Abstract
Three intensive consolidation strategies are currently proposed to younger adults with acute myeloid leukemia (AML) in first complete remission (CR): allogeneic or autologous bone marrow transplantation (BMT) and intensive consolidation chemotherapy (ICC). Patients aged 15 to 50 years with de novo AML received an induction treatment with 7 days of cytarabine and either idarubicin or rubidazone. After achievement of a CR, patients up to the age of 40 and having an HLA-identical sibling were assigned to undergo an allogeneic BMT. All the other patients received a first course of ICC with high-dose cytarabine and the same anthracycline as for induction. They were then randomly assigned to either receive a second course of ICC with amsacrine and etoposide or a combination of busulfan and cyclosphosphamide followed by an unpurged autologous BMT. Of 517 eligible patients, 367 had a CR, but only 219 (59.5%) actually received the planned intensive postremission treatment (73 allogeneic BMT, 75 autologous BMT, and 71 ICC). With a median follow-up of 62 months, the 4-year disease-free survival (DFS) of the 367 patients in CR was 39.5%. The 4-year overall survival (OS) of the 517 eligible patients was 40.5%. In multivariate analysis, DFS and OS were influenced only by the initial white blood cell count and by the French-American-British classification. The type of postremission therapy had no significant impact on the outcome. There was no difference in the 4-year DFS and OS between 88 patients for whom an allogeneic BMT was scheduled (respectively, 44% and 53%) and 134 patients of the same age category and without an HLA-identical sibling (respectively, 38% and 53%). Similarly, there was no difference in the outcome between autologous BMT and ICC. The 4-year DFS was 44% for the 86 patients randomly assigned to autologous BMT and 40% for the 78 patients assigned to ICC (P = .41). The 4-year OS was similar in the two groups (50% v 54.5%, P = .72). The median duration of hospitalization and thrombocytopenia were longer after autologous BMT (39 v 32 days, P = .006, and 109.5 v 18.5 days, P = .0001, respectively). After a first course of ICC, a second course of chemotherapy is less myelotoxic than an unpurged autologous BMT but yields comparable DFS and OS rates.
POSTREMISSION therapy remains a critical issue in acute myeloid leukemia (AML).1 2 During the past 10 years, aggressive postremission strategies have been proposed for younger patients in complete remission (CR) after induction treatment. These options include myeloablative therapy with either allogeneic bone marrow transplantation (BMT) or autologous hematopoietic stem cell support and intensive consolidation chemotherapy (ICC).
Allogeneic BMT is currently the most effective antileukemic therapy, with a relapse rate lower than 30% when applied in first CR. However, because of its toxicity, mostly related to graft-versus-host disease, patients more than 50 years of age are generally not considered as eligible for this procedure. Moreover, its use is generally restricted to patients having an HLA-identical sibling.
Autologous stem cell transplantation offers the possibility to perform the same myeloablative regimen in patients without a compatible donor and without the risks associated with graft-versus-host disease. Whereas the toxic death rate is much lower than after allogeneic BMT, the relapse rate remains higher3 either because of the graft contamination by malignant cells or of the absence of graft-versus-leukemia effect due to the donor's lymphocytes.4,5 Several uncontrolled studies on autologous transplantation with unpurged BM,6-8 purged BM,9-11 or peripheral blood stem cells12,13 have shown that 3- to 5-year disease-free survival (DFS) rates of 30% to 60% were achieved in AML in first CR. These preliminary results were confirmed by the analysis of the EBMT registry.14 However, all these studies raised the issue of patient selection.
In pilot studies with ICC, DFS rates of 30% to 50% have also been achieved, but with the same concerns regarding patient selection.15-22
Therefore, large randomized studies comparing autologous transplantation and intensive chemotherapy as postremission therapy in adult AML were necessary. The first large randomized study comparing allogeneic BMT, autologous BMT (ABMT), and ICC was recently published by the EORTC and GIMEMA groups.23 In this study, allogeneic BMT and ABMT resulted in significantly better DFS than ICC with high-dose cytarabine (HD-ARA-C). We herein report the results of a multicenter randomized study conducted by the GOELAM group also comparing ABMT and ICC.
MATERIALS AND METHODS
Patients.Patients 15 to 50 years of age with previously untreated primary AML were eligible for entry into the trial. The diagnosis was assessed by a BM aspirate showing at least 30% blast cells or, in case of myelofibrosis, by a BM biopsy. Each case was classified according to the French-American-British (FAB) System.24 No central pathology review was performed. Informed consent was obtained according to the French regulation.
Patients with a previous myelodysplasia diagnosed for more than 3 months were excluded, whereas patients with antecedent of unexplained cytopenia were included. We also excluded patients with a myeloproliferative disorder in blast crisis, patients who had previously received cytotoxic chemotherapy or radiotherapy (or both), patients with clinical or electrocardiographic signs of heart failure or coronary disease, and patients with hepatic or renal disturbances (hepatic enzymes levels over 4 times the normal values, creatinine level over 130 μmol/L).
Karyotypic abnormalities were classified as favorable [t(8; 21), t(15; 17) or inv (16)], unfavorable (−5, 5q−, −7, or multiple abnormalities), or intermediate (all other abnormalities).25 26 Central review of all available material was performed by members of the Groupe Français de Cytogénétique Hématologique.
Study design.The induction treatment consisted of a continuous infusion of ARA-C (200 mg/m2/d) for 7 consecutive days (days 1 through 7) with either idarubicin (IDR) administered intravenously on days 1 through 5 at a daily dose of 8 mg/m2 or rubidazone (RBZ) administered intravenously on days 1 through 4 at a daily dose of 200 mg/m2. RBZ is an anthracycline derivative commonly used in France at that time and yielding promising CR rates.22 27 Central randomization was stratified by center.
A BM aspiration was performed on day 17. If the marrow was hypoplastic and nonblastic, no further treatment was administered. If the marrow was hypoplastic and contained less than 50% blasts, a second induction course was administered with a combination of ARA-C over 3 days and of the anthracycline initially allocated over 2 days. If the marrow was normocellular and blastic or contained more than 50% blasts, the result was estimated as a failure and the patient was withdrawn from the study.
All patients in CR after one or two courses of induction treatment were scheduled to receive an intensive consolidation therapy. A search for an HLA-identical sibling was performed on each patient 40 years of age or younger and an allogeneic BMT was proposed to all these patients having an HLA-identical sibling. While waiting for BMT, patients generally received a standard dose consolidation chemotherapy with 150 mg/m2 amsacrine intravenously on day 1 and 100 mg/m2/d ARA-C subcutaneously on 5 consecutive days. The conditioning regimen, the prophylaxis, and treatment of graft-versus-host disease were administered according to the protocols used in each transplant center. Of 73 transplanted patients, 18 were prepared by a combination of busulfan (4 mg/kg/d on 4 consecutive days) and cyclophosphamide (50 mg/kg/d on 4 consecutive days [12 patients] or 60 mg/kg/d on 2 consecutive days [6 patients]), whereas 55 received total body irradiation-containing regimens with either 60 mg/kg/d cyclophosphamide on 2 consecutive days (41 patients) or with other chemotherapies (14 patients). The graft-versus-host prophylaxis was cyclosporine plus methotrexate (56 patients), cyclosporine plus corticosteroids (10 patients), cyclosporine plus methotrexate plus corticosteroids (3 patients), cyclosporine alone (3 patients), or methotrexate alone (1 patient). No T-cell depletion was performed.
All other patients were assigned to receive a first course of ICC consisting of HD-ARA-C (3 g/m2) administered on a 3-hour infusion every 12 hours, days 1 through 4 (total dose, 24 g/m2), combined with either IDR administered intravenously on days 5 and 6 at a daily dose of 10 mg/m2 or RBZ administered intravenously on days 5 and 6 at a daily dose of 200 mg/m2, according to the initial randomization. The first course of ICC was to be administered as soon as possible after remission achievement and no later than 75 days after induction treatment initiation.
BM was harvested after the first course of ICC without any in vitro manipulation. Patients still in CR with at least 1 × 108 nucleated cells/kg in the collected marrow were randomly assigned to receive either a second course of ICC or an ABMT. The second course of ICC consisted of amsacrine administered as a 1-hour infusion on 5 consecutive days (days 1 through 5) at a daily dose of 150 mg/m2 and etoposide administered as a 2-hour infusion on days 1 through 5 at a daily dose of 100 mg/m2. Before ABMT, the conditioning regimen was the combination of busulfan administered orally at a daily dose of 4 mg/kg for 4 consecutive days (days 9 through 6 before transplantation) and cyclophosphamide administered intravenously at a daily dose of 50 mg/kg for 4 consecutive days (days 5 through 2 before transplantation).28
Response criteria.CR was defined as a normocellular BM containing less than 5% blasts, more than 1 × 109/L granulocytes, and more than 100 × 109/L platelets. The causes for failure were early death (death during the 7 days of induction treatment), death during chemoinduced aplasia, and resistant leukemia.
Statistical analysis.Comparison between the different treatment groups were performed with the χ2 test for binary variables29 and the Kruskal-Wallis test for continuous variables.30 DFS was calculated from the date of first remission until the date of first relapse or the date of death from any cause. Overall survival (OS) was defined as the time from first randomization to the time of death. Patients who did not receive the induction treatment as indicated by the protocol were excluded from the calculation of DFS, but data were collected on these patients for the analysis of OS.
Survival curves were plotted following the Kaplan-Meier method31 and differences between the curves were analyzed with the log-rank test.32 The following parameters were analyzed for their impact on the outcome: sex, age, initial performance status according to the World Health Organization (WHO) classification, fever at the time of inclusion, initial white blood cell (WBC) count, FAB classification (2 and 3 v other subtypes and unclassified), karyotype analysis (4 groups were defined: favorable karyotypes, normal and intermediate karyotypes, unfavorable karyotypes, and missing data), first randomization arm (IDR v RBZ), number of courses needed for CR achievement (1 v 2), and type of postremission therapy. Multivariate analysis was made by a logistic regression analysis for the CR rate33 and by the Cox proportional hazard model for DFS and OS data.34 Comparison between ABMT and ICC was performed according to the intention to treat principle. Because there was no significant difference between the two induction treatment arms regarding the CR rate, the DFS, and OS, they were pooled for the comparison of postremission therapies. A direct comparison between allogeneic BMT and the other two modalities of postremission therapy was not possible because patients assigned to allogeneic BMT did not receive the first course of ICC. However, a comparison was made between the patients for whom, according to the protocol, the intention was to perform an allogeneic BMT and those who should have received the first course of ICC.
The median follow-up duration is 62 months (range, 23 to 103 months). Investigators received a follow-up questionnaire every 6 months. The final analysis was performed as of June 1996.
RESULTS
Between November 1987 and May 1994, 535 patients from 16 institutions were included in the study. Eighteen patients were considered ineligible, 9 because of an inadequate diagnosis, 7 because of age limits, and 2 because of other ineligibility criteria. The clinical characteristics of the 517 eligible patients are shown in Table 1. Thirteen patients were unevaluable, 4 because they died before having received the first day of treatment, 5 because of major protocol violation, and 4 because of a wrong randomization. There was no significant difference between the two groups (IDR or RBZ) regarding the initial characteristics.
Characteristics of the 517 Eligible Patients
| Age (median) | 15-50 (36) |
| Sex (M/F) | 256/261 |
| Performance status (0-1-2/3-4/NA) | 460/48/9 |
| Initial WBC count (109/L) (median) | 0.4-486 (13.5) |
| FAB classification (0/1/2/3/4/5/6/7/NA) | 31/111/154/44/69/88/9/6/5 |
| Karyotype | |
| Performed | 337 |
| Evaluable | 309 |
| Normal | 133 |
| Abnormal | 176 (57%) |
| Favorable | 47 |
| Intermediate | 93 |
| Unfavorable | 36 |
| Age (median) | 15-50 (36) |
| Sex (M/F) | 256/261 |
| Performance status (0-1-2/3-4/NA) | 460/48/9 |
| Initial WBC count (109/L) (median) | 0.4-486 (13.5) |
| FAB classification (0/1/2/3/4/5/6/7/NA) | 31/111/154/44/69/88/9/6/5 |
| Karyotype | |
| Performed | 337 |
| Evaluable | 309 |
| Normal | 133 |
| Abnormal | 176 (57%) |
| Favorable | 47 |
| Intermediate | 93 |
| Unfavorable | 36 |
Abbreviation: NA, not available.
Outcome and prognostic factors.Of the 504 patients evaluable for induction treatment, 367 (73%) achieved a CR, including 338 patients treated with only one course of chemotherapy (92% of the CR). There were 9 early deaths (2%), 22 deaths during aplasia (4%), and 106 (21%) patients with persisting leukemia. The outcome was not significantly different between the two induction treatment arms (IDR or RBZ). Among the parameters tested in univariate analysis for their impact on CR achievement, three were of significant value: the initial WBC count (cut-off, 30 × 109/L), the FAB classification (M2-M3 v others), and the karyotype (4 groups as defined in the Materials and Methods; Table 2). In multivariate analysis, only two parameters remained significant: the initial WBC count ( P = .0005) and the karyotype ( P = .0075).
Prognostic Factors (Univariate Analysis)
| . | CR Rate (%) . | 4-yr DFS (%) . | 4-yr OS (%) . | |||
|---|---|---|---|---|---|---|
| FAB classification | ||||||
| M2-M3 | ||||||
| Others | 78 (N = 194) | P = .05 | 53 (N = 152) | P < .0001 | 46 (N = 199) | P < .0001 |
| 69 (N = 310) | 29 (N = 215) | 18 (N = 318) | ||||
| Initial WBC count | ||||||
| <30 × 109/L | ||||||
| ≥30 × 109/L | 78 (N = 321) | P = .0004 | 42 (N = 253) | P = .005 | 42 (N = 332) | P < .001 |
| 63 (N = 183) | 31 (N = 114) | 29 (N = 185) | ||||
| Karyotype | ||||||
| Favorable | ||||||
| Normal and intermediate | ||||||
| Unfavorable | ||||||
| Missing data | 87.5 (N = 48) | P = .003 | 59 (N = 42) | P = .02 | 61 (N = 48) | P = .018 |
| 76.5 (N = 226) | 32 (N = 173) | 38 (N = 226) | ||||
| 58 (N = 36) | 47 (N = 21) | 39 (N = 36) | ||||
| 67.5 (N = 194) | 40 (N = 131) | 39 (N = 207) |
| . | CR Rate (%) . | 4-yr DFS (%) . | 4-yr OS (%) . | |||
|---|---|---|---|---|---|---|
| FAB classification | ||||||
| M2-M3 | ||||||
| Others | 78 (N = 194) | P = .05 | 53 (N = 152) | P < .0001 | 46 (N = 199) | P < .0001 |
| 69 (N = 310) | 29 (N = 215) | 18 (N = 318) | ||||
| Initial WBC count | ||||||
| <30 × 109/L | ||||||
| ≥30 × 109/L | 78 (N = 321) | P = .0004 | 42 (N = 253) | P = .005 | 42 (N = 332) | P < .001 |
| 63 (N = 183) | 31 (N = 114) | 29 (N = 185) | ||||
| Karyotype | ||||||
| Favorable | ||||||
| Normal and intermediate | ||||||
| Unfavorable | ||||||
| Missing data | 87.5 (N = 48) | P = .003 | 59 (N = 42) | P = .02 | 61 (N = 48) | P = .018 |
| 76.5 (N = 226) | 32 (N = 173) | 38 (N = 226) | ||||
| 58 (N = 36) | 47 (N = 21) | 39 (N = 36) | ||||
| 67.5 (N = 194) | 40 (N = 131) | 39 (N = 207) |
Among the 367 patients who had a CR, the probability of remaining alive and free of disease at 4 years was 39.5% ± 2.5%, with no significant difference between the two induction treatment arms. The median DFS was 18 months. In univariate analysis, the DFS was also related to the initial WBC count, to the FAB classification, and to the karyotype (Table 2). In multivariate analysis, the FAB classification was the only significant prognostic factor ( P = .0001).
The median OS of the 517 eligible patients was 22.5 months and the 4-year actuarial OS was 40.5% ± 2.5%, with no significant difference between the two induction treatment arms. In multivariate analysis, among the parameters tested, two had a significant impact on OS: the initial WBC count ( P = .003) and the FAB classification ( P = .004). The type of postremission therapy had no significant impact on DFS or OS. The relevant prognostic factors are shown in Table 2.
According to the initial WBC count and to the FAB classification, three subgroups were defined: 157 patients with an initial WBC count less than 30 × 109/L and with either M2 or M3 FAB subtype were in the low-risk group, 202 patients with an initial WBC count ≥30 × 109/L or with an FAB subtype other than M2 or M3 were in the intermediate-risk group, and 145 patients with an initial WBC count ≥30 × 109/L and with an FAB subtype other than M2 or M3 were in the high-risk group. The 4-year OS was 54.5% ± 4% in the low-risk group, 41% ± 3.5% in the intermediate-risk group, and 25% ± 4% in the high-risk group ( P < .0001). The 4-year DFS was 54% ± 4.5% in the low-risk group (128 patients), 33.5% ± 4% in the intermediate-risk group (149 patients), and 26% ± 5.5% in the high-risk group (90 patients; P < .0001).
Feasibility of postremission treatment.Of 367 patients in CR, 146 did not undergo the assigned intensive postremission therapy for the following reasons (Table 3): extrahematologic toxicity (27), protocol violation (26), refusal (24), relapse (22), poor hematologic reconstitution (21), infectious complications (19), and toxic death (7). Two patients were lost for follow-up. Thus, only 219 patients did actually receive the intensive treatment as scheduled in the protocol (59.5% of the patients in CR and 41% of the total number of included patients). Feasibility of the protocol is shown in Table 4.
Reasons for Not Completing the Protocol as Scheduled
| . | Total . | After CR Achievement . | Patients Assigned to Allogeneic BMT . | Patients Assigned to First ICC . | After First ICC . | After Randomization . | |
|---|---|---|---|---|---|---|---|
| . | . | (N = 367) . | (N = 88) . | (N = 267) . | (N = 228) . | (N = 164) . | |
| . | . | . | . | . | . | ICC (N = 78) . | ABMT (N = 86) . |
| Relapse | 22 | — | 5 | 2 | 7 | 3 | 5 |
| Infectious complications | 19 | 5 | 1 | 6 | 7 | — | — |
| Poor hematologic reconstitution | 21 | 1 | — | 2 | 18 | — | — |
| Extrahematologic toxicity | 27 | 4 | 5 | 5 | 12 | — | 1 |
| Toxic death | 7 | 1 | — | — | 6 | — | — |
| Refusal | 24 | 1 | 4 | 1 | 14 | — | 4 |
| Protocol violation | 26 | — | — | 223-150 | — | 4 | — |
| Lost for follow-up or other reasons | 2 | — | — | 1 | — | — | 1 |
| Total | 148 | 123-151 | 15 | 39 | 64 | 7 | 11 |
| . | Total . | After CR Achievement . | Patients Assigned to Allogeneic BMT . | Patients Assigned to First ICC . | After First ICC . | After Randomization . | |
|---|---|---|---|---|---|---|---|
| . | . | (N = 367) . | (N = 88) . | (N = 267) . | (N = 228) . | (N = 164) . | |
| . | . | . | . | . | . | ICC (N = 78) . | ABMT (N = 86) . |
| Relapse | 22 | — | 5 | 2 | 7 | 3 | 5 |
| Infectious complications | 19 | 5 | 1 | 6 | 7 | — | — |
| Poor hematologic reconstitution | 21 | 1 | — | 2 | 18 | — | — |
| Extrahematologic toxicity | 27 | 4 | 5 | 5 | 12 | — | 1 |
| Toxic death | 7 | 1 | — | — | 6 | — | — |
| Refusal | 24 | 1 | 4 | 1 | 14 | — | 4 |
| Protocol violation | 26 | — | — | 223-150 | — | 4 | — |
| Lost for follow-up or other reasons | 2 | — | — | 1 | — | — | 1 |
| Total | 148 | 123-151 | 15 | 39 | 64 | 7 | 11 |
Including 14 allogeneic BMT instead of first course of ICC (13 patients over 40 years of age, 1 unrelated donor).
Patients 15 to 40 years of age (HLA typing not performed).
Two hundred twenty-eight patients underwent the first ICC as planned. The median duration of neutropenia (<0.5 × 109/L) after this first course was 18 days (range, 9 to 59 days). The median duration of thrombocytopenia (<30 × 109/L) was 16 days (range, 5 to 48 days). The median length of hospitalization was 30 days in both arms. Six toxic deaths (2.5%) and 3 cases of severe cerebellar toxicity were recorded.
Allogeneic BMT.The median interval between CR achievement and allogeneic BMT was 68 days (range, 9 to 200 days). Of the 73 patients who underwent the scheduled allogeneic BMT, 22 have relapsed and 33 patients have died, 16 from procedure-related toxicity (22%) and 17 after relapse. Their 4-year DFS was 49.5% ± 6% and their 4-year OS was 55% ± 6%. The actuarial risk of relapse at 4 years was 37%. Thirteen patients 41 to 45 years of age actually received an intrafamilial allogeneic BMT instead of the first course of ICC scheduled in the protocol. The 4-year DFS and OS of the 86 patients transplanted in first CR were, respectively, 43.5% ± 5.5% and 52.5% ± 5.5%.
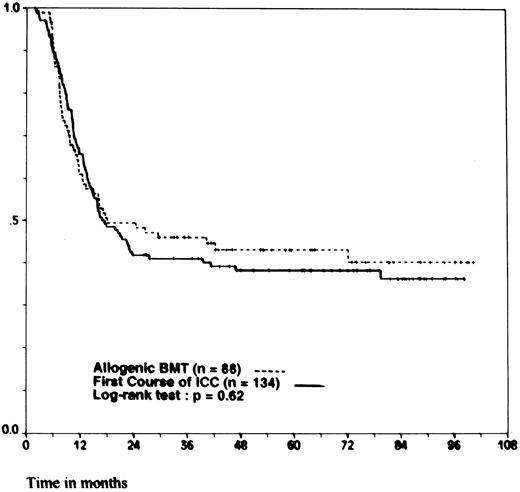
We compared the outcome of the 88 patients 40 years of age and younger for whom the intention was to perform an allogeneic BMT to that of the 134 patients of the same age category but without an HLA-identical sibling, for whom the intention was to perform a first course of ICC. There was no significant difference in the distribution of initial clinical characteristics between the two groups, except for karyotype analysis: the incidence of intermediate and unfavorable karyotypes was lower in the group of patients assigned to allogeneic BMT (17% v 47%, P = .001). The 4-year DFS was 44% ± 5.5% for allogeneic BMT and 38% ± 4% for ICC ( P = .62; Fig 1). The 4-year OS was 53% ± 5.5% for allogeneic BMT and 53% ± 4.5% for ICC ( P = .74).
Probability of DFS for patients 15 to 40 years of age. According to whether patients were assigned to allogeneic BMT (HLA-identical sibling) or to a first course of ICC (no HLA-identical sibling). Ticks indicate patients surviving in continuous CR.
Probability of DFS for patients 15 to 40 years of age. According to whether patients were assigned to allogeneic BMT (HLA-identical sibling) or to a first course of ICC (no HLA-identical sibling). Ticks indicate patients surviving in continuous CR.
We also evaluated the impact of allogeneic BMT in the three prognostic subgroups defined according to the initial characteristics found to be of significant value in the multivariate analysis (WBC count and FAB classification). In the low-risk group, the 4-year DFS was 61% ± 8.5% for the 35 patients assigned to allogeneic BMT and 51% ± 8% for the 39 patients of the same age category assigned to the first course of ICC ( P = .47). The 4-year OS was 71.5% ± 8% for allogeneic BMT and 66.5% ± 7.5% for ICC ( P = .60). In the intermediate-risk group, the 4-year DFS was 33.5% ± 9% for the 31 patients assigned to allogeneic BMT and 38% ± 6% for the 63 patients assigned for ICC ( P = .67), and the 4-year OS was 40.5% ± 9% for allogeneic BMT and 56.5% ± 6.5% for ICC ( P = .08). In the high-risk group, the 4-year DFS was 27% ± 10% for the 22 patients assigned to allogeneic BMT and 22% ± 8% for the 32 patients assigned to ICC ( P = .86) and the 4-year OS was 41% ± 10% for allogeneic BMT and 30% ± 8% for ICC ( P = .97).
Comparison of ICC and ABMT.Of the 75 patients who actually underwent ABMT according to the protocol, 34 have relapsed. Five patients have died from procedure-related toxicity (6.5%) and 29 after relapse. The 4-year DFS and OS were, respectively, 48% ± 6% and 52% ± 6%. Of the 71 patients who underwent the second course of ICC as assigned, 39 have relapsed. Two patients have died from procedure-related toxicity (3%) and 27 after relapse. The 4-year DFS and OS were, respectively, 42.5% ± 6% and 58.5% ± 6%.
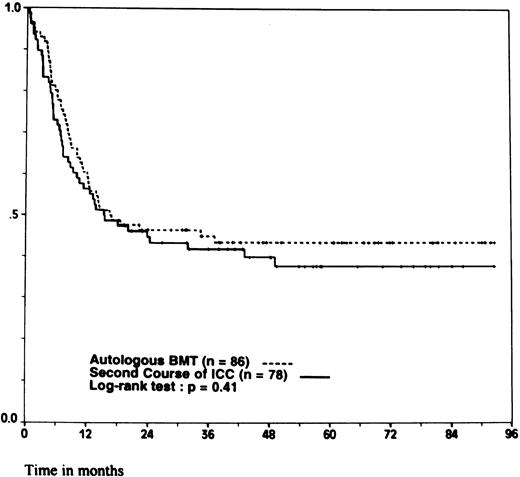
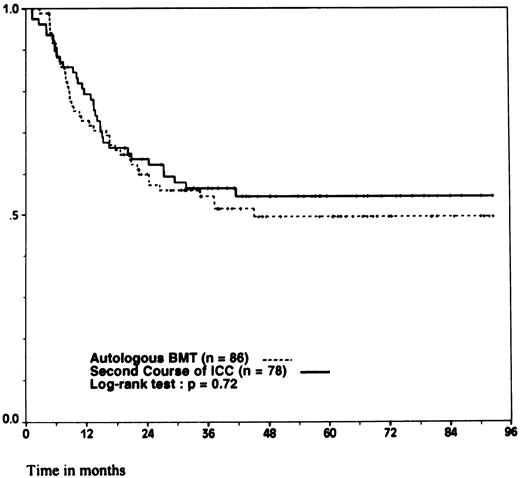
However, because 11 patients randomized in the ABMT arm and 7 patients randomized in the ICC arm did not undergo the assigned treatment, the comparison between the two groups was performed according to the intention to treat principle. There was no significant difference between the 86 patients randomly assigned to ABMT and the 78 patients randomly assigned to ICC regarding the initial clinical characteristics. The median interval between the achievement of CR and the completion of the last intensive postremission therapy as assigned by the randomization was 107 days (range, 95 to 122 days) for autologous transplantation and 91 days (range, 81 to 109 days) for intensive chemotherapy ( P = .0001). The 4-year DFS was 44% ± 5.5% for ABMT and 40% ± 5.5% for ICC ( P = .41; Fig 2). The 4-year OS was 50% ± 6% for ABMT and 54.5% ± 6% for ICC ( P = .72; Fig 3). Again, when looking at the different prognostic subgroups defined by initial WBC count and FAB classification, we found no significant difference in the outcome between ABMT and ICC. In the low-risk group, the 4-year DFS was 50% ± 9% for the 32 patients assigned to ABMT and 56.5% ± 11% for the 22 patients assigned to ICC ( P = .58) and the 4-year OS was 59% ± 9% for ABMT and 71% ± 8% for ICC ( P = .49). In the intermediate-risk group, the 4-year DFS was 38.5% ± 9% for 33 patients assigned to ABMT and 31% ± 8.5% for 35 patients assigned to ICC ( P = .7) and the 4-year OS was 42.5% ± 9% for ABMT and 55% ± 9% for ICC ( P = .66). In the high-risk group, the 4-year DFS was 38% ± 10% for the 21 patients assigned to ABMT and 28.5% ± 10% for the 21 patients assigned to ICC ( P = .36) and the 4-year OS was 46.5% ± 11% for ABMT and 40% ± 11.5% for ICC ( P = .93).
Probability of DFS. According to whether patients were randomly assigned to autologous BMT or to a second course of ICC. Ticks indicate patients surviving in continuous CR.
Probability of DFS. According to whether patients were randomly assigned to autologous BMT or to a second course of ICC. Ticks indicate patients surviving in continuous CR.
Probability of survival. According to whether patients were randomly assigned to autologous BMT or to a second course of ICC. Ticks indicate surviving patients.
Probability of survival. According to whether patients were randomly assigned to autologous BMT or to a second course of ICC. Ticks indicate surviving patients.
The probability of falsely stating that the two treatments are equivalent is 0.2023 (β = 20.23%). Therefore, the power of the study is approximately 80%. With a difference of only 4% in DFS between ICC (40%) and ABMT (44%), a total of 3,540 patients (1,770 per arm) would be required to demonstrate a statistical significance (α = 5%, β = 20%, unilateral test). This would require the inclusion of more than 10,000 patients because only 32% of the 517 eligible patients were randomized between ABMT and ICC.
The median duration of neutropenia (<0.5 × 109/L) was 25 days after ABMT and 24 days after ICC ( P = .48). The median duration of thrombocytopenia (<30 × 109/L) was 109.5 days after ABMT (range, 10 to 2,000+ days) and 18.5 days after ICC (range, 1 to 50 days; P = .0001). The median duration of hospitalization was 39 days after ABMT (range, 15 to 99 days) and 32 days after ICC (range, 22 to 52 days; P = .006).
DISCUSSION
In this study, three intensive strategies were used as postremission therapy in patients with de novo AML up to 50 years of age: allogeneic BMT or a first course of ICC followed by either an unpurged ABMT or a second course of ICC. As in previous reports on intensive postremission therapy,23,35,36 the feasibility of our protocol was an important issue. Only 59.5% of the patients achieving a CR could actually undergo the assigned intensive treatment and only 164 of the 517 eligible patients (32%) could be randomized between ABMT and ICC. Some of the exclusions were explained by patients' or even physicians' psychologic reticences regarding autologous transplantation. This study was initiated in 1987 when autologous transplantation was not as widely offered as it is currently. However, the majority of withdrawals were due to complications of the first intensive consolidation (hematologic or extrahematologic toxicities, poor hematologic reconstitution precluding BM harvest with sufficient hematopoietic quality). The use of hematopoietic growth factors could partly resolve these problems by reducing the morbidity and mortality related to hematologic toxicity of intensive chemotherapy. Several controlled studies have shown that the use of granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) in AML is not deleterious.37 38
In our hands, allogeneic BMT was not superior to other types of intensive therapy, which contrasts with several published studies.23,36,39-41 The DFS rate after allogeneic BMT appeared to be somewhat inferior to those previously reported. Our results are not explained by a higher transplant-related mortality (22% toxic deaths) but mainly by an unexpected actuarial risk of relapse of 37% at 4 years. We fail to explain this unusual relapse rate, but the contrast between a relatively low toxic death rate and a high relapse rate reflects a major concern in allogeneic BMT. Attempts to reduce the toxicity, the incidence, and the severity of graft-versus-host disease have resulted in increased relapse rates.42 Conversely, attempts to enhance the antileukemic efficacy of preparative regimens have frequently induced higher toxicity.43 In our series, patients were generally transplanted early without having received ICC, which would have possibly reduced the relapse rate but induced a higher mortality rate after allogeneic BMT.44
The main objective of this study was to compare ABMT and ICC as postremission therapy in adult AML. Each of these two options was administered after a first course of ICC with HD-ARA-C plus an anthracycline. With this approach, we failed to show any significant superiority of ABMT. The 4-year DFS and OS were, respectively, 44% and 50% for ABMT and 40% and 54.5% for ICC. Although these results are comparable to those obtained in children by the Pediatric Oncology Group,45 they differ from other studies comparing chemotherapy and autologous transplantation as postremission therapy in adult AML.23,39,40 46 Those studies indeed showed at least a longer DFS for patients assigned to ABMT.
However, the design of three of them was different. In two series, autologous stem cell transplantation was compared with conventional dose chemotherapy.39,40 The large study conducted by the CALGB has shown that, for patients 60 years of age and younger, the probability of remaining alive in remission after 4 years is significantly higher with the HD-ARA-C than with low or intermediate doses of ARA-C.35 This study, which confirms the results of a previous small randomized trial,47 supports the concept of dose-response effect and indicates that ABMT should be compared with intensive chemotherapy rather than with standard-dose maintenance treatment. The British MRC also conducted a large randomized trial testing the impact of ABMT.46 However, in this study, autologous transplantation was performed after several courses of consolidation and was compared with no treatment.
The design of our study was more comparable to that of the EORTC/GIMEMA trial.23 In both studies, the randomization between ABMT and a second course of ICC took place after a first course of HD-ARA-C containing intensive consolidation. The EORTC/GIMEMA groups reported a 4-year DFS significantly better for ABMT (48%) as compared with ICC (30%). The principal difference between the two studies is the apparently better outcome after ICC in our study, which could be explained by several considerations.
Firstly, in our first course of intensive chemotherapy, ARA-C was administered at much higher doses (24 g total dose for all our patients, instead of 6 g for the majority of the patients in the EORTC/GIMEMA trial). The dose we used was in the range of the highest of the three dose levels used in the CALGB trial, whereas the dose of 6 g was rather in the range of the intermediate dose that yielded significantly lower results.35 It should be noted that, even with this high-dose regimen, the toxic death rate of this course was only 2.5% in our series.
Secondly, the anthracycline used both in induction and in the first consolidation were either IDR or RBZ instead of daunorubicin. There was no significant difference in DFS and OS between these two anthracycline derivatives. The efficacy of RBZ has never been compared with that of daunorubicin in a randomized study. However, several randomized studies show a superiority of IDR over daunorubicin at least in younger patients.48-50
Finally, the second course of ICC introduced two drugs, namely amsacrine and etoposide, not previously administered to patients. The antileukemic activity of these two drugs has been shown51-53 and, using sequentially a number of active drugs, could limit the risk of selection of resistant clones.
Our results with 2 courses of ICC are close to those published by the CALGB with 4 courses of HD-ARA-C.35 Therefore, we can assume that the chemotherapy arm of the EORTC/GIMEMA trial was not optimal. Our data suggest that, in terms of DFS, the benefit of unpurged ABMT as compared with the best available ICC is only marginal and that the demonstration of a statistical significance would need a very large study or a meta-analysis of randomized trials. Moreover, ABMT appears to be a valuable approach for some patients in first relapse or in second remission, with 30% to 50% relapse-free survival in series of selected patients.54,55 In terms of OS, ICC yields results as least equal to those achieved with ABMT, thanks to a better salvage of relapsed patients.23
Finally, in our study, the duration of hospitalization was significantly shorter after ICC. Most importantly, as the marrow was collected after the first course of ICC, in an attempt of in vivo purging, the duration of thrombocytopenia was much longer after ABMT than after ICC (median duration, 109.5 v 18.5 days). This slow hematopoietic recovery would be an even more critical issue if, as suggested by the results of the European survey,14 purged marrow was to be preferred over unpurged marrow. Rapid hematopoietic reconstitution is observed with autologous transplantation using G-CSF-mobilized peripheral blood stem cells.56 However, the impact of peripheral blood stem cell transplantation on the outcome of patients with AML is not yet known. There is currently no evidence that this source of hematopoietic progenitors is superior to BM in terms of DFS or OS.57 58
We conclude that ICC should be considered as the standard postremission therapy in adults with AML in first CR. Several issues regarding ICC should be addressed in future trials such as the optimal number of courses, the impact of more intensive induction treatments,59,60 or the role of multiple drug resistance reversal.61 Alternatively, new modalities of autologous stem cell transplantation should be compared with this simple and relatively safe postremission therapy.62
ACKNOWLEDGMENT
The authors are greatly indebted to Mariette Mercier, Valérie Polin, and Erard Gilles for help in statistical analysis and to Charlotte Avet Loiseau for editing the manuscript.
Supported by a grant from Programme Hospitalier de Recherche Clinique.
Presented in part at the 36th Annual Meeting of the American Society of Hematology, Nashville, TN, December 1994 and at the 32nd annual meeting of the American Society of Clinical Oncology, Philadelphia, PA, May 1996.
Address reprint requests to Jean-Luc Harousseau, MD, Department of Hematology, Hotel Dieu Hospital, 1, Place Alexis Ricordeau, 44035 Nantes, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal