Abstract
Bone marrow transplantation (BMT) can cure patients with high-risk or recurrent acute lymphoblastic leukemia (ALL). Those lacking a related donor can receive either autologous or histocompatible unrelated donor (URD) marrow. Autotransplantation may result in higher risk of relapse, whereas URD allografts, although associated with serious posttransplant toxicities, may reduce relapse risk. Six years (1987 to 1993) of consecutive autologous BMT (University of Minnesota, Dana Farber Cancer Institute; n = 214) were compared with URD transplants (National Marrow Donor Program; n = 337). Most transplants (70% autologous, 48% URD) were in early remission (first or second complete remission [CR1 or CR2]); 376 patients (75% autologous, 64% URD) were less than 18 years old. Autologous BMT led to significantly lower transplant-related mortality (TRM; relative risk [RR] 0.35; P = .001). URD transplantation offered greater protection against relapse (autologous RR 3.1; P = .001). Patients greater than 18 years old, women, and BMT recipients beyond CR2 had higher TRM, whereas adults, BMT recipients in CR2+, or BMT recipients during 1991 through 1993 had significantly more relapse. After 25 months median follow-up, 100 URD and 56 autologous recipients survive leukemia free. URD BMT in CR2 resulted in superior disease-free survival (DFS), especially for adult patients. Multivariate analysis showed superior DFS for children, men, and BMT during CR1 or 2. Autologous and URD BMT can extend survival for a minority of patients unlikely to be cured by chemotherapy, and the results with either technique are comparable. Greater toxicity and TRM after URD BMT are counterbalanced by better protection against relapse. Prospective studies addressing additional clinical variables are needed to guide clinical decision making about transplant choices for patients with ALL.
ADVANCES IN CHEMOTHERAPY have been able to cure the majority of children and many adults with acute lymphoblastic leukemia (ALL).1-5 For those who relapse or for certain high-risk subgroups, allogeneic related donor bone marrow transplantation (BMT) yields extended disease-free survival (DFS) for a large number of patients, both children and adults.6-12 For those lacking a histocompatible related donor, autologous marrow, collected during remission and cryopreserved, can be used for reconstitution after high-dose conditioning.13-22 More recently, by using the donor search and identification network of the National Marrow Donor Program (NMDP), phenotypically histocompatible unrelated donor marrow (URD) can be used for allogeneic BMT.23-25 Because excess toxicity (rejection and graft-versus-host disease [GVHD]) is expected after URD allotransplantation,26,27 but also reduced chances of posttransplant relapse because of the graft-versus-leukemia (GVL) effect,25 28 the comparative safety and ultimate efficacy of the two transplant techniques is uncertain. Therefore, we analyzed the detailed results of 6 years (1987 to 1993) of consecutive autologous transplantation from the University of Minnesota (UM) and the Dana Farber Cancer Institute (DFCI) compared with URD results over the same 6-year time period from the NMDP with particular attention to risks of transplant-related mortality (TRM), relapse, and DFS. The results of this comparative analysis may be used to facilitate clinical decision making about the relative efficacy of either transplant technique for patients with ALL.
MATERIALS AND METHODS
The results of 6 years of consecutive patients transplanted for ALL were compiled. Autologous BMT results were obtained from UM (n = 121) and the DFCI (n = 93) including prospectively collected records from all patients with ALL undergoing autotransplantation at the two institutions.15,18,19,21,29 30 These data were compared with the centralized records of the NMDP (n = 337), which contain demographic and outcome data for all patients receiving transplants facilitated by the NMDP. All patients transplanted between September 1, 1987 (the initiation of the NMDP) and August 31, 1993 were analyzed. All patients had been followed for a minimum of 18 months at the time the data set was closed (May 1995). Initial prospective data collection through the NMDP did not include determination of the date of achieving first remission, the diagnostic leukocyte count, or the site of relapse. Therefore, neither the duration of first complete remission (CR1), diagnostic leukocyte count, nor relapse site was available as a prognostic element for analysis. NMDP data were retrospectively audited on site (at the transplant centers) for completeness and accuracy, and the data files from the two autotransplant centers (UM and DFCI) were merged electronically and reverified for completeness and accuracy before analysis.
Statistical analysis was performed in the University of Minnesota Bone Marrow Transplant Database and Biostatistical Facility by using SAS (SAS Institute Inc, Cary, NC) software (version 6.09, 1992) running on Digital Equipment Corporation Vax VMS System (Maynard, MA). Differences between groups were assessed using χ2 analysis. Posttransplant outcomes (relapse, TRM, and DFS) were determined using Kaplan-Meier product limit estimates31 with 95% confidence limits derived from the standard errors. Estimates of outcome ± 95% confidence limits are shown at the last event. Comparisons between groups were performed by using the log-rank test. Multiple variable analyses were performed using the Cox model,32 stratified across remission groups as indicated. Recursive partitioning analysis33 was performed to determine the outcomes in successive cohorts divided by prognostic factors applied in order of statistical significance. Cox model analyses of each subset stratified across remission cohorts were used to assess the statistical significance of each partition.
Autologous transplants were performed as previously reported by using supportive care and conditioning regimens described from the two institutions15,18,19,21,29,30 and as shown in Table 1. The multicenter transplant techniques for the URD transplants included those in place at each transplant center, meeting the experience and quality standards of the NMDP.23 34 Fifty-one percent of URD recipients were phenotypically matched with their donors at human leukocyte antigen-A (HLA-A), HLA-B, and HLA-DR by serological techniques. No high resolution DNA sequence level matching was performed for these initial NMDP URD transplants. No center-specific analyses were performed in either the autologous or the URD cohort.
Patient Characteristics
| . | Autologous (%) . | Unrelated (%) . | P . |
|---|---|---|---|
| Total | 214 | 337 | |
| Sex | |||
| Male | 142 (66) | 203 (60) | .15 |
| Female | 72 (34) | 134 (40) | |
| Age | |||
| 0-18 yrs | 160 (75) | 216 (64) | .009 |
| 19+ yrs | 54 (25) | 121 (36) | |
| Remission No. | |||
| 1* | 51 (23.8) | 52 (15.6) | <.001 |
| [18% ≤ 18; 6% > 18] | [9% ≤ 18; 7% > 18] | ||
| 2 | 98 (45.8) | 106 (31.7) | |
| [35% ≤ 18; 11% > 18] | [24% ≤ 18; 7% > 18] | ||
| 3+ | 53 (24.8) [18% ≤ 18; 7% > 18] | 93 (27.8) [21% ≤ 18; 7% > 18] | |
| Relapse† | 12 (5.6) | 83 (24.9) | |
| [5% ≤ 18; 1% > 18] | [10% ≤ 18; 14% > 18] | ||
| Year of BMT | |||
| 1987-1990 | 142 (66.4) | 94 (27.9) | <.001 |
| 1991-1993 | 72 (33.6) | 243 (72.1) | |
| Conditioning Regimen‡ | |||
| Cy + TBI | 37 (17.3) | 125 (37) | <.001 |
| Cy + TBI + other drugs | 170 (79.4) | 183 (54) | |
| Chemotherapy without TBI | 7 (3.3) | 29 (9) |
| . | Autologous (%) . | Unrelated (%) . | P . |
|---|---|---|---|
| Total | 214 | 337 | |
| Sex | |||
| Male | 142 (66) | 203 (60) | .15 |
| Female | 72 (34) | 134 (40) | |
| Age | |||
| 0-18 yrs | 160 (75) | 216 (64) | .009 |
| 19+ yrs | 54 (25) | 121 (36) | |
| Remission No. | |||
| 1* | 51 (23.8) | 52 (15.6) | <.001 |
| [18% ≤ 18; 6% > 18] | [9% ≤ 18; 7% > 18] | ||
| 2 | 98 (45.8) | 106 (31.7) | |
| [35% ≤ 18; 11% > 18] | [24% ≤ 18; 7% > 18] | ||
| 3+ | 53 (24.8) [18% ≤ 18; 7% > 18] | 93 (27.8) [21% ≤ 18; 7% > 18] | |
| Relapse† | 12 (5.6) | 83 (24.9) | |
| [5% ≤ 18; 1% > 18] | [10% ≤ 18; 14% > 18] | ||
| Year of BMT | |||
| 1987-1990 | 142 (66.4) | 94 (27.9) | <.001 |
| 1991-1993 | 72 (33.6) | 243 (72.1) | |
| Conditioning Regimen‡ | |||
| Cy + TBI | 37 (17.3) | 125 (37) | <.001 |
| Cy + TBI + other drugs | 170 (79.4) | 183 (54) | |
| Chemotherapy without TBI | 7 (3.3) | 29 (9) |
Abbreviations: Cy, Cyclophosphamide; TBI, total body irradiation; BMT, bone marrow transplantation; URD, unrelated donor marrow.
Percent shown in brackets for children ≤18; adults >18.
Includes BMT in relapse and primary induction failure (autologous 0; URD 3).
χ 2 analysis; TBI v no TBI; P = .02.
RESULTS
Characteristics of the patients treated are shown in Table 1. Most patients were men. A greater proportion of autologous recipients were less than 18 years (P = .009). Autologous transplant recipients were more likely to be treated in earlier remissions (P < .001) but a large proportion in both groups were in CR1 or CR2 (69.6% autologous, 47.3% URD). Very few autologous but nearly a quarter of URD recipients were transplanted in relapse. In both groups over 90% of patients received total body irradiation before transplantation.
After a median 25 months of follow-up (range 2 to 72 months; autologous 2 to 72, median 34; URD 3 to 69, median 25), 177 of the 551 patients survive and 156 was free of leukemia (56 autologous and 100 URD). A total of 200 patients died without relapse (34 of 214 [15.9%] autologous and 168 of 337 [49.9%] URD). Posttransplant relapse occurred in 195 patients (126 [58.9%] autologous and 69 [20.5%] URD). Univariate Kaplan-Meier analyses of the risks of relapse, TRM, and DFS are shown (Table 2) for subsets of patients undergoing transplantation in different remission or relapse status. Posttransplant leukemia relapse was significantly more frequent (1.5-fold to 4.5-fold more) for those undergoing autologous transplantation in CR2, CR3, or in relapse. A similar, but not statistically significant difference was observed for transplantation in CR1 with (1.6-fold) more frequent relapse after autologous grafts (P = .12).
Outcomes After BMT for ALL: Univariate Analysis
| (n) . | Relapse . | Transplant-Related Mortality . | Disease-Free Survival . | |||
|---|---|---|---|---|---|---|
| . | (% ± 95% CI) . | P . | (% ± 95% CI) . | P . | (% ± 95% CI) . | P . |
| CR1 | ||||||
| Auto (51) | 53 ± 16% | .12 | 19 ± 15% | .0001 | 42 ± 15% | .03 |
| URD (52) | 33 ± 23% | 57 ± 14% | 32 ± 14% | |||
| CR2 | ||||||
| Auto (98) | 76 ± 10% | .0001 | 14 ± 9% | .0001 | 20 ± 9% | .02 |
| URD (106) | 17 ± 9% | 48 ± 12% | 42 ± 11% | |||
| CR3 | ||||||
| Auto (40) | 74 ± 20% | .05 | 21 ± 13% | .04 | 19 ± 16% | .85 |
| URD (79) | 47 ± 16% | 58 ± 13% | 23 ± 10% | |||
| CR4+ | ||||||
| Auto (13) | 60 ± 31% | .29 | 23 ± 23% | .02 | 31 ± 25% | .30 |
| URD (14) | 73 ± 45% | 10% | 0 | |||
| In relapse | ||||||
| Auto (12) | 90 ± 19% | .0001 | 27 ± 27% | .37 | 0 | .10 |
| URD (83) | 60 ± 16% | 63 ± 13% | 16 ± 8% | |||
| (n) . | Relapse . | Transplant-Related Mortality . | Disease-Free Survival . | |||
|---|---|---|---|---|---|---|
| . | (% ± 95% CI) . | P . | (% ± 95% CI) . | P . | (% ± 95% CI) . | P . |
| CR1 | ||||||
| Auto (51) | 53 ± 16% | .12 | 19 ± 15% | .0001 | 42 ± 15% | .03 |
| URD (52) | 33 ± 23% | 57 ± 14% | 32 ± 14% | |||
| CR2 | ||||||
| Auto (98) | 76 ± 10% | .0001 | 14 ± 9% | .0001 | 20 ± 9% | .02 |
| URD (106) | 17 ± 9% | 48 ± 12% | 42 ± 11% | |||
| CR3 | ||||||
| Auto (40) | 74 ± 20% | .05 | 21 ± 13% | .04 | 19 ± 16% | .85 |
| URD (79) | 47 ± 16% | 58 ± 13% | 23 ± 10% | |||
| CR4+ | ||||||
| Auto (13) | 60 ± 31% | .29 | 23 ± 23% | .02 | 31 ± 25% | .30 |
| URD (14) | 73 ± 45% | 10% | 0 | |||
| In relapse | ||||||
| Auto (12) | 90 ± 19% | .0001 | 27 ± 27% | .37 | 0 | .10 |
| URD (83) | 60 ± 16% | 63 ± 13% | 16 ± 8% | |||
Shown are Kaplan-Meier estimates ± 95% confidence limits within each cohort divided by remission number and type of transplant. P values represent log-rank univariate comparisons.
Abbreviations: BMT, bone marrow transplantation; ALL, acute lymphoblastic leukemia; CI, confidence interval; Auto, autologous; URD, unrelated donor marrow.
The greater toxicity of URD transplantation reflecting greater hazards of rejection and GVHD plus posttransplant immunodeficiency and opportunistic infection resulted in significantly more frequent TRM. As shown, TRM was significantly more frequent (2.8- to 4-fold greater) for URD recipients in any remission and a similar, but not significantly worse, outcome was observed for those receiving URD allotransplants while in relapse.
These discordant, unfavorable outcomes for the two transplant techniques yielded differing net results of DFS after transplantation (Table 2). For those transplanted in CR1, autologous transplantation was significantly superior with a 10% greater likelihood of posttransplant DFS (42% autologous transplants v 32% URD; P = .03). In contrast, for those transplanted in CR2 a 20% greater likelihood of DFS was observed in recipients of URD transplantation (20% autologous v 42% URD; P = .02), primarily reflecting the markedly increased relapse hazard for the autologous recipients in this group. There were no significantly different outcomes in DFS observed between autologous and URD transplant recipients treated in later remission or in relapse.
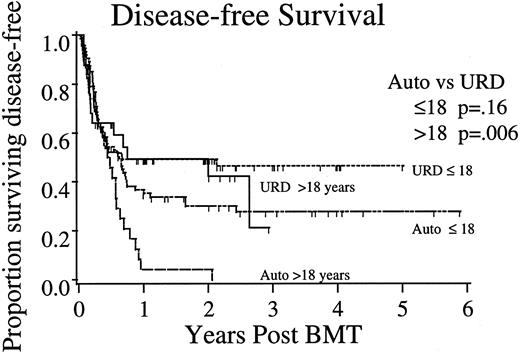
Importance of patient age. To further evaluate the impact of age on posttransplant outcome, the largest and most uniform cohort of patients, those undergoing BMT in CR2, were analyzed comparing the three posttransplant endpoints in the autologous and URD transplant subgroups; adults greater than 18 years of age compared with children 18 years or less (Fig 1). For patients transplanted during CR2, in children 18 years or less, DFS after URD (47% ± 12%; 95% confidence interval [CI]) was better but not statistically different from that observed after autologous (28% ± 14%) transplantation (P = .16). Females in this younger group had poorer outcome (stratified log rank analysis, P = .023). In adults, URD transplantation yielded significantly better DFS (autologous 0% v URD 42% ± 22%; P = .006), whereas sex had no additional impact on the outcome (P = .78).
DFS after transplantation for ALL in CR2. Shown are Kaplan-Meier projections of outcome for autologous and unrelated donor allogeneic marrow recipients divided by age <18 years. P values shown represent log-rank tests of significance between autologous and URD transplants within age strata.
DFS after transplantation for ALL in CR2. Shown are Kaplan-Meier projections of outcome for autologous and unrelated donor allogeneic marrow recipients divided by age <18 years. P values shown represent log-rank tests of significance between autologous and URD transplants within age strata.
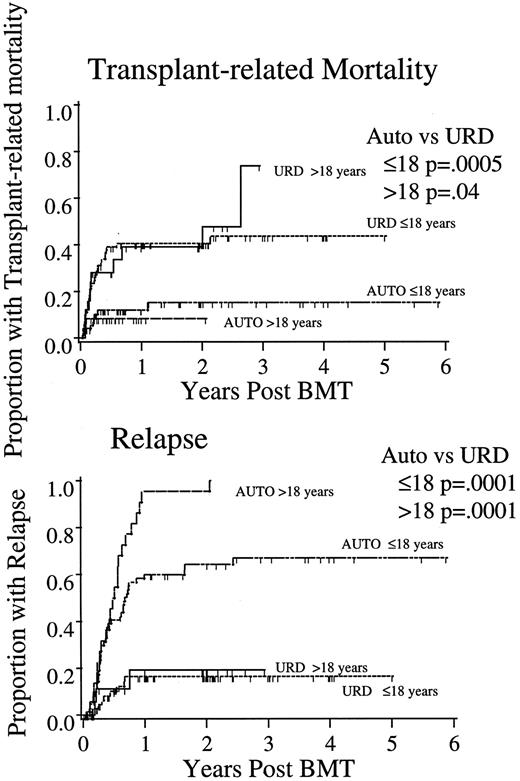
Consistent with the earlier analyses, URD transplantation led to greater TRM in both age groups (CR2 ≤ 18: autologous 15% ± 10%, URD 44% ± 12%; P = .0005; CR2 > 18: autologous 8% ± 11%, URD 48% ± 24%; P = .04; Fig 2). Sex had no impact on the risk of TRM in any subgroup (P > .5). The relapse risk was lower for all recipients of URD compared with autologous transplantation in CR2, both in children (autologous, 67% ± 13% v URD, 12% ± 9%; P = .0001; adults 100% v 19% ± 20%; P = .0001). Relapse was more common in women undergoing BMT in CR2 (P = .01), especially in girls.
TRM and relapse after autologous or URD BMT for ALL in CR2. P values as in Fig 1.
TRM and relapse after autologous or URD BMT for ALL in CR2. P values as in Fig 1.
The greater TRM after URD BMT represents a powerful competing hazard in comparative analyses of protection against relapse. To examine this GVL effect with less confounding by these competing hazards, we analyzed the risks of relapse in the subset of patients (CR1 and CR2) surviving relapse-free beyond day 100 after transplantation. In this cohort (100 URD [63% of the total] and 108 autologous [72%]), the risk of relapse beyond day 100 was still significantly less in recipients of URD BMT, both in children (13.6% ± 9.2% URD v 53.5% ± 12.1% autologous; P = .001) and in adults (26.1% ± 24.4% URD v 85.4% ± 16.8% autologous; P = .001).
Multivariate analysis. Recognizing previously reported prognostic factors expected to differentially alter posttransplant outcomes using these two techniques, we analyzed these same three endpoints with Cox model multivariable analysis considering patient age, patient gender, year of transplant, and differing remission status in the regression models. As shown (Table 3), autologous BMT was associated with a significantly higher risk of posttransplant relapse as was transplantation for adults over 18 years old and those transplanted during later remission or in relapse. The risk of relapse was greater in 1991 to 1993, as well. The risk of transplant-related mortality was independently and significantly higher in URD recipients, adults, females, and those transplanted at or beyond third remission or in relapse. Despite additional experience and advances in donor selection and patient management techniques, TRM was not significantly lower in the latter era, from 1991 to 1993.
Outcome After BMT: Multivariate Analysis
| A.Relapse (unfavorable factors) | |||
| RR of Relapse | 95% CI | P | |
| Autologous | 3.12 | 2.17-4.49 | .001 |
| Age > 18 yrs | 1.42 | 1.03-1.95 | .028 |
| Female | 1.29 | 0.95-1.74 | .096 |
| 1991-1993 | 1.46 | 1.06-2.01 | .019 |
| CR1 | 0.59 | 0.38-0.91 | .015 |
| CR2 | 1.0 | — | — |
| CR3+ | 1.46 | 1.02-2.09 | .034 |
| In relapse | 1.46 | 1.06-2.01 | .019 |
| B.Transplant-related mortality (unfavorable factors) | |||
| RR of death in remission | 95% CI | P | |
| URD | 2.83 | 1.88-4.27 | .001 |
| Age > 18 yrs | 1.72 | 1.27-2.33 | .001 |
| Female | 1.37 | 1.02-1.82 | .03 |
| 1987-1990 | 1.13 | 0.82-1.56 | .44 |
| CR1 | 1.21 | 0.78-1.87 | .38 |
| CR2 | 1.0 | — | — |
| CR3+ | 1.66 | 1.14-2.42 | .006 |
| In relapse | 1.6 | 1.06-2.41 | .022 |
| C.Disease-free survival (favorable factors) | |||
| RR of relapse or death | 95% CI | P | |
| URD | 0.91 | 0.71-1.15 | .42 |
| Age ≤ 18 | 0.63 | 0.51-0.78 | .001 |
| Male | 0.76 | 0.61-0.93 | .007 |
| 1987-1990 | 0.88 | 0.71-1.1 | .26 |
| CR1 | 0.87 | 0.64-1.18 | .36 |
| CR2 | 1.0 | — | — |
| CR3+ | 1.57 | 1.21-2.02 | .001 |
| In relapse | 1.74 | 1.29-2.36 | .001 |
| A.Relapse (unfavorable factors) | |||
| RR of Relapse | 95% CI | P | |
| Autologous | 3.12 | 2.17-4.49 | .001 |
| Age > 18 yrs | 1.42 | 1.03-1.95 | .028 |
| Female | 1.29 | 0.95-1.74 | .096 |
| 1991-1993 | 1.46 | 1.06-2.01 | .019 |
| CR1 | 0.59 | 0.38-0.91 | .015 |
| CR2 | 1.0 | — | — |
| CR3+ | 1.46 | 1.02-2.09 | .034 |
| In relapse | 1.46 | 1.06-2.01 | .019 |
| B.Transplant-related mortality (unfavorable factors) | |||
| RR of death in remission | 95% CI | P | |
| URD | 2.83 | 1.88-4.27 | .001 |
| Age > 18 yrs | 1.72 | 1.27-2.33 | .001 |
| Female | 1.37 | 1.02-1.82 | .03 |
| 1987-1990 | 1.13 | 0.82-1.56 | .44 |
| CR1 | 1.21 | 0.78-1.87 | .38 |
| CR2 | 1.0 | — | — |
| CR3+ | 1.66 | 1.14-2.42 | .006 |
| In relapse | 1.6 | 1.06-2.41 | .022 |
| C.Disease-free survival (favorable factors) | |||
| RR of relapse or death | 95% CI | P | |
| URD | 0.91 | 0.71-1.15 | .42 |
| Age ≤ 18 | 0.63 | 0.51-0.78 | .001 |
| Male | 0.76 | 0.61-0.93 | .007 |
| 1987-1990 | 0.88 | 0.71-1.1 | .26 |
| CR1 | 0.87 | 0.64-1.18 | .36 |
| CR2 | 1.0 | — | — |
| CR3+ | 1.57 | 1.21-2.02 | .001 |
| In relapse | 1.74 | 1.29-2.36 | .001 |
Shown are the unfavorable factors (for relapse and transplantrelated mortality) and the favorable factors (for disease-free survival) independently associated with each endpoint in Cox model multivariate analysis. The relative risk, 95% CI and significance probability for each factor considered is shown. For remission number, the relative risks are shown compared with the reference group transplanted during CR2. For year of transplant, the groups are divided 1987-1990; 1991-1993.
Abbreviations: BMT, bone marrow transplantation; RR, relative risk; CI, confidence interval; CR, complete remission; URD, unrelated donor marrow.
Multivariate analysis of DFS identified significantly lower risks of relapse or death and, thus, improved DFS for children 18 years or younger, men, and those patients undergoing transplantation in CR1 or CR2. URD transplantation was not associated with significantly improved DFS in this regression model (P = .42).
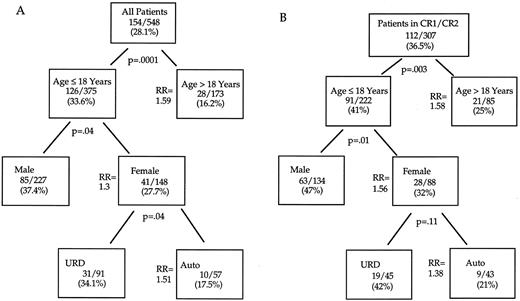
Recursive partitioning analysis. Complex interactions of these variables may complicate their application to clinical decision making. Multivariate regression models describe the independent strength of hazards, but do not quantify the proportion of patients achieving endpoints in cohorts with one or several identified risk factors. Therefore, we used recursive partition analysis to analyze the differential impact of these factors within significantly distinct clinical subgroups on the important endpoint of DFS in all patients (Fig 3A) or in those transplanted in CR1 or CR2 (Fig 3B). As shown, 28.1% of all patients survive disease free. Adults have significantly poorer outcomes (RR of relapse or death 1.59; P = .0001). Within the adult cohort, neither type of transplant (autologous v URD) nor gender could discriminate subgroups with significantly different risks of DFS posttransplant. Among transplant recipients 18 years old or younger, boys had significantly superior outcome with 37% surviving disease free compared with only 28% of girls. Within this cohort of male children undergoing transplantation, autologous and URD BMT yielded similar outcomes. In female children, URD transplant was associated with a significantly superior DFS compared with recipients of autografts (34% URD v 18% autologous; P = .04). No other factors could further discriminate differences in DFS within these varying clinical cohorts. Within the favorable group undergoing BMT in CR1 or CR2 (Fig 3B) an additional recursive partition analysis showed significantly better DFS in children and males. Similar to the findings observed in the entire patient group, in girls, a trend towards improved DFS after URD versus autologous BMT was also apparent (RR, 1.38; P = .11).
DFS after BMT: Recursive partitioning analysis. (A) All patients. (B) BMT in CR1 or CR2. Shown in each box are the number and crude percentage of patients surviving leukemia-free after BMT. The relative risks (of relapse or death) reflect Cox model tests of significance within the partition subgroup stratified over remission status. No additional factors identified subgroups with significantly different outcome after either autologous or URD allogeneic BMT.
DFS after BMT: Recursive partitioning analysis. (A) All patients. (B) BMT in CR1 or CR2. Shown in each box are the number and crude percentage of patients surviving leukemia-free after BMT. The relative risks (of relapse or death) reflect Cox model tests of significance within the partition subgroup stratified over remission status. No additional factors identified subgroups with significantly different outcome after either autologous or URD allogeneic BMT.
DISCUSSION
High-dose chemotherapy, usually with total body irradiation, followed by transplantation of either autologous or URD allogeneic marrow can cure a substantial, but still unsatisfactory fraction of children and adults with ALL. This analysis shows differential toxicities and outcomes of the two transplantation options when performed in different remission status and for different subgroups of patients. The consistently greater toxicity and TRM after URD transplantation is somewhat less severe in children, but is disturbingly high in both age groups. Thus, substantive improvements in prompt donor availability and in transplant technique are required before the greater antileukemia potential of URD transplantation can be exploited for larger numbers of patients with ALL. Phenotypically closer donor/recipient matching, currently using high resolution DNA sequence-based HLA typing, may reduce some posttransplant hazards of GVHD23 as well as persisting immunodeficiency and opportunistic infection.24,35 Additionally, newer posttransplant immunosuppressives36 or T lymphocyte depletion of the donor graft37 might reduce the TRM, although these improvements await testing in formal prospective and randomized comparative trials.
Alternatively, autologous transplantation has been reported to consolidate and extend remission for a fraction of ALL patients with only modest TRM.13-22 Previous reports have suggested that autotransplant results are superior for patients displaying clinically favorable characteristics of their underlying ALL. These include lower diagnostic leukocyte count, longer initial remission duration,13,18,19 and perhaps lesser residual leukemia burden as assayed by clonogenic leukemia precursor assays29 or molecular techniques. These predictive factors, referable to the original leukemia rather than to transplant technology, highlight the pitfalls of selection bias in analysis of either transplant technique. Additional modifications of autografting methods including in vitro purging or posttransplant immunotherapy may further improve on currently reported results.
In this multicenter analysis, we could not fully address all factors that may have influenced clinical decision making in allocation of patients to either autologous or URD BMT. The propensity for early relapse and short later remissions in ALL could lead some centers to offer early autotransplants to those highest-risk patients expected to have the briefest duration of remission and who would, therefore, be unable to tolerate the delay inherent in donor search and identification. Conversely, allografts with URD are only feasible for those patients who remain in remission long enough for a donor to be identified.38 This may reflect an inherent remission durability and, thus, leukemia sensitivity to treatment that might, in part, account for the superior protection against relapse observed after URD transplantation. The importance of first remission duration on outcome after URD transplantation has not yet been reported. Additional case matching for relevant clinical factors (duration of CR1, diagnostic leukocyte count, cytogenetic or immunophenotypic subsets, and site of relapse) may further refine subsequent comparative analyses of these two transplant techniques. Improvements in transplant outcome by modification of conditioning regimens, posttransplant immunologic or immunotoxin therapy for the autologous recipients, or better histocompatibility matching and GVHD prophylaxis for the URD recipients may advance outcomes in both cohorts.
Although the inherent delays in donor searching and limited availability of suitable histocompatible unrelated donors, especially for certain minority and ethnic subgroups, may make a formal randomized prospective trial of these two techniques infeasible for patients with ALL, careful clinical decision making must account for the differential hazards but also differential antileukemia potency of these two transplant therapies. Future work must better define these issues and hopefully improve outcomes to broaden the applicability of successful transplantation therapy for patients with ALL.
Supported in part by National Cancer Institute Grants No. CA21737, CA68484, and CA66996 and by the National Marrow Donor Program and the Baxter Corp.
Address reprint requests to Daniel J. Weisdorf, MD, Box 480, University of Minnesota Hospital, 420 Delaware St SE, Minneapolis, MN 55455.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal