAPK signaling pathway in PC12 cells by interacting with Grb2/SOS.22,40 RAFTK/Pyk2 activation also appeared to couple stress signals to the c-Jun-NH2 terminal kinase (JNK) pathway.27 The tyrosine phosphorylation, activation, and association of RAFTK with Grb2 after VRP stimulation suggested that it may constitute a novel component of the FLT4 receptor-mediated signaling pathway leading to JNK activation. In concert with this hypothesis, we found that JNK was activated upon VRP stimulation of HEL-JW cells. JNK has been shown to be activated by both stress and mitogenic stimuli.29-31,41,42 In hematopoietic cells, JNK is activated by several hematopoietic growth factors.43 The activation of JNK by VRP indicates that JNK is also involved in FLT4 receptor-mediated signaling pathways in hematopoietic cells.
In these studies, we also observed that VRP stimulation enhanced the tyrosine phosphorylation of the cytoskeletal protein paxillin, and its association with RAFTK. Paxillin is a prominent component of the focal adhesion complex which participates in adherent contacts with the extracellular matrix35-37 and has also been shown to play an important role in signal transduction in hematopoietic cells.36 It is a substrate for FAK, another member of the focal adhesion kinase family, and also binds to several adaptor proteins and tyrosine kinases including Crk, Crkl, Src, and Bcr/Abl.35,36,44,45 RAFTK was found to associate with paxillin through its C-terminal proline-rich domain. FAK, which is similar to RAFTK, has also been shown to associate with paxillin through its proline-rich C-domain.34 The phosphorylation of paxillin and its association with RAFTK may help to explain the effects of VRP/VEGF-C on the migration of endothelial cells.19
In conclusion, we have mapped in hematopoietic cells the signaling events that are activated upon treatment with the novel VRP ligand for the FLT4 receptor. Our observations indicate that VRP activates RAFTK as well as the Shc/Grb2/SOS signaling pathway in HEL-JW cells. These studies further show that RAFTK activation may be involved in facilitating the transmission of surface signals to the cytoskeleton and activation of transcriptional factors through JNK stimulation. These data provide insight into the reported functions of VRP in modulating cell growth and/or migration.
ACKNOWLEDGMENT
We thank our colleagues Mel A. Ona and William C. Hatch for their technical assistance, and James Lee and William I. Wood of Genentech, Inc for providing purified recombinant VRP. We are grateful to Janet Delahanty for editing this manuscript, Evelyn Gould for her assistance in preparing the figures, Youngsun Jung for her typing assistance, and Delroy Heath for facilitating our receipt of the needed reagents for the experiments. We also give special thanks to Ronald Ansin for his support of our research program. This article is dedicated to the memory of our colleague Dr Dananagoud Hiregowdara.
Supported in part by National Institutes of Health (Bethesda, MD) Grant Nos. HL 53745-02, HL 43510-07, HL 55187-01, HL 51456-02, and HL 55445-01.
Address reprint requests to Jerome E. Groopman, MD, Divisions of Experimental Medicine and Hematology/Oncology, Harvard Institutes of Medicine — BIDMC, 4 Blackfan Circle, Boston, MA 02115.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal