Abstract
Mice lacking granulocyte colony-stimulating factor (G-CSF) are neutropenic with reduced hematopoietic progenitors in the bone marrow and spleen, whereas those lacking granulocyte-macrophage colony-stimulating factor (GM-CSF) have impaired pulmonary homeostasis and increased splenic hematopoietic progenitors, but unimpaired steady-state hematopoiesis. These contrasting phenotypes establish unique roles for these factors in vivo, but do not exclude the existence of additional redundant functions. To investigate this issue, we generated animals lacking both G-CSF and GM-CSF. In the process of characterizing the phenotype of these animals, we further analyzed G-CSF– and GM-CSF–deficient mice, expanding the recognized spectrum of defects in both. G-CSF–deficient animals have a marked predisposition to spontaneous infections, a reduced long-term survival, and a high incidence of reactive type AA amyloidosis. GM-CSF–deficient mice have a modest impairment of reproductive capacity, a propensity to develop lung and soft-tissue infections, and a similarly reduced survival as in G-CSF–deficient animals. The phenotype of mice lacking both G-CSF and GM-CSF was additive to the features of the constituent genotypes, with three novel additional features: a greater degree of neutropenia among newborn mice than in those lacking G-CSF alone, an increased neonatal mortality rate, and a dominant influence of the lack of G-CSF on splenic hematopoiesis resulting in significantly reduced numbers of splenic progenitors. In contrast to newborn animals, adult mice lacking both G-CSF and GM-CSF exhibited similar neutrophil levels as G-CSF–deficient animals. These findings demonstrate that the additional lack of GM-CSF in G-CSF–deficient animals further impairs steady-state granulopoiesis in vivo selectively during the early postnatal period, expand the recognized roles of both G-CSF and GM-CSF in vivo, and emphasize the utility of studying multiply deficient mouse strains in the investigation of functional redundancy.
THE BIOLOGIC ACTIVITIES of the hematopoietic growth factors granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) overlap significantly both in vitro1 and following pharmacologic administration in vivo,2,3 raising the possibility of significant functional redundancy of their physiologic roles.4 Initially, this postulated redundancy was explored through the creation of mice lacking either G-CSF5 or GM-CSF.6,7 G-CSF–deficient mice have reduced hematopoietic progenitors in the bone marrow and spleen and neutropenia.5 Despite the potent hematopoietic capacity of GM-CSF, mice lacking this factor manifest no detectable deficiency in steady-state hematopoiesis, but have increased splenic hematopoietic progenitors and an impaired pulmonary surfactant clearance resulting in abnormalities resembling human alveolar proteinosis.6-8 These contrasting phenotypes establish that at least one of the in vivo roles of each factor is unique and indispensable, but do not exclude the existence of additional redundant functions. For instance, the residual granulopoiesis in G-CSF–deficient mice may be a result of the actions of GM-CSF. Furthermore, the normal neutrophil level in GM-CSF–deficient mice does not establish that GM-CSF has no physiologic role in steady-state granulopoiesis, but indicates that in the presence of regulators such as G-CSF any such role is dispensable. A definitive means to explore these issues in vivo is to generate mice simultaneously lacking both factors. In such mice, observation of a novel defect or exacerbation of the phenotypic features of singly deficient animals would unequivocally establish partial in vivo redundancy.9
We therefore generated mice lacking both G-CSF and GM-CSF. These mice have features consistent with the superimposition of the previously reported and newly recognized abnormalities of the two constituent genotypes; moreover, mice lacking both G-CSF and GM-CSF are more severely neutropenic up to 2 weeks of age than those lacking G-CSF alone, indicating that GM-CSF is physiologically available to support granulopoiesis in the early postnatal period.
MATERIALS AND METHODS
Mice.The G-CSF locus is designated “G,” and the GM-CSF locus “GM.” At each of these loci, homozygous wild-type, heterozygous, and homozygous null mice are designated “+/+,” “+/−,” and “−/−,” respectively. For the sake of brevity, where an animal is homozygous wild-type at a given locus, the genotype of the animal at that locus is not specifically stated unless it creates potential ambiguity.
The G−/− and GM−/− mice used were previously described,5,6 and were generated from 129/OLA-derived embryonic stem cells and C57BL/6-derived blastocysts. Similarly outbred 129/OLA × C57BL/6 G+/+GM+/+ mice were used as controls. G+/−GM+/− mice were generated by interbreeding G−/−GM+/+ and G+/+GM−/− strains. Doubly heterozygous progeny were identified by polymerase chain reaction (PCR) screening and interbred to yield G−/−GM−/− animals, which were then mated. PCR genotyping at the G and GM loci was performed on tail DNA of 21-day-old mice as previously described.5 6 All G−/−GM−/− mice studied were progeny of G−/−GM−/− × G−/−GM−/− matings. Mice were housed in a conventional facility unless stated otherwise, fed food pellets and water ad libitum, and killed if distressed. Experimentation followed the guidelines of the Animal Welfare Committee of the National Health and Medical Research Council and was approved by the institutional Animal Ethics Committee.
Hematologic analysis.In mice aged at least 14 days, retro-orbital venous plexus blood was sampled using EDTA-coated microhematocrit tubes (Clay Adams, Parsippany, NJ), diluted immediately 1:4 in 2 mg/mL EDTA in mouse-tonicity phosphate-buffered saline, and analyzed using a Sysmex-K1000 automated counter (Toa Medical Electronics Co, Kobe, Japan). Peripheral blood smears were stained with May-Grünwald/Giemsa, and manual differential cell counts of at least 200 nucleated cells were performed. Absolute neutrophil levels were then calculated using the total leukocyte count after appropriate correction for nucleated red blood cells where present. Before 14 days of age, jugular venous blood was collected as previously described10 and diluted between 1:5 and 1:7.5. Differential analyses of May-Grünwald/Giemsa–stained cytospin smears of spleen and peritoneal-wash cell suspensions were based on manual counts of 100 to 200 cells. Bone marrow differential analyses were based on the mean of two manual counts of 500 cells from duplicate smears. All differential analyses were performed by a single investigator. Only apparently healthy animals were sampled for these studies.
Progenitor assays.In vitro hematopoietic colony-forming cells were enumerated in triplicate semisolid agar cultures as described previously.11 Colony formation was stimulated with pokeweed mitogen–stimulated spleen-conditioned medium ([SCM] 10% vol/vol) or the following purified bacterially synthesized recombinant proteins singly or in combination: human G-CSF 10 ng/mL (Amgen, Melbourne, Australia), murine GM-CSF 10 ng/mL (Dr N. Nicola, Cooperative Research Centre for Cellular Growth Factors, Parkville, Australia), and pegylated rat stem cell factor (SCF) 100 ng/mL (Amgen, Thousand Oaks, CA). Cultures were incubated for 7 days at 37°C in a fully humidified atmosphere of 5% CO2 in air. Colonies were defined as clones containing at least 50 cells, except for megakaryocytic colonies, which were defined as clones containing at least three megakaryocytes, and were counted using a dissecting microscope at 35-fold magnification. To classify colonies, whole plates were fixed in 2.5% glutaraldehyde in normal saline, dried, stained sequentially with acetylcholinesterase, Luxol Fast Blue, and hematoxylin, mounted, and examined at 200- to 400-fold magnification.
Histologic analysis.The histologic survey of 8- to 12-week-old G−/−GM−/− animals examined 10% buffered Formalin-fixed, paraffin-embedded sections of lung, heart, thymus, lymph node, liver, kidney, pancreas, stomach, small and large intestine, spleen, bone marrow, bone shaft, brain, and eye stained with hematoxylin and eosin. For some analyses, lungs were harvested en bloc and inflated with 1 mL 10% buffered Formalin following tracheal cannulation.
Pulmonary pathologic changes were scored using a histologic grading scale incorporating both the severity and extent of abnormalities. The severity of alveolar surfactant deposition and pulmonary lymphoid accumulation were scored separately on a qualitative scale from 1 to 4. For scoring surfactant deposition, affected areas were graded as follows: grade 1, sparse focal granular deposits within a minority of alveoli; grade 2, more uniform and extensive granular deposits within the majority of alveoli; grade 3, extensive granular deposits, becoming confluent in some alveoli; and grade 4, uniformly confluent deposits appearing to completely fill affected alveoli. In the lungs of healthy wild-type mice, there were occasionally small perivascular clusters of small mature lymphocytes. Pulmonary lymphoid accumulation was scored as grade 1 if there were lymphocytic foci slightly larger than usually seen in normal mice but less than 10 cells thick in section; grade 2, segmental lymphoid foci of greater than 10-cell thickness in section, or narrow circumferential accumulations surrounding some vessels; grade 3, more extensive circumferential lesions, which may completely bridge the areas between adjacent vessels or bronchi; and grade 4, extensive areas of lymphoid accumulation no longer confined to the perivascular or peribronchial zones, but extending interstitially into surrounding lung parenchyma. These grading schema had good intraobserver reproducibility, with at least 80% of specimens scored identically for both proteinosis and lymphoid accumulation on repeated analyses with a maximum discrepancy of one grade between analyses (n = 44). Visual estimations of the percentage of both vascular involvement by lymphoid aggregates and lung fields affected by abnormal surfactant accumulation were also reproducible, each with a mean difference of less than 15% on repeated analyses.
Congo red staining and analysis of the potassium permanganate sensitivity of Congo red staining were performed as previously described,12,13 and were examined under crossed polarizing filters (Nikon, Tokyo, Japan). To be considered positive for amyloid, tissues were required to stain positively with Congo red and to demonstrate green birefringence under crossed polarizing filters. The severity of amyloidosis was graded from 1 (slight) to 3 (extensive) using the criteria of Janigan.14
Microbiologic analysis.Tissue samples for microbiologic analysis were obtained immediately following killing, prepared for examination by light-microscopy after Gram staining, and processed for bacterial culture using standard methods.15 Animals found dead were not subject to microbiologic analysis.
Survival analysis.For the survival studies, a cohort of 219 mice were set aside in the conventional animal house environment and prospectively evaluated without intervention. Animals that were clearly distressed were killed. A group of 32 apparently healthy mice were culled in error at 34 to 44 weeks of age, and were censored at the time of culling. All other animals were evaluated until death or until illness necessitating killing, and those still alive at the most recent analysis were censored at that date.
Statistics.Numeric data are presented as the mean ± 1 SD unless stated otherwise. Comparisons between nominal data were made using Fisher's exact test or the χ2 test and between ordinal data using the Mann-Whitney U test or Kruskal-Wallis test as appropriate. P values less than .05 after appropriate correction for multiple comparisons using the Holm modification of the Bonferroni procedure16 were regarded as significant. Survival data were analyzed using the method of Kaplan and Meier and compared using the log-rank test. Multivariate regression analysis was performed on Winstat 3.0 software (Kalmia Co, Cambridge, MA) using a forward stepwise procedure with incorporation of all independent variables with a univariate P value less than .05.
RESULTS
Viability and fertility of G−/−GM−/− mice.Mendelian inheritance patterns predict that an average of one in 16 offspring (6.25%) from G+/−GM+/− × G+/−GM+/− matings should be of the doubly nullizygous genotype (G−/−GM−/−). Of 342 pups born to such matings surviving to weaning and hence to genotyping, only nine (2.6%; 95% confidence interval, 1.2% to 4.9%) were confirmed to be G−/−GM−/−. This frequency is lower than predicted, confirming selective loss of G−/−GM−/− animals. These data do not separate the relative contribution of prenatal and postnatal loss of pups. However, the increased neonatal mortality rate among viable G−/−GM−/− pups born to doubly nullizygous mothers (Table 1) appears insufficient to completely account for the skewed distribution of genotypes among successfully weaned animals. This suggests that there may additionally be a selective loss of G−/−GM−/− embryos.
Litter Size and Neonatal Survival
| Parameter . | Genotype . | |||
|---|---|---|---|---|
| . | Wild-Type . | G−/− . | GM−/− . | G−/−GM−/− . |
| No. of mating pairs active | 29 | 9 | 20 | 10 |
| Total no. of litters born | 96 | 25 | 45 | 25 |
| No. of pups born per litter (mean ± SD)† | 8.6 ± 3.0 | 8.0 ± 2.9 | 7.3 ± 3.5* | 7.0 ± 2.5* |
| No. of pups weaned per litter (mean ± SD)† | 6.5 ± 3.9 | 7.0 ± 3.0 | 5.6 ± 3.3 | 4.3 ± 3.3* |
| Crude neonatal mortality rate (%) | 24 | 12* | 23 | 39* |
| Percentage of male pups at weaning (mean ± SD) | 49 ± 20 | 43 ± 17 | 55 ± 23 | 43 ± 22 |
| Parameter . | Genotype . | |||
|---|---|---|---|---|
| . | Wild-Type . | G−/− . | GM−/− . | G−/−GM−/− . |
| No. of mating pairs active | 29 | 9 | 20 | 10 |
| Total no. of litters born | 96 | 25 | 45 | 25 |
| No. of pups born per litter (mean ± SD)† | 8.6 ± 3.0 | 8.0 ± 2.9 | 7.3 ± 3.5* | 7.0 ± 2.5* |
| No. of pups weaned per litter (mean ± SD)† | 6.5 ± 3.9 | 7.0 ± 3.0 | 5.6 ± 3.3 | 4.3 ± 3.3* |
| Crude neonatal mortality rate (%) | 24 | 12* | 23 | 39* |
| Percentage of male pups at weaning (mean ± SD) | 49 ± 20 | 43 ± 17 | 55 ± 23 | 43 ± 22 |
Data collected for all mating pairs of the given genotypes active over a continuous 6-month period in the same conventional animal house environment. Mating pairs were all crosses between pairs of the genotype shown in the respective column.
P < .05 v wild-type.
P < .05 for heterogeneity across all genotypes.
G−/−GM−/− mice appeared healthy, developed normally, and were fertile, but displayed a modest impairment of reproductive capacity and reduced neonatal survival (Table 1). The lack of GM-CSF was the major variable influencing litter size. G−/−GM−/− and G+/+GM−/− mating pairs produced litters of a similar mean size, but both were smaller than wild-type litters. By contrast, there was no reduction in the size of litters born to G−/− matings. The early survival of G−/−GM−/− pups was also significantly impaired. These differences were unrelated to gender. It was not possible to determine the specific cause of these neonatal deaths, as few animals were available for histologic analysis (due to maternal cannibalism). However, the timing of these deaths among G−/−GM−/− pups coincided with a period of marked neutropenia (detailed later), raising the possibility that they were related to infections.
Hematologic analysis of G−/−GM−/− mice.The initial evaluation of hematopoiesis consisted of a comparison of six pairs of 8- to 12-week-old G−/−GM−/− and wild-type mice. While no differences in total femoral cellularity were evident, the cellular composition differed markedly. In G−/−GM−/− mice, both early (myelocytes and promyelocytes, 5.6% ± 1.3%) and late (metamyelocytes and polymorphonuclear forms, 20.0% ± 3.4%) neutrophil precursors were reduced relative to those in wild-type animals (11.4% ± 3.7% and 32.8% ± 4.8%, respectively). The percentage of bone marrow eosinophils was lower in G−/−GM−/− mice, although the absolute difference was small (1.4% ± 1.0% v 2.6% ± 1.0%). The reduction in myeloid precursors in G−/−GM−/− animals was accompanied by a proportional increase in morphologically mature lymphocytes (47.8% ± 5.4% v 30.5% ± 5.1%). The percentage of erythroid and monocytic cells was not disturbed in G−/−GM−/− animals. These alterations in bone marrow cellular composition are similar in nature and degree to those previously described in mice lacking G-CSF alone.5
There was no difference in body weight, spleen weight, cell yield from peritoneal lavage, or cellular composition of peritoneal lavage or spleen cell suspensions between these 8- to 12-week-old G−/−GM−/− and control animals (data not shown).
Bone marrow hematopoietic progenitors.Consistent with the reduction in morphologically recognizable neutrophil precursors in the bone marrow of G−/−GM−/− animals, the total number of colony-forming cells responsive to all stimuli tested was lower than in control mice, although these differences did not attain statistical significance (Table 2). The most prominent reduction observed among G−/−GM−/− mice was in the number of macrophage colonies formed in response to GM-CSF. In further experiments with a total of eight mice per genotype, no significant differences in the number or type of colonies formed in response to G-CSF either alone or in combination with GM-CSF were found between G−/−GM−/− and control animals (data not shown).
Colony-Forming Cells in the Bone Marrow and Spleen of Wild-Type, G−/−, GM−/−, and G−/−GM−/− Mice
| Cell Source . | Stimulus . | Genotype . | Total . | Granulocyte . | Macrophage . | Granulocyte/Macrophage . |
|---|---|---|---|---|---|---|
| Bone marrow | SCM | Wild-type | 48 ± 5 | 12 ± 8 | 12 ± 6 | 20 ± 3 |
| G−/− | 31 ± 12 | 7 ± 2 | 11 ± 7 | 10 ± 3* | ||
| GM−/− | 27 ± 14 | 5 ± 2 | 10 ± 6 | 11 ± 6 | ||
| G−/−GM−/− | 40 ± 8 | 9 ± 4 | 13 ± 6 | 15 ± 5 | ||
| GM-CSF | Wild-type | 53 ± 14 | 13 ± 7 | 13 ± 5 | 28 ± 10 | |
| G−/− | 24 ± 5* | 6 ± 1 | 6 ± 1* | 12 ± 4* | ||
| GM−/− | 34 ± 15 | 10 ± 2‡ | 8 ± 6 | 16 ± 10 | ||
| G−/−GM−/− | 32 ± 14 | 10 ± 6 | 4 ± 3* | 17 ± 6 | ||
| SCF | Wild-type | 25 ± 10 | 12 ± 7 | 1 ± 1 | 10 ± 5 | |
| G−/− | 21 ± 6 | 11 ± 4 | 1 ± 1 | 8 ± 5 | ||
| GM−/− | 18 ± 9 | 9 ± 2 | 0 ± 0‡ | 7 ± 6 | ||
| G−/−GM−/− | 19 ± 7 | 11 ± 3 | 1 ± 1 | 6 ± 3 | ||
| Spleen | SCM | Wild-type | 16 ± 11 | 2 ± 2 | 4 ± 3 | 7 ± 5 |
| G−/− | 10 ± 4 | 1 ± 1 | 2 ± 1 | 5 ± 2 | ||
| GM−/− | 31 ± 22‡ | 3 ± 2 | 7 ± 8 | 17 ± 10‡ | ||
| G−/−GM−/− | 8 ± 3† | 1 ± 0† | 2 ± 1 | 4 ± 2† | ||
| GM-CSF | Wild-type | 12 ± 6 | 2 ± 2 | 2 ± 2 | 8 ± 3 | |
| G−/− | 3 ± 3* | 0 ± 0 | 1 ± 2 | 2 ± 1* | ||
| GM−/− | 19 ± 12‡ | 4 ± 3 | 1 ± 1 | 13 ± 8‡ | ||
| G−/−GM−/− | 3 ± 2*† | 1 ± 1 | 0 ± 0*† | 2 ± 2*† | ||
| SCF | Wild-type | 11 ± 8 | 5 ± 4 | 1 ± 1 | 4 ± 4 | |
| G−/− | 6 ± 3 | 2 ± 2 | 1 ± 0 | 3 ± 2 | ||
| GM−/− | 26 ± 21‡ | 12 ± 11 | 2 ± 2 | 4 ± 4 | ||
| G−/−GM−/− | 3 ± 2* | 1 ± 1 | 0 ± 1 | 1 ± 1*† | ||
| SCF + GM-CSF | Wild-type | 28 ± 14 | 6 ± 3 | 3 ± 1 | 18 ± 11 | |
| G−/− | 17 ± 11 | 2 ± 1* | 2 ± 2 | 13 ± 10 | ||
| GM−/− | 62 ± 38‡ | 7 ± 5‡ | 5 ± 1*‡ | 45 ± 31‡ | ||
| G−/−GM−/− | 10 ± 6*† | 2 ± 1 | 2 ± 1† | 6 ± 4† |
| Cell Source . | Stimulus . | Genotype . | Total . | Granulocyte . | Macrophage . | Granulocyte/Macrophage . |
|---|---|---|---|---|---|---|
| Bone marrow | SCM | Wild-type | 48 ± 5 | 12 ± 8 | 12 ± 6 | 20 ± 3 |
| G−/− | 31 ± 12 | 7 ± 2 | 11 ± 7 | 10 ± 3* | ||
| GM−/− | 27 ± 14 | 5 ± 2 | 10 ± 6 | 11 ± 6 | ||
| G−/−GM−/− | 40 ± 8 | 9 ± 4 | 13 ± 6 | 15 ± 5 | ||
| GM-CSF | Wild-type | 53 ± 14 | 13 ± 7 | 13 ± 5 | 28 ± 10 | |
| G−/− | 24 ± 5* | 6 ± 1 | 6 ± 1* | 12 ± 4* | ||
| GM−/− | 34 ± 15 | 10 ± 2‡ | 8 ± 6 | 16 ± 10 | ||
| G−/−GM−/− | 32 ± 14 | 10 ± 6 | 4 ± 3* | 17 ± 6 | ||
| SCF | Wild-type | 25 ± 10 | 12 ± 7 | 1 ± 1 | 10 ± 5 | |
| G−/− | 21 ± 6 | 11 ± 4 | 1 ± 1 | 8 ± 5 | ||
| GM−/− | 18 ± 9 | 9 ± 2 | 0 ± 0‡ | 7 ± 6 | ||
| G−/−GM−/− | 19 ± 7 | 11 ± 3 | 1 ± 1 | 6 ± 3 | ||
| Spleen | SCM | Wild-type | 16 ± 11 | 2 ± 2 | 4 ± 3 | 7 ± 5 |
| G−/− | 10 ± 4 | 1 ± 1 | 2 ± 1 | 5 ± 2 | ||
| GM−/− | 31 ± 22‡ | 3 ± 2 | 7 ± 8 | 17 ± 10‡ | ||
| G−/−GM−/− | 8 ± 3† | 1 ± 0† | 2 ± 1 | 4 ± 2† | ||
| GM-CSF | Wild-type | 12 ± 6 | 2 ± 2 | 2 ± 2 | 8 ± 3 | |
| G−/− | 3 ± 3* | 0 ± 0 | 1 ± 2 | 2 ± 1* | ||
| GM−/− | 19 ± 12‡ | 4 ± 3 | 1 ± 1 | 13 ± 8‡ | ||
| G−/−GM−/− | 3 ± 2*† | 1 ± 1 | 0 ± 0*† | 2 ± 2*† | ||
| SCF | Wild-type | 11 ± 8 | 5 ± 4 | 1 ± 1 | 4 ± 4 | |
| G−/− | 6 ± 3 | 2 ± 2 | 1 ± 0 | 3 ± 2 | ||
| GM−/− | 26 ± 21‡ | 12 ± 11 | 2 ± 2 | 4 ± 4 | ||
| G−/−GM−/− | 3 ± 2* | 1 ± 1 | 0 ± 1 | 1 ± 1*† | ||
| SCF + GM-CSF | Wild-type | 28 ± 14 | 6 ± 3 | 3 ± 1 | 18 ± 11 | |
| G−/− | 17 ± 11 | 2 ± 1* | 2 ± 2 | 13 ± 10 | ||
| GM−/− | 62 ± 38‡ | 7 ± 5‡ | 5 ± 1*‡ | 45 ± 31‡ | ||
| G−/−GM−/− | 10 ± 6*† | 2 ± 1 | 2 ± 1† | 6 ± 4† |
Four mice were studied per genotype with each cell source and stimulus. All comparisons were made using the Mann-Whitney U test. Results were rounded to the nearest whole number after analysis and are expressed as the mean ± 1 SD number of colony-forming cells of triplicate determinations per 25,000 unselected bone marrow cells and per 200,000 unselected spleen cells. The sum of granulocyte, macrophage, and granulocyte-macrophage colonies may not equal total colonies, as mixed-lineage (megakaryocytic and granulocytic/monocytic or eosinophilic and granulocytic/monocytic), pure blast, and pure megakaryocytic colonies were quantified separately but are not tabulated. The sum of these additional colony types constituted a mean of 5.0% ± 3.8% of total bone marrow colonies and 7.9% ± 5.8% of total spleen colonies, and none of the 3 genotypes studied differed significantly from the wild-type.
P < .05 v wild-type.
P < .05 v GM−/−.
P < .05 v G−/−.
Concordant with the initial study of GM−/− mice,6 no significant differences compared with the control mice were noted in the number or type of colonies formed in response to any of the stimuli tested. Also consistent with the initial report,5 G−/− animals in the present study showed a significant reduction in total colony-forming cells responsive to GM-CSF (45% of control), due to the formation of fewer pure macrophage and mixed granulocyte-macrophage colonies, and fewer mixed granulocyte-macrophage colonies formed in response to SCM.
There were no significant differences between G−/− and G−/−GM−/− mice in any of the colony-forming assays performed, demonstrating that the diminution of bone marrow progenitors in G−/−GM−/− mice is solely a consequence of G-CSF deficiency.
Splenic hematopoietic progenitors.The initial description of GM−/− mice noted an increase in the number of progenitors in the spleen.6 Although not statistically significantly greater than in wild-type mice, a similar tendency was evident with all stimuli tested in the current studies (mean total colonies, 201% of wild-type; Table 2). Conversely, G−/− mice had significantly fewer splenic progenitors responsive to GM-CSF than control mice, with a trend toward a similar reduction with the other stimuli tested. These divergent tendencies toward increased splenic progenitors among GM−/− mice and diminished splenic progenitors among both G−/− and G−/−GM−/− animals resulted in significant differences between GM−/− and both G−/− and G−/−GM−/− mice for most comparisons (Table 2).
In G−/−GM−/− mice, there were significant reductions in the total number of colony-forming cells in the spleen in response to GM-CSF, SCF, and the two stimuli combined. All classes of colonies were similarly affected. In additional studies using G-CSF alone or in combination with GM-CSF, the total number of splenic progenitors was also significantly lower in G−/−GM−/− compared with wild-type (data not shown). These findings are consistent with a “dominant” influence of the lack of G-CSF on the hematopoietic status of G−/−GM−/− animals, and suggest that the lack of GM-CSF does not result in any additive effect on the splenic progenitor numbers in G−/− mice, as there were no statistically significant differences observed between G−/− and G−/−GM−/− mice.
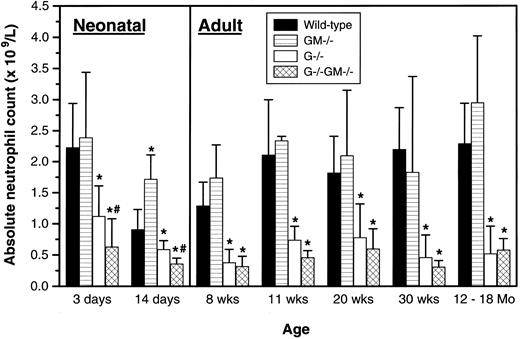
Peripheral blood neutrophil levels.Peripheral blood cell counts were studied in cohorts of mice between the ages of 3 days and 12 to 18 months, and are arbitrarily divided into those dealing with mature adult hematopoiesis (8 weeks to 12 to 18 months) and the period from 3 days to 2 weeks of age that we have termed “neonatal” (Fig 1).
Peripheral blood absolute neutrophil counts. At age 3 days, 8 to 15 mice per genotype were assayed on 5 separate days; at age 14 days, 6 to 8 mice on 1 day; at age 8 weeks, 8 to 14 mice on 3 days; at age 11 weeks, 8 to 12 mice on 3 days; at age 20 weeks, 14 to 24 mice on 3 days; and at age 30 weeks, 8 to 14 mice on 3 days. The columns labeled 12-18 Mo comprised results from 7 to 9 mice per genotype ranging from 13 to 18 months of age analyzed on 2 separate days. The gender of animals studied at ≤14 days of age could not be determined by external examination, and all older mice studied were male. Results were pooled and are shown as the mean ± 1 SD. All comparisons are between mice of a given age cohort. *P < .05 v wild-type; #P < .05, G−/− v G−/−GM−/−.
Peripheral blood absolute neutrophil counts. At age 3 days, 8 to 15 mice per genotype were assayed on 5 separate days; at age 14 days, 6 to 8 mice on 1 day; at age 8 weeks, 8 to 14 mice on 3 days; at age 11 weeks, 8 to 12 mice on 3 days; at age 20 weeks, 14 to 24 mice on 3 days; and at age 30 weeks, 8 to 14 mice on 3 days. The columns labeled 12-18 Mo comprised results from 7 to 9 mice per genotype ranging from 13 to 18 months of age analyzed on 2 separate days. The gender of animals studied at ≤14 days of age could not be determined by external examination, and all older mice studied were male. Results were pooled and are shown as the mean ± 1 SD. All comparisons are between mice of a given age cohort. *P < .05 v wild-type; #P < .05, G−/− v G−/−GM−/−.
Adult.No consistent differences in hemoglobin level, red blood cell count, hematocrit, platelet count, and total or differential white blood cell count, including the eosinophil count, were observed between GM−/− and control mice throughout the age range studied (data not shown). The neutropenia of G−/− mice persisted at a relatively constant level throughout adult life (mean absolute neutrophil count, 30% of wild-type). During the adult period, G−/−GM−/− mice were chronically neutropenic to a similar degree as G−/− mice (mean absolute neutrophil count, 24% of wild-type), with no other consistent abnormalities (data not shown). Although the absolute neutrophil count of G−/−GM−/− mice was numerically lower than that of G−/− mice at 8, 11, 20, and 30 weeks of age, none of these differences attained statistical significance. Thus, there is no evidence of further impairment of adult steady-state granulopoiesis due to the additional lack of GM-CSF in G-CSF–deficient mice.
Neonatal.Examination of the peripheral blood of mice up to 14 days of age showed similar trends, but with three novel findings. Unlike the situation in adult mice, young G−/−GM−/− animals were more severely neutropenic than G−/− mice at both 3 and 14 days of age. At 14 days of age, GM−/− mice had significantly greater neutrophil counts than control animals. When expressed relative to wild-type animals of the same age to control for observed changes in neutrophil counts with maturation, young G−/− mice were also relatively less neutropenic than adult G−/− animals (a mean of 58% v 30% of wild-type levels). No other differences between genotypes for any other parameters were noted.
Mice of all four genotypes exhibited the normal dynamic alteration in neutrophil levels during the first 8 weeks of life,10 indicating that neither maternal nor endogenous G-CSF or GM-CSF are essential for these fluctuations.
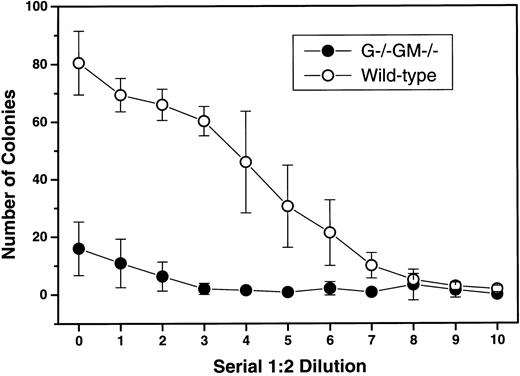
Colony-stimulating capacity of SCM from G−/−GM−/− mice.SCM prepared from G−/−GM−/− mice was 80% less potent than SCM prepared from control animals; the maximum number of colonies produced was 16 ± 9 versus 81 ± 11 (Fig 2). All classes of myeloid colonies were similarly reduced, although pure granulocytic colonies were still formed in response to G−/−GM−/− SCM despite lacking both G-CSF and GM-CSF.
Reduced colony-stimulating capacity of SCM from G−/−GM−/− mice. Pokeweed mitogen–stimulated SCM was prepared from 4 G−/−GM−/− mice and age- and sex-matched wild-type mice using standard methods,11 and assayed for colony-stimulating capacity using G+/+GM+/+ C57BL/6 bone marrow in triplicate semisolid agar cultures with serial 1:2 dilutions of SCM as stimuli. Colonies were scored at 7 days. Results are shown as the mean ± 1 SD for 4 experiments per genotype. At the highest SCM concentration, the mean absolute numbers of pure granulocyte (1.4 v 12.9), pure macrophage (3.2 v 24.4), and mixed granulocyte/macrophage (10.0 v 42.6) colonies formed were all lower with G−/−GM−/− media v wild-type.
Reduced colony-stimulating capacity of SCM from G−/−GM−/− mice. Pokeweed mitogen–stimulated SCM was prepared from 4 G−/−GM−/− mice and age- and sex-matched wild-type mice using standard methods,11 and assayed for colony-stimulating capacity using G+/+GM+/+ C57BL/6 bone marrow in triplicate semisolid agar cultures with serial 1:2 dilutions of SCM as stimuli. Colonies were scored at 7 days. Results are shown as the mean ± 1 SD for 4 experiments per genotype. At the highest SCM concentration, the mean absolute numbers of pure granulocyte (1.4 v 12.9), pure macrophage (3.2 v 24.4), and mixed granulocyte/macrophage (10.0 v 42.6) colonies formed were all lower with G−/−GM−/− media v wild-type.
Characterization of pulmonary disease in G−/−GM−/− mice.The major histologic abnormalities in eight apparently healthy 8- to 12-week-old G−/−GM−/− mice were confined to the lungs. Although a single 12-week-old male animal had widespread amyloid deposition (discussed later), other organs in these animals had no consistent abnormalities evident.
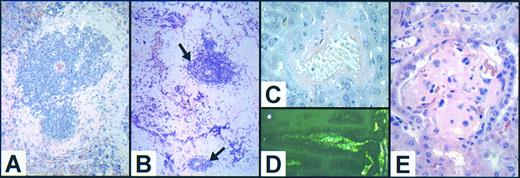
The lungs contained a variable degree of perivascular lymphoid accumulation, preferentially affecting hilar vessels, and widespread intra-alveolar surfactant accumulation (Fig 3B to D), as described in mice lacking GM-CSF (Fig 3A).6 7 Neither the extent nor the severity of these changes were greater than in an age-matched cohort of GM−/− mice.
Illustrative sections of the range of severity of the observed pulmonary pathology. (A) Grade 2 proteinosis: relatively uniform and moderately extensive granular deposits in the majority of alveoli in affected areas in a 12-month-old male GM−/− mouse; note abnormally distended alveolar macrophage (arrow). (B) Grade 4 proteinosis: uniformly confluent deposits appearing to completely fill affected alveoli in a 21-week-old female G−/−GM−/− mouse. (C) Grade 2 lymphoid accumulation: moderately large segmental lymphoid focus < 10 cells thick in a 43-week-old female G−/−GM−/− mouse. (D) Grade 4 lymphoid accumulation: extensive areas of lymphoid accumulation no longer confined to the perivascular zone, but extending interstitially into the surrounding lung parenchyma in a 29-week-old female G−/−GM−/− mouse; note confluent areas of surfactant accumulation within alveoli (grade 4 proteinosis). (All sections are stained with hematoxylin and eosin [H&E]; A and B are original magnification ×1,360, and C and D are ×340.)
Illustrative sections of the range of severity of the observed pulmonary pathology. (A) Grade 2 proteinosis: relatively uniform and moderately extensive granular deposits in the majority of alveoli in affected areas in a 12-month-old male GM−/− mouse; note abnormally distended alveolar macrophage (arrow). (B) Grade 4 proteinosis: uniformly confluent deposits appearing to completely fill affected alveoli in a 21-week-old female G−/−GM−/− mouse. (C) Grade 2 lymphoid accumulation: moderately large segmental lymphoid focus < 10 cells thick in a 43-week-old female G−/−GM−/− mouse. (D) Grade 4 lymphoid accumulation: extensive areas of lymphoid accumulation no longer confined to the perivascular zone, but extending interstitially into the surrounding lung parenchyma in a 29-week-old female G−/−GM−/− mouse; note confluent areas of surfactant accumulation within alveoli (grade 4 proteinosis). (All sections are stained with hematoxylin and eosin [H&E]; A and B are original magnification ×1,360, and C and D are ×340.)
Given the propensity for pulmonary infection in these mice, we examined the influence of environment on the development of these lung changes. A group of eight 20-week-old male G−/−GM−/− mice raised in a conventional animal house environment were compared with six age- and sex-matched mice raised in specific pathogen–free (SPF) conditions. No discernible influence of environment was evident at this single time point analyzed in adulthood (data not shown).
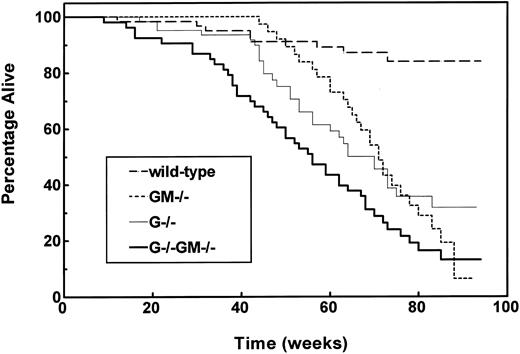
Long-term survival and causes of death.Cohorts of control, G−/−, GM−/−, and G−/−GM−/− mice balanced for gender were selected after weaning, maintained in a conventional environment, and evaluated until death or until illness necessitating killing. At the time of analysis, the median age of surviving animals was 79 weeks (range, 65 to 94), and a total of 111 animals had died. No differences in survival by gender within any of the genotypes were apparent, so subsequent analyses combined genders. As expected, the median survival of wild-type mice had not been reached, and their 18-month actuarial survival rate was 84% ± 5% (Fig 4).
Impaired survival of growth factor–deficient mouse strains. Mixed-gender cohorts of wild-type (n = 60), GM−/− (n = 44), G−/− (n = 62), and G−/−GM−/− (n = 53) mice were set aside after successful weaning and studied until death or distress necessitating killing. Survival is plotted according to the method of Kaplan and Meier, and P values are based on comparisons using the log-rank test. Survival of G−/−, GM−/−, and G−/−GM−/− strains was impaired relative to wild-type mice (P < .0001). Survival of G−/−GM−/− mice was inferior to both G−/− and GM−/− animals (each P ≤ .022).
Impaired survival of growth factor–deficient mouse strains. Mixed-gender cohorts of wild-type (n = 60), GM−/− (n = 44), G−/− (n = 62), and G−/−GM−/− (n = 53) mice were set aside after successful weaning and studied until death or distress necessitating killing. Survival is plotted according to the method of Kaplan and Meier, and P values are based on comparisons using the log-rank test. Survival of G−/−, GM−/−, and G−/−GM−/− strains was impaired relative to wild-type mice (P < .0001). Survival of G−/−GM−/− mice was inferior to both G−/− and GM−/− animals (each P ≤ .022).
The survival of G−/−, GM−/−, and G−/−GM−/− animals was significantly inferior to that of wild-type animals; median survival durations were 70, 71, and 56 weeks, respectively (Fig 4). The survival of G−/− and GM−/− mice was comparable; however, G−/−GM−/− mice had a significantly shorter survival than either GM−/− or G−/− animals. The temporal pattern of mortality also differed between genotypes. The survival of G−/− and GM−/− mice did not diverge significantly from that of control animals until 45 and 60 weeks of age, respectively, whereas the survival of G−/−GM−/− mice was significantly inferior to that of wild-type mice from 16 weeks onward.
At death, G−/−GM−/− animals were more cachectic than the other genotypes; the body weight was 25 ± 4 g for G−/−GM−/−, 31 ± 7 g for wild-type, 30 ± 7 g for G−/−, and 32 ± 7 g for GM−/− animals. The apparent cause of death and histologic findings at autopsy differed according to genotype (Table 3).
Analysis of Survival Cohorts of Wild-Type, G−/−, GM−/−, and G−/−GM−/− Mice
| Genotype . | No. of Deaths . | Mean Age at Death (wk)3-152 . | Apparent Primary Cause . | Infections Encountered . | Other Findings . |
|---|---|---|---|---|---|
| . | (no. examined)3-150 . | . | of Death (no. of mice) . | (% animals affected) . | . |
| Wild-type | 8 (4) | 44 ± 20 | Bowel tumor (2) | None evident | |
| Renal cyst (1) | |||||
| No cause evident (1) | |||||
| G−/− | 31 (24) | 53 ± 16 | Infection and amyloid (10) | Any infection (63%) | No pulmonary infections found |
| Infection alone (5) | Superficial cellulitis (38%) | Amyloidosis in 54% | |||
| No cause evident (4) | Abscess formation (hepatic, intraabdominal, or perianal) (25%) | Thickening of alveolar septae in 79% | |||
| Tumors (lymphoma, soft-tissue, pancreatic) (3) | Bacterial isolates: S aureus × 1, P mirabilis × 1, Pasturella haemolytica × 1, Enterococci × 1 | ||||
| Amyloid without infection (2) | |||||
| GM−/− | 29 (19) | 66 ± 12 | Proteinosis and lung infection (8) | Any infection (84%) | All animals had lung proteinosis3-151 |
| Extrapulmonary infection (7) | Pulmonary infection (53%) | Amyloidosis in 16% | |||
| Lung proteinosis alone (3) | Soft-tissue infections (42%) | ||||
| Tumor (uterine) (1) | Bacterial isolates: S aureus × 1, Pasturella pneumotropica × 3, Streptococcus sp. × 2 | ||||
| G−/−GM−/− | 43 (25) | 49 ± 19 | Extrapulmonary infection (8) | Any infection (64%) | All animals had lung proteinosis; lymphoid accumulation more severe and widespread than in GM−/− 3-151 |
| Proteinosis and lung infection (7) | Pulmonary infection (36%) | Thickening of alveolar septae in 33% | |||
| Lung proteinosis alone (6) | Soft-tissue infection (36%) | Amyloidosis in 25% | |||
| No cause evident (3) | Bacterial isolates: S aureus × 1 | ||||
| Tumor (lung) (1) |
| Genotype . | No. of Deaths . | Mean Age at Death (wk)3-152 . | Apparent Primary Cause . | Infections Encountered . | Other Findings . |
|---|---|---|---|---|---|
| . | (no. examined)3-150 . | . | of Death (no. of mice) . | (% animals affected) . | . |
| Wild-type | 8 (4) | 44 ± 20 | Bowel tumor (2) | None evident | |
| Renal cyst (1) | |||||
| No cause evident (1) | |||||
| G−/− | 31 (24) | 53 ± 16 | Infection and amyloid (10) | Any infection (63%) | No pulmonary infections found |
| Infection alone (5) | Superficial cellulitis (38%) | Amyloidosis in 54% | |||
| No cause evident (4) | Abscess formation (hepatic, intraabdominal, or perianal) (25%) | Thickening of alveolar septae in 79% | |||
| Tumors (lymphoma, soft-tissue, pancreatic) (3) | Bacterial isolates: S aureus × 1, P mirabilis × 1, Pasturella haemolytica × 1, Enterococci × 1 | ||||
| Amyloid without infection (2) | |||||
| GM−/− | 29 (19) | 66 ± 12 | Proteinosis and lung infection (8) | Any infection (84%) | All animals had lung proteinosis3-151 |
| Extrapulmonary infection (7) | Pulmonary infection (53%) | Amyloidosis in 16% | |||
| Lung proteinosis alone (3) | Soft-tissue infections (42%) | ||||
| Tumor (uterine) (1) | Bacterial isolates: S aureus × 1, Pasturella pneumotropica × 3, Streptococcus sp. × 2 | ||||
| G−/−GM−/− | 43 (25) | 49 ± 19 | Extrapulmonary infection (8) | Any infection (64%) | All animals had lung proteinosis; lymphoid accumulation more severe and widespread than in GM−/− 3-151 |
| Proteinosis and lung infection (7) | Pulmonary infection (36%) | Thickening of alveolar septae in 33% | |||
| Lung proteinosis alone (6) | Soft-tissue infection (36%) | Amyloidosis in 25% | |||
| No cause evident (3) | Bacterial isolates: S aureus × 1 | ||||
| Tumor (lung) (1) |
Animals found dead were not subject to histologic or microbiologic analysis.
Both the mean severity and extent of lymphoid perivascular accumulation were greater in G−/−GM−/− mice than in GM−/− mice (3.0 v 2.1 and 79% v 44%, respectively, both P ≤ .0004), whereas the mean severity and extent of surfactant accumulation were not significantly different in G−/−GM−/− and GM−/− animals at the time of death (2.7 v 2.7 and 73% v 83%, respectively, both P > .1).
Mean age ± 1 SD of all animals dying, regardless of whether found dead or killed.
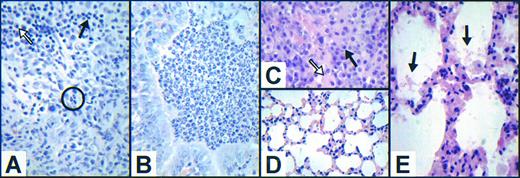
G−/−.At death, 63% of G−/− mice had microbiologic or histologic evidence of infection. The cellular infiltrate at sites of infection was composed predominantly of lymphocytes and macrophages, with scattered plasma cells (Fig 5A). Despite peripheral blood neutrophil counts as high as 1.12 × 109/L, neutrophils were infrequent at sites of infection compared with the cellular infiltrate seen in GM−/− animals (Fig 5B). None of 24 G−/− animals had histologic evidence of pulmonary infection, although in 79% of the animals there were foci of alveolar septal hypercellularity with hyperplasia of cuboidal type II alveolar cells in the absence of inflammatory infiltrate (Fig 5D). These changes were not related to the presence of infection at other sites, amyloid deposition either within the lungs or elsewhere, or gender, and no such abnormalities were seen in the lungs of six apparently healthy G−/− mice aged 6 to 7 months.
Variable cellular composition of inflammatory infiltrates and novel aspects of pulmonary pathology in growth factor–deficient mice. (A) Cellular inflammatory infiltrate surrounding an intraabdominal abscess in a 73-week-old female G−/− mouse illustrating the scarcity of neutrophils (circled) and the predominance of lymphocytes (solid arrow) and macrophages (hollow arrow). Cultures from this abscess grew both Proteus mirabilis and Enterococcus species. (B) Representative view of the cellular inflammatory infiltrate within a bronchiole in an area of pneumonia in a 71-week-old GM−/− mouse illustrating the predominance of morphologically normal neutrophils. (C) Cellular inflammatory infiltrate surrounding a pulmonary abscess in a 37-week-old female G−/−GM−/− mouse illustrating the scarcity of neutrophils, with the majority of cells being lymphocytes (solid arrow) and macrophages (hollow arrow). (D) Representative field of lung abnormality in a 45-week-old female G−/− animal showing marked hypercellularity of alveolar septae in the absence of pulmonary infection (compare with appearance of normal alveolar septae in GM−/− mice in Fig 3A). (E) Similar appearance of alveolar septae in a 64-week-old male G−/−GM−/− mouse with relatively minor surfactant accumulation (arrows). (All sections are stained with H & E; C is original magnification ×2,040, and all others are ×1,360.)
Variable cellular composition of inflammatory infiltrates and novel aspects of pulmonary pathology in growth factor–deficient mice. (A) Cellular inflammatory infiltrate surrounding an intraabdominal abscess in a 73-week-old female G−/− mouse illustrating the scarcity of neutrophils (circled) and the predominance of lymphocytes (solid arrow) and macrophages (hollow arrow). Cultures from this abscess grew both Proteus mirabilis and Enterococcus species. (B) Representative view of the cellular inflammatory infiltrate within a bronchiole in an area of pneumonia in a 71-week-old GM−/− mouse illustrating the predominance of morphologically normal neutrophils. (C) Cellular inflammatory infiltrate surrounding a pulmonary abscess in a 37-week-old female G−/−GM−/− mouse illustrating the scarcity of neutrophils, with the majority of cells being lymphocytes (solid arrow) and macrophages (hollow arrow). (D) Representative field of lung abnormality in a 45-week-old female G−/− animal showing marked hypercellularity of alveolar septae in the absence of pulmonary infection (compare with appearance of normal alveolar septae in GM−/− mice in Fig 3A). (E) Similar appearance of alveolar septae in a 64-week-old male G−/−GM−/− mouse with relatively minor surfactant accumulation (arrows). (All sections are stained with H & E; C is original magnification ×2,040, and all others are ×1,360.)
GM−/−.All mice examined had typical features of the previously reported lung pathology.6 7 The alveolar septae were uniformly normal (Fig 3A). Microbiologic or histologic evidence of infection was present in 84% of the animals, and involved the lung as lobar or bronchial pneumonia or pulmonary abscess formation in 53% of the mice. Inflammatory cellular infiltrates at the sites of infection contained predominantly neutrophils, with few lymphocytes, macrophages, and plasma cells (Fig 5B). The peripheral blood neutrophil count was significantly elevated in six infected mice analyzed (mean, 4.51 × 109/L).
G−/−GM−/−.The histologic findings in G−/−GM−/− animals combined features of both constituent genotypes, but with more severe and widespread pulmonary perivascular lymphoid accumulation (Table 3). One third of the animals showed focal areas of hypercellularity of the alveolar septae (Fig 5E), as noted in G−/− mice. Microbiologic or histologic evidence of infection was present in 64% of the mice, and involved the lungs in 36% of the animals. As in G−/− mice, morphologically recognizable neutrophils were infrequent at sites of infection (Fig 5C), despite peripheral blood neutrophil counts as high as 1.54 × 109/L (mean, 0.56 × 109/L; n = 6). The only organism cultured from these sites was Staphylococcus aureus, although Gram-negative bacilli were seen within lung abscesses.
Nature and manifestation of amyloidosis.In the initial histologic survey of 8- to 12-week-old G−/−GM−/− mice, a single animal was found to have moderate splenomegaly and hepatomegaly due to deposition of acellular eosinophilic material. Congo red staining confirmed this to be amyloid, prompting a more complete survey among animals individually lacking G-CSF or GM-CSF. Although not previously noted,5 G-CSF–deficient animals were also found to have a high incidence of amyloid.
The gross and histologic manifestations of amyloidosis were similar in affected animals of all genotypes studied. Amyloidotic animals had a pale and enlarged spleen (242 ± 133 and 173 ± 91 mg for animals with and without histologic evidence of amyloid, respectively, P = .034). The frequency and severity of organ involvement was surveyed by microscopic examination of sections from the heart, lungs, bowel, spleen, liver, and kidneys of 50 affected mice (Table 4). The most commonly and severely involved organs were the liver and spleen, with no differences in the pattern of organ involvement evident between genotypes.
Manifestations of Amyloidosis
| Genotype . | Incidence of . | No. of Affected . | Percentage of Organs Positive in Affected Animals (mean severity score*) . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Amyloid (%) . | Mice Examined . | Liver . | Spleen . | Kidney . | Bowel . | Heart . | Lung . |
| Wild-type | 4 | 1 | 0 (0) | 0 (0) | 100 (1.0) | 0 (0) | 0 (0) | 100 (1.0) |
| GM−/− | 10 | 4 | 100 (1.5) | 100 (1.3) | 50 (0.5) | 50 (0.8) | 75 (0.8) | 50 (0.5) |
| G−/− | 46 | 22 | 86 (1.3) | 86 (1.8) | 67 (1.1) | 73 (1.1) | 73 (0.8) | 36 (0.4) |
| G−/−GM−/− | 32 | 23 | 91 (1.3) | 91 (1.6) | 64 (0.7) | 71 (1.1) | 48 (0.5) | 57 (0.6) |
| Total | 27 | 50 | 88 (1.3) | 88 (1.6) | 65 (0.9) | 69 (1.0) | 60 (0.6) | 48 (0.5) |
| Genotype . | Incidence of . | No. of Affected . | Percentage of Organs Positive in Affected Animals (mean severity score*) . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Amyloid (%) . | Mice Examined . | Liver . | Spleen . | Kidney . | Bowel . | Heart . | Lung . |
| Wild-type | 4 | 1 | 0 (0) | 0 (0) | 100 (1.0) | 0 (0) | 0 (0) | 100 (1.0) |
| GM−/− | 10 | 4 | 100 (1.5) | 100 (1.3) | 50 (0.5) | 50 (0.8) | 75 (0.8) | 50 (0.5) |
| G−/− | 46 | 22 | 86 (1.3) | 86 (1.8) | 67 (1.1) | 73 (1.1) | 73 (0.8) | 36 (0.4) |
| G−/−GM−/− | 32 | 23 | 91 (1.3) | 91 (1.6) | 64 (0.7) | 71 (1.1) | 48 (0.5) | 57 (0.6) |
| Total | 27 | 50 | 88 (1.3) | 88 (1.6) | 65 (0.9) | 69 (1.0) | 60 (0.6) | 48 (0.5) |
There were no statistically significant differences evident in either the distribution or severity of amyloidosis between genotypes.
Severity of amyloid deposition was scored using the criteria of Janigan,14 where 1 = slight, 2 = moderate, and 3 = extensive, as originally defined.
In mild to moderate cases (grade 1 to 2), splenic amyloid deposits were predominantly perifollicular with preservation of the white pulp (Fig 6A). However, in more severe cases (grade 3), the residual lymphoid follicles were atrophic (Fig 6B) and subcapsular hematopoiesis was absent. In the liver, amyloid was deposited predominantly surrounding central veins (Fig 6C) and at portal triads, but in severe cases it radiated into the hepatic lobules between plates of hepatocytes. There was no associated inflammatory infiltrate or hepatocyte necrosis. In the bowel, amyloid deposits were limited to the submucosa (Fig 6D), and in the kidney, they were most evident in the glomerular vasculature (Fig 6E) and papillae. In other organs, deposition was limited to the wall and perivascular tissues of small- to medium-sized blood vessels. Pulmonary amyloid deposits were always clearly separate and distinct from any coincident surfactant accumulation.
Range of manifestations of amyloidosis in growth factor–deficient mice. (A) Moderate (grade 2) splenic amyloid in an infected 50-week-old female G−/− mouse demonstrating the usual pattern of perifollicular localization of homogeneous acellular eosinophilic material with relative preservation of the splenic white pulp (original magnification ×340, H & E). (B) Extensive (grade 3) confluent splenic amyloid deposits in a 67-week-old female G−/−GM−/− animal with residual atrophic lymphoid follicles (arrows). The mouse was culled due to the presence of a soft-tissue abscess that grew S aureus (original magnification ×340, H & E). (C) Congo red positively stained hepatic amyloid deposition around the central vein in an 81-week-old female G−/−GM−/− mouse (original magnification ×1,360, Congo red). (D) Intestinal amyloid within the submucosa of villi showing characteristic green birefringence when viewed under crossed polarizing filters (same animal as C); original magnification ×1,360, Congo red). (E) Renal amyloid resulting in obliteration of the normal glomerular vasculature in a 48-week-old male G−/−GM−/− mouse (original magnification ×1,360, H & E).
Range of manifestations of amyloidosis in growth factor–deficient mice. (A) Moderate (grade 2) splenic amyloid in an infected 50-week-old female G−/− mouse demonstrating the usual pattern of perifollicular localization of homogeneous acellular eosinophilic material with relative preservation of the splenic white pulp (original magnification ×340, H & E). (B) Extensive (grade 3) confluent splenic amyloid deposits in a 67-week-old female G−/−GM−/− animal with residual atrophic lymphoid follicles (arrows). The mouse was culled due to the presence of a soft-tissue abscess that grew S aureus (original magnification ×340, H & E). (C) Congo red positively stained hepatic amyloid deposition around the central vein in an 81-week-old female G−/−GM−/− mouse (original magnification ×1,360, Congo red). (D) Intestinal amyloid within the submucosa of villi showing characteristic green birefringence when viewed under crossed polarizing filters (same animal as C); original magnification ×1,360, Congo red). (E) Renal amyloid resulting in obliteration of the normal glomerular vasculature in a 48-week-old male G−/−GM−/− mouse (original magnification ×1,360, H & E).
The amyloid deposits were the serum amyloid A (AA), or reactive type, since Congo red staining was abolished by pretreatment with potassium permanganate in two representative cases of affected G−/− and G−/−GM−/− mice (data not shown).
Factors associated with the presence of amyloidosis.A total of 186 animals were examined for the presence of amyloidosis (26 control, 48 G−/−, 40 GM−/−, and 72 G−/−GM−/−). Of these, 123 were culled for illness, and 63 were apparently healthy and killed specifically for study. Amyloid was seen most frequently among G−/− and G−/−GM−/− mice (Table 4), although such an analysis is confounded by variation in other potentially significant risk factors between genotypes.
To more accurately assess the specific characteristics associated with the development of amyloidosis, a forward stepwise multivariate regression analysis was performed (Table 5) incorporating the following potentially contributory factors: G-CSF genotype (+/+ v −/−), GM-CSF genotype (+/+ v −/−), status at analysis (culled for illness v killed healthy), gender, presence of infection, environment (all as discrete variables), and age (as a continuous variable). Overall, nullizygosity for G-CSF, male gender, and culling due to illness were independently associated with the presence of amyloidosis. The lack of GM-CSF had no influence on the risk of developing amyloid.
Multivariate Analysis of Risk Factors for Amyloidosis
| Variable . | Multivariate P . |
|---|---|
| Whole cohort (N = 186)5-150 | |
| Lack of G-CSF | .00007 |
| Male gender | .0012 |
| Animals culled for illness | .0056 |
| Uninfected animals (n = 101)5-151 | |
| Lack of G-CSF | .0086 |
| Male gender | .0031 |
| Variable . | Multivariate P . |
|---|---|
| Whole cohort (N = 186)5-150 | |
| Lack of G-CSF | .00007 |
| Male gender | .0012 |
| Animals culled for illness | .0056 |
| Uninfected animals (n = 101)5-151 | |
| Lack of G-CSF | .0086 |
| Male gender | .0031 |
GM-CSF genotype (+/+ v −/−), age (as a continuous variable), environment (standard animal house v SPF), and the presence of infection were examined as independent variables and did not enter the final multivariate model.
GM-CSF genotype (+/+ v −/−), age (as a continuous variable), status at analysis (culled for illness v killed healthy for study), and environment (standard animal house v SPF) were examined as independent variables and did not enter the final multivariate model.
Given the association of chronic infection with amyloidosis17 18 and the predisposition of growth factor–deficient strains to infection, 101 animals without gross, histologic, or microbiologic evidence of infection were analyzed separately (22 wild-type, 22 G−/−, 22 GM−/−, and 35 G−/−GM−/−; Table 5). Among these “uninfected” animals, a lack of G-CSF and male gender were predictive of amyloidosis. Status at analysis was no longer significant, possibly due to its strong correlation with the presence of infection. It is notable that the SPF environment did not measurably reduce the incidence of amyloidosis. Of 22 SPF-raised animals examined (all apparently healthy males with a mean age of 10 months: four control, four G−/−, four GM−/−, and 10 G−/−GM−/−), six had evidence of amyloid (two G−/− and four G−/−GM−/−).
DISCUSSION
Despite numerous in vitro studies documenting the biologic capacity of hematopoietic colony-stimulating factors, their physiologic roles in vivo remain incompletely defined. The existence of spontaneous null mutants19 and the generation of gene-targeted mice lacking either factors5-7,20 or their receptors21-26 have provided important insights into the unique functions of these cytokines and their signal transduction pathways. G-CSF and GM-CSF have significant granulopoietic activity in vitro, many aspects of which are common to both factors. However, mice deficient in each of these factors have characteristic but dissimilar features, establishing the existence of unique functional roles for G-CSF and GM-CSF in vivo.5-7 We generated mice lacking both G-CSF and GM-CSF to further explore the possibility of shared in vivo functions.
Although normal lung fibroblasts can be induced to produce G-CSF in vitro,27 the lungs do not constitutively produce this factor in vivo.28 Together with the reported lack of pulmonary abnormalities in mice lacking either G-CSF5 or the G-CSF receptor,26 this demonstrates that G-CSF does not play a critical role in lung homeostasis in the unperturbed state. However, the high rate of focal histologic abnormalities of the alveolar septae among animals of both strains lacking G-CSF (G−/−GM+/+ and G−/−GM−/−) killed due to illness suggests that G-CSF may have an important indirect role in pulmonary function under some circumstances. The abundance of type II pneumocytes noted in mice lacking G-CSF resembles the changes described in the syndrome of “diffuse alveolar damage,”29 the features of which can be produced experimentally by intratracheal administration of lipopolysaccharide30 or overexpression of tumor necrosis factor-α.31 The absence of such abnormalities in infected and moribund GM−/− mice suggests that a component of the host response influenced by G-CSF rather than an underlying infection itself is critical in the pathogenesis of these pulmonary changes.
Lung abnormalities attributable to the deficiency of GM-CSF were not exacerbated by the additional lack of G-CSF. This is similar to findings in mice lacking either functional receptors for interleukin-5 (IL-5)21,22 or both IL-3 and functional IL-5 receptors25 in addition to GM-CSF, where there was no detectable exacerbation of the lung disease attributable to the loss of the actions of GM-CSF alone.21,22 25
The degree of steady-state neutropenia in adult G−/−GM−/− mice was no more severe than in animals lacking G-CSF alone, demonstrating that GM-CSF is not essential for the residual granulopoiesis observed in adult G−/− mice. Further, in contrast to op/op mice, which lack active M-CSF,32 33 G−/− and G−/−GM−/− mice show no evidence of hematopoietic recovery up to 18 months of age. The age-dependent correction of some of the hematopoietic defects associated with op/op mice illustrates the potential plasticity of the hematopoietic system and its capacity to use alternative compensatory mechanisms, presumably in the form of growth factors with partially overlapping functions. The lack of any age-related correction of the phenotypes of any of the three factor-deficient strains in the current analysis indicates that the phenomenon of age-dependent amelioration in factor-deficient animals is probably exceptional.
The greater degree of neutropenia up to 2 weeks of age in G−/−GM−/− animals compared with singly G-CSF–deficient mice establishes that under certain circumstances GM-CSF can contribute to steady-state neutrophil production in vivo, even though GM−/− mice are not neutropenic at any time point. The failure to demonstrate any such worsening of neutropenia in adult G−/−GM−/− mice is consistent with the notion that the contribution of factors regulating hematopoiesis may vary during different developmental periods. This notion is also supported by the finding that young G-CSF–deficient mice are proportionally less neutropenic than adult animals of the same genotype.
The factors controlling the patterned changes in hepatic, splenic, and medullary hematopoiesis through the fetal and neonatal periods are unknown.34 Since all strains examined manifest the normal dynamic alterations in neutrophil levels during the first 8 weeks of life, neither G-CSF nor GM-CSF appears essential for these changes, although available data had suggested that their involvement was possible.35-41 Murine fetal tissues do not produce significant quantities of G-CSF or GM-CSF,35 but maternal G-CSF can cross the placenta and remain biologically active.36 Also, both of these factors,35,37-40 as well as M-CSF,35 41 are produced locally within the placenta and pregnant uterus and may potentially act systemically to influence hematopoiesis during the fetal and early neonatal periods.
In mice lacking both G-CSF and GM-CSF, the reduction in both marrow and splenic progenitor numbers and the percentage of bone marrow myeloid precursors were no greater than in G-CSF–deficient mice. Previous reports and the current analysis describe increased numbers of splenic progenitors in GM−/− mice,6 but a reduction in G−/− mice.5 It is therefore noteworthy that the number of splenic progenitors in G−/−GM−/− mice was significantly lower than in wild-type mice but indistinguishable from that in G-CSF–deficient animals, demonstrating that G-CSF is necessary for, and may be responsible for, the enhanced splenic hematopoiesis in GM−/− mice.
The initial report of GM-CSF–deficient mice found no impairment of reproductive capacity based on the study of five litters,6 and no data have been published on the fertility of mice lacking the GM-CSF receptor β-common chain.21,22,25 The current analysis demonstrates a modest reduction in the litter size of GM−/− animals. This suggests an indispensable physiologic role for GM-CSF in optimizing reproductive function, and is consistent with the observation that administration of a GM-CSF–neutralizing antibody to pregnant mice results in an increased rate of spontaneous abortion.42 The expression and secretion of GM-CSF by placental tissue35,38,40 and the responsiveness of both trophoblast43 and nontrophoblast placental cells to GM-CSF44 suggest that GM-CSF has a role in placental formation or function. This is supported by the capacity of injected GM-CSF to increase both placental and fetal weight and enhance fertility in CBA × DBA/2 mice, which have a high background rate of spontaneous abortion.42,45 A possible explanation for these observations is suggested by the finding that in vitro the perfused ovaries of GM−/− mice have a blunted progesterone response to gonadotropin stimulation.46 Additionally, if the breeding data suggesting a selective loss of doubly nullizygous embryos among the progeny of double heterozygote crosses are confirmed, this would be consistent with a specific role for embryo-derived GM-CSF in these processes. However, the methods used cannot definitively establish the contribution of prenatal versus postnatal events, and such a role remains speculative.
Mice lacking either GM-CSF or G-CSF individually had significantly impaired long-term survival. In GM-CSF–deficient mice, this was attributable to both pulmonary and soft-tissue infections and “alveolar proteinosis-like” lung disease. The finding of a significant peripheral blood neutrophilia in a number of GM−/− mice with sporadic infections and the prominent neutrophilic infiltration at sites of infection suggest that GM-CSF is not required for augmented granulopoiesis or neutrophil localization in response to some stimuli.25 47 However, the increased incidence of soft-tissue infections observed in GM-CSF–deficient animals despite apparently adequate peripheral blood neutrophilia and neutrophil localization suggests a possible impairment in some aspect of microbial killing.
G-CSF–deficient mice had similarly increased mortality, predominantly due to severe infections. Surprisingly, there were no pulmonary infections seen despite prolonged neutropenia, consistent with the primary role of the alveolar macrophage in the prevention of pulmonary infection. In G−/− animals, neutrophils were infrequent at sites of infection, possibly due to the steady-state peripheral blood neutropenia and impaired granulopoietic response to microbial challenge5 or a defect in neutrophil localization. However, no such localization defect was detected in response to either intraperitoneal casein47 or thioglycollate.26
Many of the pathologic features of the doubly deficient G−/−GM−/− mice are consistent with the additive effects of the constituent genotypes. Thus, these animals manifest a high rate of soft-tissue and pulmonary infections, together with alveolar proteinosis-like lung disease, hypercellularity of the alveolar septae, and a mortality rate significantly greater than either of the singly deficient strains. In G−/−GM−/− animals, as in those lacking G-CSF alone, neutrophils were infrequent at sites of infection.
Finally, mice of both strains lacking G-CSF had a greatly increased propensity to develop reactive AA-type amyloidosis, with the dominant role of G-CSF, independent of GM-CSF genotype and the associated predisposition to infection, confirmed in multivariate analyses. There is a low background incidence of spontaneous AA-type amyloidosis in many mouse strains, with a higher rate beyond 12 months of age, particularly among fighting males and in the presence of infection.17,18 48 We found a 4% incidence of amyloidosis among wild-type mice, whereas 10% of GM−/− mice, many of which had evidence of infection, were affected. Although male gender and the need for animals to be culled due to illness were also independently associated with the incidence of amyloid, the absence of G-CSF, and the resulting chronic neutropenia, was the most significant predictor identified.
Other animal models have previously implicated chronic neutropenia in the pathogenesis of amyloidosis. Gray collie dogs with cyclic neutropenia develop AA-type amyloidosis,49,50 as do the progeny of hybrid mice selected and interbred on the basis of leukopenia.51 52 While not dismissing the contribution of underlying infection, these models suggest that neutropenia per se may predispose to the development of AA-type amyloidosis.
The major constituent of tissue deposits in AA-type amyloidosis is a cleavage product of serum amyloid-A protein, which is synthesized by the liver as an acute-phase protein under the control of IL-1α, IL-6,53-56 and possibly other gp 130-linked cytokines.57 One hypothesis that may explain the link between neutropenia and amyloidosis is the counterregulatory increase in the activity of one or more of the factors regulating serum amyloid-A in response to chronic neutropenia. Teleologically, such a response could be explained by the capacity of one or more of these cytokines to enhance granulopoiesis.58 Studies to investigate this hypothesis are ongoing, and the susceptibility of mice deficient in both G-CSF and IL-6 to amyloidosis will be useful in addressing this issue.
ACKNOWLEDGMENT
The authors thank Dr E. Stanley for his role in the generation of the GM−/− mice used in this study, Dr V. Sinickas for microbiologic analyses, Dr A. Grigg for access to the Sysmex automated analyzer, the animal house staff for animal husbandry, V. Feakes for preparation of histologic sections and special stains, Dr I. Gordon for statistical guidance, and Prof A. Burgess for his critical reading of the manuscript.
Supported in part by a National Health and Medical Research Council Medical Postgraduate Research Scholarship (J.F.S.).
Address reprint requests to John F. Seymour, MB, BS, Ludwig Institute for Cancer Research, Melbourne Tumour Biology Branch, PO Box 2008 Royal Melbourne Hospital, Victoria, 3050 Australia.

![Fig. 3. Illustrative sections of the range of severity of the observed pulmonary pathology. (A) Grade 2 proteinosis: relatively uniform and moderately extensive granular deposits in the majority of alveoli in affected areas in a 12-month-old male GM−/− mouse; note abnormally distended alveolar macrophage (arrow). (B) Grade 4 proteinosis: uniformly confluent deposits appearing to completely fill affected alveoli in a 21-week-old female G−/−GM−/− mouse. (C) Grade 2 lymphoid accumulation: moderately large segmental lymphoid focus < 10 cells thick in a 43-week-old female G−/−GM−/− mouse. (D) Grade 4 lymphoid accumulation: extensive areas of lymphoid accumulation no longer confined to the perivascular zone, but extending interstitially into the surrounding lung parenchyma in a 29-week-old female G−/−GM−/− mouse; note confluent areas of surfactant accumulation within alveoli (grade 4 proteinosis). (All sections are stained with hematoxylin and eosin [H&E]; A and B are original magnification ×1,360, and C and D are ×340.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3037/4/m_bl_0001f3.jpeg?Expires=1769099901&Signature=sS4gvuqL2PTJq3tFVBasp~8Z3EJD0vhi5U2Eq2FQHm3a4GhJf~tZrcVtfRhiyKga2qRoncwnDT8MRZEbcBaLGnsDlvL46e2CjdxnG2Hyp~y6i5ho9D4Lr864IBKRGemaDDgUCV-~IYhl0WgKzApVbGa4sLLUKXwwzV~bYlbpdZ4jm90IBi2F2rkqKvsJRwwj-ktAD9KyS2ZFhMY-osRZOY1k19I~E~lvCD3-B2eaFcS3xHe~kt5XJuwvU2YKEBoBnLF7h39JOZp1HMYAe0lWXv7oJxsGDFkavgB3iPubg9mHfWD6SA9lZdQBaibVOyTg9MPuLdteiO5Uaqmxb92q4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal