Abstract
With a combined phase-contrast and fluorescence video imaging system, changes in morphology and cytosolic [Ca2+]i were investigated of fura-2–loaded platelets during adhesion to fibrinogen or collagen matrices. The Ca2+ signals were, on the level of single platelets, compared to the secretion and procoagulant responses, using fluorescent-labeled AK-6 antibody against P-selectin and labeled annexin V for detection of surface-exposed phosphatidylserine (PS), respectively. Platelets in contact with fibrinogen developed filapods and spread over the matrix, in most of the cells without detectable Ca2+ signal. Thrombin induced repetitive spiking in [Ca2+]i , followed by the expression of P-selectin but not of PS on the platelet surface. Platelet interaction with collagen resulted in spreading and transformation of the cells into blebbing, “balloon”-like structures (diameter about 5 μm). The latter morphological changes were accompanied by high and prolonged increases in [Ca2+]i , by the exposure of both P-selectin and PS, and by the ability of the platelets to convert prothrombin into thrombin. Thrombin addition accelerated the onset of the Ca2+ signals and the appearance of surface-exposed PS. Collagen-induced PS exposure was slightly reduced by treatment of the platelets with aspirin, and strongly inhibited by suppression of the Ca2+ responses with prostaglandin E1 or the Ca2+ chelator, dimethyl-BAPTA. Inhibition of protein tyrosine phosphorylation with genistein, U73343, or wortmannin resulted in spiking Ca2+ responses in many of the platelets and in almost complete reduction of bleb formation and PS exposure. In contrast, genistein did not suppress bleb formation and PS exposure of platelets stimulated with the Ca2+ ionophore A23187. We conclude that a collagen but not fibrinogen matrix acts as a potent activator of the procoagulant response through activation of tyrosine kinases and subsequent generation of sustained intracellular Ca2+ signals.
PLATELETS CONTROL the hemostatic process by a variety of mechanisms. After injury of a vessel wall, collagen fibers in the subendothelium become exposed to the blood stream and serve as initiation sites for platelet adhesion and activation. Aggregation of the adherent cells with other platelets through bridges of integrin αIIbβ3 receptors and fibrinogen leads to the formation of a hemostatic plug. In a final stage of activation, platelets expose negatively charged phosphatidylserine (PS) at their outer membrane surface and, thereby, transform into procoagulant cells. The surface-exposed PS serves as a catalytic site for the assembly of tenase, prothrombinase and other coagulation complexes, thus allowing formation of activated factor X, thrombin and other coagulation factors.1-3 This late, procoagulant response is often but not always associated with the shedding of microvesicles from the platelet plasma membrane.3-7
In platelets activated with agonists of G protein-coupled receptors, such as thrombin, platelet-activating factor, adenosine diphosphate (ADP) and thromboxane A2 , one of the early intracellular signals is an elevation in cytosolic [Ca2+]i , usually mediated by activation of phospholipase C-β and -γ isoforms. It is well-accepted that this [Ca2+]i increase is an important factor in the initiation and propagation of platelet responses like exocytosis of α and other secretory granules, generation of prostaglandins and thromboxanes, and formation of multiplatelet aggregates.8-10 Less well understood, however, is the role of elevated [Ca2+]i in late platelet reactions such as in the induction of the procoagulant response. It has been appreciated that PS exposure and microvesiculation are basically Ca2+-dependent events, since both are elicited by Ca2+-mobilizing agents such as the Ca2+ ionophores, ionomycin and A23187,2,11,12 the endomembrane Ca2+ATPase inhibitor, thapsigargin,7,13 and the complement membrane attack complex C5b-9.4,6 On the other hand, many phospholipase C-activating receptor agonists that induce a potent Ca2+ response are unable to elicit PS exposure or microvesiculation. Conversely, collagen is only a weak Ca2+ mobilizing agonist for suspended platelets, whose effect is greatly dependent on thromboxane A2 generation, whereas it does evoke a (small) procoagulant reaction even in the absence of thromboxane formation.2,11,12 Thus, others factors than only elevation in [Ca2+]i may control the platelet procoagulant and microvesiculation activities.13 Since collagen is the most important, physiological activator of the procoagulant response so far, the mechanism by which it acts is important to be clarified.
Only few studies have focused on the activation properties of platelets in direct contact with natural adhesive proteins. Platelets bound to fibrinogen or collagen undergo considerable morphological changes, develop filapods, and spread through their integrin receptors αIIbβ3 and α2β1 , respectively.14-17 These morphological changes are accompanied by extensive redistribution of the actin cytoskeleton,18,19 and lead to the creation of focal contact sites with the adhesive matrices.20 There is accumulating evidence that platelet spreading is associated with the activation of several protein tyrosine kinases, including p72syk and the focal adhesion kinase, p125FAK.14,15,21-25 However, reports are conflicting as to whether platelet binding to immobilized fibrinogen26-28 or collagen17,24 29-31 results in activation of phospholipase C. How the potential Ca2+ signals of these spreading platelets are related to activation of tyrosine kinases and downstream effects such as secretion and PS exposure, is still unknown.
The present study was undertaken to compare, on the level of single cells, the morphological changes of platelets adhering to fibrinogen or collagen matrices with the onset of specific activation processes. Using a microscope system equipped with two cameras to continuously record transillumination (absorption) as well as epi-illumination (fluorescence) images, we were able to monitor various types of responses during the course of the adhesion process. Phase-contrast absorption images reported on changes in platelet morphology, and sets of fluorescent images gave information on the handling of [Ca2+]i , the secretion process and the surface exposure of PS for individual platelets. Secretion of α-granules was detected by the accumulation on the platelet surface of fluorescent-labeled AK-6 antibody against P-selectin. Exposure of PS was measured with a fluorescent derivative of annexin V, ie, a Ca2+-dependent protein that specifically binds to PS-containing membranes.7,32 33 Our results show that platelets on fibrinogen extensively spread often without detectable Ca2+ or secretion signals, whereas platelets on collagen form previously unrecognized, blebbing structures and show potent Ca2+, secretion and procoagulant responses that are dependent on activation of tyrosine kinases. Thus, the adhesive proteins fibrinogen and collagen seem to have distinct functions in the hemostatic process, by providing a surface for maximal adhesion and by offering a structure where platelets easily transform into procoagulant structures, respectively.
MATERIALS AND METHODS
Platelet preparation and adhesion measurement.Blood was taken from healthy volunteers who had not taken medication during 2 weeks. Platelet-rich plasma (PRP) was prepared and incubated with fura-2 acetoxymethyl ester (3 μmol/L) in the presence of apyrase (0.1 U ADPase/mL) at 37°C, as described before.26 Lysine acetyl salicylate (aspirin, 100 μmol/L) and 5,5′-dimethyl BAPTA acetoxymethyl ester (100 μmol/L) were added to the PRP, where indicated. The platelets were washed twice, and suspended in HEPES buffer pH 7.4 composed of (in mmol/L): NaCl 136, glucose 5, Na-HEPES 10, KCl 2.7, MgCl2 2, 0.1% (wt/vol) bovine serum albumin and apyrase (0.2 U ADPase/mL).13 Suspensions were adjusted to 5 × 107 platelets/mL.
Round glass coverslips (diameter 22 mm) were cleaned and exposed to a solution of 10 mg/mL fibrinogen for 10 minutes, as described before.34 Otherwise, cleaned coverslips were sprayed with a solution of 100 μg/mL Horm collagen (Nycomed, Munich, Germany) using a 100-GXF air brush (Badger, Franklin Park, IL). Collagen-coated coverslips were allowed to dry on air before use.
The collagen- or fibrinogen-containing coverslips were mounted in open, round perfusion chambers (height 7.5 mm, inner diameter 19.5 mm), and repetitively rinsed with saline and once with HEPES buffer pH 7.4. The perfusion chamber was then filled with a suspension of fura-2–loaded platelets (300 μL, 1.5 × 107 cells) supplemented with 2 mmol/L CaCl2 and 0.50 μg/mL fluorescein isothiocyanate (FITC)-labeled annexin V.11,33 In some experiments, the annexin V was replaced by FITC-labeled AK-6 monoclonal antibody against P-selectin (Laboratory of Blood Transfusion Service, Amsterdam, The Netherlands) to monitor platelet secretion. Platelet agonists, obtained from sources as described elsewhere,26 34 were added during the fluorescence measurements in volumes of usually 300 μL HEPES buffer pH 7.4. A23187, genistein, prostaglandin E1 and wortmannin were purchased from Sigma (St Louis, MO), U73343 (1-[6-[[(17β)-3 - m e t h o x y e s t r a - 1 , 3 , 5 ( 1 0 ) - t r i e n - 1 7 - y l ;cb a m i n o ;cb h e x y l ;cb - 2 , 5 -pyrrolidinedione) was from Biomol (Plymouth Meeting, PA) and 5,5′-dimethyl BAPTA acetoxymethyl ester was obtained from Molecular Probes (Leiden, The Netherlands).
Fluorescence video imaging of single platelets.A coverslip-containing perfusion chamber was placed on an inverted microscope (Nikon diaphot 200; Nikon, Tokyo, Japan) and held at room temperature. Fluorescence from fura-2 and FITC-labeled annexin V or antibody was measured quasi-simultaneously with a combined fluorescence imaging and microphotometric system (FIMS) that was connected to the microscope and commanded by a UNIX/Quanticell-driven computer system (Applied Imaging, Sunderland, Tyne & Wear, UK). The light from a Xenon lamp (Nikon) passed a computer-controlled excitation and neutral density filter wheel, and reached the microscope through a UV-transparent liquid light guide and a dichroic long-pass filter. Samples were observed with a Nikon 40× phase-contrast quartz oil-immersion objective, except were indicated otherwise. The emission light passed a 1:1 beam splitter, a computer-controlled emission filter wheel, and finally, reached a low-light-level intensified, charge-coupled device camera working at standard video rate (Photonic Sciences, Robertsbridge, Sussex, UK). Fura-2 signals were detected with alternating 340- and 380-nm excitation filters (15-nm half-bandwiths), a 400-nm dichroic mirror, and a 510-nm band-pass emission filter (half-bandwith 40 nm); FITC fluorescence was observed with a 485-nm excitation filter (half-bandwith 22 nm), a 505-nm dichroic long-pass filter, and a 530-nm emission filter (half-bandwith 30 nm). With these optical conditions, there was no interference of the fura-2 and FITC-fluorescence signals. Four to eight fluorescence images were hardware-averaged and background-subtracted, ratio images were calculated in case of fura-2 fluorescence, and the results were stored to a 128 MByte real-time image processor using Quanticell software (Applied Imaging). Averaged, background-subtracted (ratio) images could thus be obtained real-time every 1 to 3 seconds. Geometric regions, matching individual cells, were off-line analyzed for changes in fluorescence, basically as described before.34
Experiments with FITC-labeled annexin V and AK-6 antibody were performed in the continuous presence of the fluorescent compound. Accumulation of fluorescence at the surface of individual platelets was followed in time using 0.2- or 0.4-neutral density (ND) gray filters and an optimal camera gain, at which background fluorescence was slighly above zero. At this setting, fluorescence of >99% of the nonadherent platelets was at background level. For measurements of fura-2 fluorescence, elevated levels of [Ca2+]i were expressed as increases in ratio of fluorescence at 340-nm/380-nm excitation, for reasons indicated previously.26
Phase-contrast video imaging of single platelets.At regular time intervals, fluorescence measurements of platelets on coverslip were alternated with phase-contrast recordings. Coverslips were then transilluminated with white light, and phase-contrast transmission images were recorded with a FTLC-450 charge-coupled device camera (Pulnix, Basingstoke, UK) that was connected to the second arm of the 1:1 beam splitter of the FIMS equipment. The camera signals were directly sent to a videotape recording system.
Measurement of prothrombinase activity.Coverslips coated with fibrinogen or collagen were incubated with platelet suspensions in HEPES buffer pH 7.45 supplemented with 3 mmol/L CaCl2 , 200 pmol/L factor Va, and 20 pmol/L factor Xa. Prothrombinase measurements were started by the addition of 200 nmol/L prothrombin, and thrombin activity was measured in incubation samples with chromogenic substrate, S2238.35
RESULTS
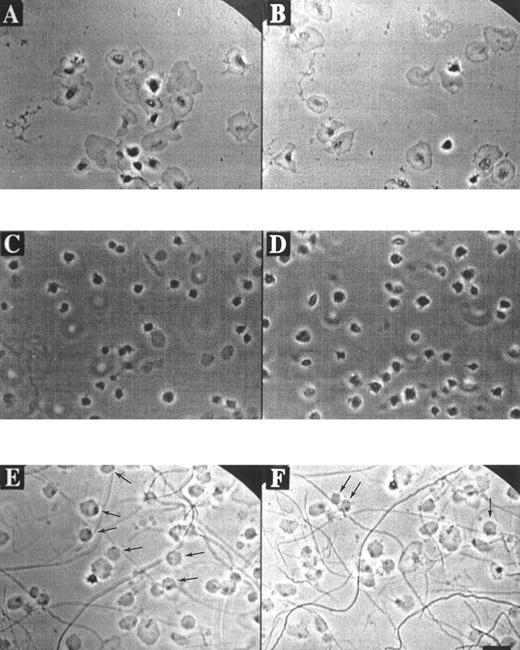
Morphological and Ca2+ responses of single platelets adhering to fibrinogen or collagen.Phase-contrast video imaging recordings indicated that fura-2–loaded platelets adhering to a fibrinogen matrix in the presence of ADP-degrading apyrase (0.2 U/mL), gradually developed filapods and broad lamellae. Spreading over the fibrinogen surface started after about 5 minutes of adhesion, and was completed after 20 minutes (Fig 1A and B). When this experiment was performed in the presence of apyrase and the integrin αIIbβ3 antagonist, H-Arg-Gly-Asp-Ser-OH (10 μmol/L), ie, conditions resembling those of earlier studies,26 34 platelets still bound to fibrinogen and developed filapods, but showed little signs of spreading (Fig 1C and D).
Morphology of platelets on fibrinogen or collagen matrices. Platelets in suspension were allowed to adhere to a coverslip coated with fibrinogen (A through D) or collagen (E and F ) in the presence of 2 mmol/L CaCl2 and 0.2 U/mL apyrase. Phase-contrast images were continuously recorded with a bright-field camera. Video prints are shown from various microscopic fields after 20 minutes of adhesion to immobilized fibrinogen in the absence (A and B) or presence (C and D) of 10 μmol/L H-Arg-Gly-Asp-Ser-OH. Video prints are also shown from two fields after 20 minutes of adhesion to collagen (E and F ). Note the rounded, balloon-like platelets with small dark spots, attached to the collagen fibers (E and F, arrows). Bar indicates 5 μm.
Morphology of platelets on fibrinogen or collagen matrices. Platelets in suspension were allowed to adhere to a coverslip coated with fibrinogen (A through D) or collagen (E and F ) in the presence of 2 mmol/L CaCl2 and 0.2 U/mL apyrase. Phase-contrast images were continuously recorded with a bright-field camera. Video prints are shown from various microscopic fields after 20 minutes of adhesion to immobilized fibrinogen in the absence (A and B) or presence (C and D) of 10 μmol/L H-Arg-Gly-Asp-Ser-OH. Video prints are also shown from two fields after 20 minutes of adhesion to collagen (E and F ). Note the rounded, balloon-like platelets with small dark spots, attached to the collagen fibers (E and F, arrows). Bar indicates 5 μm.
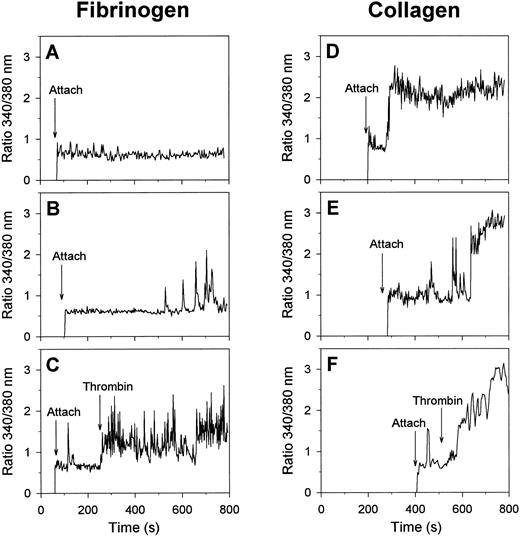
Visualization of changes in [Ca2+]i in single, fura-2 –loaded platelets was performed by recording of ratio images of fluorescence at 340- and 380-nm excitation. In the absence of H-Arg-Gly-Asp-Ser-OH, most of the platelets that were spreading on fibrinogen remained low in [Ca2+]i for at least 15 minutes (Fig 2A). A minority of about 10% of the cells on fibrinogen showed irregular spiking in [Ca2+]i after a lag-time as long as 433 ± 60 seconds (mean ± SEM, 38 platelets) (Fig 2B). When α-thrombin was added, most of the spreading platelets responded by immediate trains of spikes (Fig 2C), closely resembling the spikes with thrombin earlier observed with unspread platelets.26 These results suggest that platelet spreading on fibrinogen, mediated by the αIIbβ3 integrins, can proceed independently of [Ca2+]i increases and without altered responsiveness of the cells to thrombin.
Calcium responses of single platelets evoked by adhesion to fibrinogen or collagen matrices. Fura-2–labeled platelets were allowed to adhere to fibrinogen (A through C) or collagen (D through F ) surfaces in the presence of 2 mmol/L CaCl2 and 0.2 U/mL apyrase; 2 nmol/L α-thrombin was given during the experiments where indicated. Timepoints of attachment are marked by arrows. Representative traces of individual platelets are given from at least 10 independent experiments.
Calcium responses of single platelets evoked by adhesion to fibrinogen or collagen matrices. Fura-2–labeled platelets were allowed to adhere to fibrinogen (A through C) or collagen (D through F ) surfaces in the presence of 2 mmol/L CaCl2 and 0.2 U/mL apyrase; 2 nmol/L α-thrombin was given during the experiments where indicated. Timepoints of attachment are marked by arrows. Representative traces of individual platelets are given from at least 10 independent experiments.
Strikingly different morphological and Ca2+ responses were observed when the platelets came into contact with collagen matrices. The adhesion to collagen resulted in a short phase of filapod formation, lasting for about 5 minutes, after which the partially spread platelets rounded off and transformed into blebbing, balloon-like cells with a diameter of about 5 μm (Fig 1E and F ). Bleb formation was most frequently seen with platelets that were bound to the visible collagen fibers. Continuous phase-contrast recordings showed that almost all balloon-shaped platelets contained small round structures that appeared to rotate along the surface membrane. After this transformation, the blebbing cells gradually detached from the collagen matrix, and finally lost all contact with it. Similar balloon-like structures were observed, when nonadherent platelets were activated with the Ca2+ ionophores A23187 or ionomycin in the presence of extracellular CaCl2 (data not shown).
Although the moment of contact of a platelet with collagen was not accompanied by a Ca2+ signal, most of the platelets responded later by a sudden increase in [Ca2+]i with a lag-time of 160 ± 15 seconds (mean ± SEM, 54 cells), ie, before the formation of blebs. The Ca2+ response of 79% of the platelets consisted of a high and prolonged [Ca2+]i increase (Fig 2D), that was sometimes preceded by a short train of [Ca2+]i spikes (Fig 2E). In the remaining 21% of the cells, the Ca2+ response was spiking or oscillating in shape (total of 61 analyzed cells). When α-thrombin was added, those collagen-bound platelets that were still low in [Ca2+]i responded by a potent Ca2+ signal (Fig 2F ), whereas platelets with elevated [Ca2+]i did not respond (data not shown). Apparently, thrombin mainly accelerated the onset of the Ca2+ signals in platelets that had not yet undergone a Ca2+ response.
Exposure of P-selectin and PS on the surface of fibrinogen- and collagen-bound platelets.Because the secretion of storage granules is a well-described Ca2+-dependent response,8 9 we followed this process in the adhering platelets by recording the surface appearance of granular P-selectin using a FITC-labeled monoconal antibody, AK-6. Platelets that were spreading on fibrinogen in the presence of this antibody displayed levels of FITC fluorescence that were close to that of the background. In many cells, stimulation with α-thrombin resulted in a rapid, fourfold to sixfold increase in FITC fluorescence (Fig 3A), indicative of thrombin-evoked P-selectin expression. Platelets that were adhering to collagen, however, started to accumulate FITC AK-6 fluorescence even in the absence of coagonist after a delay time of about 3 minutes (Fig 3B).
Exposure of P-selectin on the surface of single platelets during adhesion to fibrinogen or collagen matrices. Fura-2–loaded platelets were allowed to adhere to immobilized fibrinogen (A) or collagen (B) in the presence of 2 mmol/L CaCl2 and FITC-labeled AK-6 antibody against P-selectin. Arrows indicate timepoint of addition of 2 nmol/L thrombin or timepoint of attachment to collagen. Fluorescence images were taken during 5 to 10 minutes, and traces represent changes in FITC fluorescence of individual platelets. These platelets were high in [Ca2+]i at the end of the FITC fluorescence recordings. Traces are representative of >35 platelets out of three independent experiments.
Exposure of P-selectin on the surface of single platelets during adhesion to fibrinogen or collagen matrices. Fura-2–loaded platelets were allowed to adhere to immobilized fibrinogen (A) or collagen (B) in the presence of 2 mmol/L CaCl2 and FITC-labeled AK-6 antibody against P-selectin. Arrows indicate timepoint of addition of 2 nmol/L thrombin or timepoint of attachment to collagen. Fluorescence images were taken during 5 to 10 minutes, and traces represent changes in FITC fluorescence of individual platelets. These platelets were high in [Ca2+]i at the end of the FITC fluorescence recordings. Traces are representative of >35 platelets out of three independent experiments.
Annexin V has widely been used to monitor the procoagulant response, because of its capability to selectively bind to PS exposed at the surface of procoagulant platelets.11,12,32,33 We performed series of dual probe measurements, using fura-2-loaded platelets and FITC-labeled annexin V, to compare the intracellular Ca2+ signals with the surface appearance of PS. Firstly, sets of images were recorded of fura-2 fluorescence ratio and FITC fluorescence from a single microscopic field. Pseudo-colored fura-2 images showed that many of the platelets that had spread on fibrinogen were low in [Ca2+]i (Fig 4A, ie, the cells represented by a dark-blue color). These platelets did not bind FITC-labeled annexin V, but became FITC positive after addition of the Ca2+ mobilizers, ionomycin (Fig 4B) or A23187 (not shown). This result is in agreement with the high annexin V binding affinity of Ca2+ ionophore-stimulated platelets in suspension.7,11 12 The potent [Ca2+]i -elevating effect of thrombin on most of the fibrinogen-bound platelets (Fig 4C) was only occasionally accompanied by FITC-annexin V binding (Fig 4D). Platelets that were in contact with collagen behaved differently, in that many developed FITC-annexin V binding sites and that almost all of the annexin V-positive cells had elevated, suprabasal levels of [Ca2+]i (Fig 4E and F, ie, cells not represented by a dark-blue color in F ).
Pseudo-color fluorescence video images of fura-2–loaded platelets adhering to fibrinogen or collagen matrices in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V. (A) Fura-2 ratio image of platelets adhering to fibrinogen during 20 minutes. (B) FITC-annexin V intensity image of platelets adhering to fibrinogen during 20 minutes and subsequent stimulation with 10 μmol/L ionomycin. (C and D) Corresponding fura-2 ratio image (C) and FITC-annexin V intensity image (D) from one microscopic field of platelets adhering to fibrinogen during 20 minutes in the presence of 2 nmol/L α-thrombin. (E and F ) Corresponding fura-2 ratio image (E) and FITC-annexin V intensity image (F ) from one microscopic field with platelets adhering to collagen during 20 minutes. The false-color rainbow of fura-2 fluorescence ratio was set such that dark blue indicates basal levels of [Ca2+]i and that color changes from light blue, green, yellow to red point to increasing levels of [Ca2+]i . Color changes from blue to red also indicate increasing intensities of FITC fluorescence.
Pseudo-color fluorescence video images of fura-2–loaded platelets adhering to fibrinogen or collagen matrices in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V. (A) Fura-2 ratio image of platelets adhering to fibrinogen during 20 minutes. (B) FITC-annexin V intensity image of platelets adhering to fibrinogen during 20 minutes and subsequent stimulation with 10 μmol/L ionomycin. (C and D) Corresponding fura-2 ratio image (C) and FITC-annexin V intensity image (D) from one microscopic field of platelets adhering to fibrinogen during 20 minutes in the presence of 2 nmol/L α-thrombin. (E and F ) Corresponding fura-2 ratio image (E) and FITC-annexin V intensity image (F ) from one microscopic field with platelets adhering to collagen during 20 minutes. The false-color rainbow of fura-2 fluorescence ratio was set such that dark blue indicates basal levels of [Ca2+]i and that color changes from light blue, green, yellow to red point to increasing levels of [Ca2+]i . Color changes from blue to red also indicate increasing intensities of FITC fluorescence.
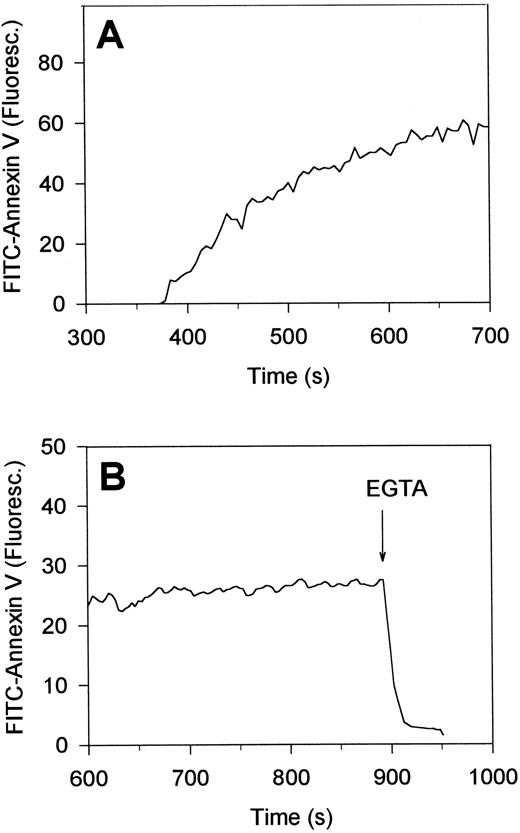
Time-dependent measurements showed that platelets in contact with collagen for about 5 minutes, ie, about 2 minutes after the first [Ca2+]i increase, started to assemble FITC-annexin V. FITC-fluorescence levels per platelet increased 25- to 50-fold with a t1/2 of 70 ± 20 seconds (mean ± SEM, 22 cells) (Fig 5A). As required, this accumulation of annexin V was Ca2+-dependent, because the addition of a surplus of EGTA resulted in complete loss of fluorescence within 10 seconds (Fig 5B). Taken together, these annexin V binding studies suggest that platelet interaction with collagen, but not with fibrinogen, leads to PS exposure, and thus, formation of procoagulant sites on the platelet surface. This was confirmed by incubation studies with factors Va and Xa, CaCl2 and prothrombin, indicating that the collagen-bound platelets were indeed capable of stimulating thrombin formation (data not shown).
Reversibility of the accumulation of FITC-annexin V on the surface of single, collagen-bound platelets. Platelets were allowed to adhere to immobilized collagen in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V (A). After 15 minutes of adhesion, 10 mmol/L EGTA was added to displace annexin V from the platelet surface (B). Changes in FITC-fluorescence intensity of single platelets were recorded by video fluorescence imaging techniques. Time settings are relative to the timepoints of interaction of the platelets with collagen.
Reversibility of the accumulation of FITC-annexin V on the surface of single, collagen-bound platelets. Platelets were allowed to adhere to immobilized collagen in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V (A). After 15 minutes of adhesion, 10 mmol/L EGTA was added to displace annexin V from the platelet surface (B). Changes in FITC-fluorescence intensity of single platelets were recorded by video fluorescence imaging techniques. Time settings are relative to the timepoints of interaction of the platelets with collagen.
As another way of comparing changes in fura-2 and FITC-annexin V fluorescence, the fractions of platelets were counted in one microscopic field that were high in [Ca2+]i and bound to FITC-annexin V. After 20 minutes of adhesion to fibrinogen and activation with thrombin, 88% of the platelets were elevated in [Ca2+]i , while less than 3% of these platelets had accumulated FITC-annexin V (Table 1). Of the platelets in contact with collagen, 57% was high in [Ca2+]i and a similar percentage was FITC-annexin V positive. These percentages increased to 76% to 80% when thrombin was added to the cells on collagen. Glass artifacts could be excluded, because platelet contact with uncovered glass coverslips did not result in appreciable Ca2+ responses or annexin V binding within this time interval (Table 1).
Fractions of Ca2+-Responsive and Annexin V-Binding Platelets After Adhesion to Fibrinogen or Collagen Matrices
| . | . | . | Increase in . | Binding of . |
|---|---|---|---|---|
| . | . | . | [Ca2+]i . | Annexin V . |
| Coverslip . | Thrombin . | No. of . | . | |
| . | . | . | . | . |
| Coating . | (nmol/L) . | Analyzed Cells . | (% of analyzed cells) . | |
| . | . | . | . | . |
| Fibrinogen | 0 | 310 | 11.9 | 1.6 |
| 2 | 290 | 87.6 | 2.8 | |
| Collagen | 0 | 410 | 56.8 | 57.8 |
| 2 | 120 | 75.8 | 80.0 | |
| None* | 0 | 125 | 6.9 | 2.6 |
| . | . | . | Increase in . | Binding of . |
|---|---|---|---|---|
| . | . | . | [Ca2+]i . | Annexin V . |
| Coverslip . | Thrombin . | No. of . | . | |
| . | . | . | . | . |
| Coating . | (nmol/L) . | Analyzed Cells . | (% of analyzed cells) . | |
| . | . | . | . | . |
| Fibrinogen | 0 | 310 | 11.9 | 1.6 |
| 2 | 290 | 87.6 | 2.8 | |
| Collagen | 0 | 410 | 56.8 | 57.8 |
| 2 | 120 | 75.8 | 80.0 | |
| None* | 0 | 125 | 6.9 | 2.6 |
Fura-2–loaded platelets were allowed to adhere to fibrinogen-or collagen-coated surfaces in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V during 20 minutes. Thrombin was added after 5 minutes of incubation where indicated. Images of fura-2 fluorescence ratio and FITC-fluorescence intensity were then recorded from the same microscopic field, and percentages of platelets with elevated [Ca2+]i were determined as well as the percentages of the FITC-positive cells. Cumulated data are given from 3 to 4 independent experiments.
Coverslips were prewashed with HEPES buffer pH 7.4 containing 0.1% (wt/vol) bovine serum albumin.
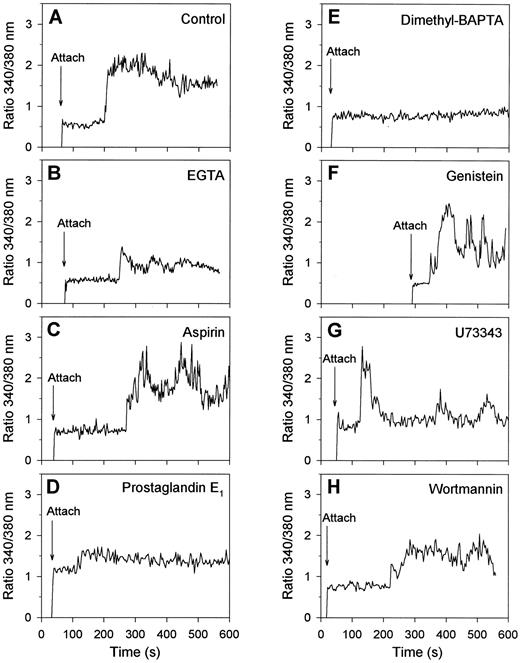
Regulation of collagen-induced exposure of PS by protein tyrosine kinases and [Ca2+]i . Having assigned that many of the collagen-bound platelets with elevated [Ca2+]i expose PS and become procoagulant, we aimed to get more insight into the regulation of this procoagulant response. Therefore, fura-2–loaded platelets were treated with a variety of inhibiting compounds and the effects were measured on [Ca2+]i transients and FITC-annexin V binding. When extracellular CaCl2 was absent and replaced by EGTA, platelets spread normally on collagen, but showed a low-amplitude, usually spiking Ca2+ signal with a lag time of about 2 minutes (Fig 6A and B). These platelets did not form blebs (data not shown). Treatment of the platelets with aspirin to inhibit thromboxane-dependent events resulted in a somewhat delayed Ca2+ response, which in 50% of the cells was continuously high and in the remaining 50% was low or spiking (Fig 6C). However, aspirin treatment resulted in only a small reduction of the fraction of platelets that had elevated [Ca2+]i and bound FITC-annexin V after 15 minutes of adhesion (Fig 7). Downregulation of the platelet Ca2+ responses by treatment with cyclic AMP-elevating prostaglandin E1 (Fig 6D) or with the intracellular Ca2+ chelator, dimethyl BAPTA (Fig 7E), resulted, as predicted,4 in greatly decreased Ca2+ signals, but also in a considerable reduction in the number of FITC-annexin V-positive cells (Fig 7). Bleb formation was not observed in these platelets.
Effects of activation inhibitors on Ca2+ responses of single platelets bound to collagen. Fura-2–loaded platelets were treated with the indicated drugs and allowed to adhere to collagen in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V during 15 minutes. Changes in fura-2 fluorescence ratio and FITC-annexin V fluorescence intensity were recorded from one microscopic field as described in Materials and Methods. Shown are typical Ca2+ response patterns of single platelets, representative of 36 to 76 cells, for the following conditions: control (A); CaCl2 replaced by 1 mmol/L EGTA (B); platelets pretreated with 100 μmol/L aspirin (C) or dimethyl BAPTA (E) while loading with fura-2; platelets incubated with 5 μmol/L prostaglandin E1 for 5 minutes (D), with 100 μmol/L genistein for 30 minutes (F ), with 20 μmol/L U73343 for 5 minutes (G), or with 10 μmol/L wortmannin for 5 minutes (H).
Effects of activation inhibitors on Ca2+ responses of single platelets bound to collagen. Fura-2–loaded platelets were treated with the indicated drugs and allowed to adhere to collagen in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V during 15 minutes. Changes in fura-2 fluorescence ratio and FITC-annexin V fluorescence intensity were recorded from one microscopic field as described in Materials and Methods. Shown are typical Ca2+ response patterns of single platelets, representative of 36 to 76 cells, for the following conditions: control (A); CaCl2 replaced by 1 mmol/L EGTA (B); platelets pretreated with 100 μmol/L aspirin (C) or dimethyl BAPTA (E) while loading with fura-2; platelets incubated with 5 μmol/L prostaglandin E1 for 5 minutes (D), with 100 μmol/L genistein for 30 minutes (F ), with 20 μmol/L U73343 for 5 minutes (G), or with 10 μmol/L wortmannin for 5 minutes (H).
Effects of activation inhibitors on Ca2+ responses and annexin V binding capacity of platelets adhering to collagen. Fura-2–loaded platelets were treated with various drugs and allowed to adhere to collagen in the presence of 2 mmol/L CaCl2 and FITC-annexin V, exactly as described for Fig 6. Given are the percentages of platelets that were elevated in [Ca2+]i (A), and stained positively with FITC-annexin V (B) after 15 minutes of adhesion. Data are averaged values ± SEM from three to five independent experiments (350 to 600 cells).
Effects of activation inhibitors on Ca2+ responses and annexin V binding capacity of platelets adhering to collagen. Fura-2–loaded platelets were treated with various drugs and allowed to adhere to collagen in the presence of 2 mmol/L CaCl2 and FITC-annexin V, exactly as described for Fig 6. Given are the percentages of platelets that were elevated in [Ca2+]i (A), and stained positively with FITC-annexin V (B) after 15 minutes of adhesion. Data are averaged values ± SEM from three to five independent experiments (350 to 600 cells).
For studies of the possible involvement of protein tyrosine kinases, many of the commonly used inhibitors were unsuitable because of their high fluorescence. We instead used three nonfluorescent compounds that, in earlier experiments with platelet suspensions, were shown to abolish completely agonist-evoked protein tyrosine phosphorylation: genistein (100 μmol/L), a common tyrosine kinase inhibitor36; U73343 (20 μmol/L), which blocks activation of tyrosine kinases upstream of cytosolic phospholipase A2 activation35; and wortmannin, an inhibitor of phosphoinositide 3-kinase at lower concentrations but also acting as tyrosine kinase inhibitor in the micromolar range (10 μmol/L).37 Treatment with these compounds did not influence the initial adhesion of platelets to collagen, in agreement with published data.17 However, genistein, U73343 and wortmannin reduced the number of platelets showing a continuously high Ca2+ signal, and increased the number with a spiking or low Ca2+ response to 61%, 89%, and 70%, respectively (30 to 65 analyzed platelets, Fig 6F through H shows representative traces). After 15 minutes of adhesion, the number of Ca2+-elevated cells was not significantly influenced by genistein treatment, but this was more than halved with U73343 or wortmannin. Nevertheless, all three compounds greatly reduced the fraction of platelets that bound FITC-annexin V (Fig 7) and transformed into balloon-like cells (data not shown).
To determine whether tyrosine kinases might be involved in regulation of PS exposure independently of their effects on Ca2+ signaling, we studied whether genistein could influence FITC-annexin V binding of platelets on fibrinogen that were stimulated with a submaximal dose of A23187 (1 μmol/L). However, treatment with genistein (100 μmol/L) reduced the fraction of annexin V-positive platelets insignificantly from 22.0% ± 5.6% to 17.8% ± 3.5% (mean ± SEM, 4 experiments). Similarly, genistein treatment did not suppress A23187-induced bleb formation of platelets (data not shown).
DISCUSSION
Ca2+ responses following platelet interaction with fibrinogen or collagen matrices.This study establishes that initial adhesion of individual, fura-2–loaded platelets with immobilized fibrinogen or collagen does not result in elevation in [Ca2+]i . In addition, filapod formation and spreading of platelets on fibrinogen can successfully proceed without detectable [Ca2+]i increase, provided that ADPase-degrading apyrase is present. In cases where adhering platelets do show a Ca2+ signal, ie, in a minority of the platelets on fibrinogen and in most of the cells on collagen, these typically occur after a delay time of several minutes (Fig 2). Knowing that the first interaction of platelets with fibrinogen9,23,38 and collagen,14,17,39 is mediated by αIIbβ3 and α2β1 integrins, respectively, these results thus suggest that occupation of these integrin receptors does not directly stimulate phospholipase C. This strongly contrasts to the immediate Ca2+ signals that are elicited in single platelets by typical, phospholipase C-linked agonists like thrombin.19,26,34 These data are compatible with the findings from others that platelets loaded with intracellular Ca2+-chelator18,28 or preincubated with [Ca2+]i -reducing prostaglandin E138 can form lamellar extensions and spread on a fibrinogen matrix. They also add to the conclusion of Haimovich et al20,25 that ADP secreted by platelets on fibrinogen contributes to the Ca2+/protein kinase C-dependent phosphorylation on tyrosine of p125FAK. Stimulatory effects of released ADP may thus explain why spreading on fibrinogen or collagen is only partially correlated with elevation in [Ca2+]i .17 28
The procoagulant response after platelet interaction with collagen or fibrinogen matrices.The procoagulant response of platelets comprises a set of separate but often associated events. One of these is the collapse of phospholipid asymmetry of the outer membrane, putatively due to activation of a Ca2+-dependent phospholipid scramblase and concomitant inhibition of an aminophospholipid translocase in the plasma membrane. This results in the appearance of procoagulant PS at the platelet surface, allowing the activation of prothrombinase and other coagulation complexes.1-3,40 In addition, the plasma membrane fuses with the intracellular membranes, forms blebs and sheds microvesicles,4,12,13 which processes may or may not involve calpain- (and thus Ca2+-) mediated proteolysis of cytoskeletal proteins.5-7 Earlier experiments with platelet suspensions have led to the conclusion that collagen, even in the presence of stimulatory thrombin, is only a weak activator of the procoagulant response when compared to Ca2+ ionophores.2,3 11 In the present study, where we focused on platelets in direct contact with collagen, it was established that adhesion to collagen is strongly stimulatory for all elements of the procoagulant response. Thus, binding of many of the platelets to collagen fibers results in: (1) accumulation of fluorescent anti-P-selectin antibody as well as annexin V (Figs 3 and 4), indicative for exocytosis and PS exposure, respectively; (2) capacity of the platelets to catalyze the conversion of prothrombin into thrombin by reconstituted prothrombinase; (3) changes in morphology to blebbing, balloon-like cells (Fig 1). In contrast, adhesion to fibrinogen results in spread platelets, which only after activation by thrombin bind anti-P-selectin, and need activation by Ca2+ ionophores to bind annexin V and form blebbing structures. Accordingly, the earlier suggested weak procoagulant effect of collagen in suspended platelets can now be interpreted as a potent effect on those few platelets that are in direct contact with collagen fibers, and little contribution of the cells that are not bound to collagen.
It should be emphasized that to recognize the blebbing, balloon-like platelets (diameter about 5 μm), we needed to collect phase-contrast images at high magnification, preferably by continuous video recording. The blebbing cells were best characterized by the presence of dark structures (which may or may not be microvesicles) moving along the surface of the balloons. These structures were observed in platelets attached to collagen, but also in Ca2+ ionophore-activated platelets. Platelets of similar morphology were found after the perfusion of PRP over fibrinogen-coated coverslips.41 Typically, the blebbing cells were often loosely attached to the immobilizing surface. Although rounded, blebbing cells were not recognized on scanning electron micrographs of platelets in contact with collagen, putatively because of their disappearance during sample preparation, these balloon-shaped platelets (diameter 5 to 10 μm) were identified by three-dimensional scans of platelets labeled with FITC-annexin V using confocal fluorescence microscopy (J. Briedé and T. Lindhout, unpublished results, March 1997). Obviously, the resolution of light microscopy is too low to identify microvesicles of <0.1 μm. However, we speculate that the blebbing platelets provide the sources of microvesicles that have previously been detected in suspensions of collagen- or A23187-stimulated platelets using flow cytometry.2-7 11
Regulation of the collagen-evoked procoagulant response by [Ca2+]i and protein tyrosine kinases.Virtually all collagen-bound platelets that exposed PS appeared to be increased in [Ca2+]i (Fig 4E and F and Table 1). After the conclusion from others concerning A23187- or thapsigargin-activated platelets,3,5-7,11-13 42 this suggests that the collagen-evoked Ca2+ response is an important factor in the induction of PS exposure. This was confirmed by the observation that 79% of the platelets in contact with collagen showed high and prolonged increases in [Ca2+]i (Fig 2D through F ). In contrast, the thrombin-evoked Ca2+ response of platelets on fibrinogen usually consisted of trains of [Ca2+]i spikes (Fig 2C), which in these cells were not accompanied by PS exposure (Fig 4C). Further evidence was obtained by treatment of platelets with various [Ca2+]i -reducing agents, such as EGTA (abolishing influx of external Ca2+), prostaglandin E1 (increasing intracellular cyclic AMP), or dimethyl BAPTA (chelating cytosolic Ca2+). These treatments greatly reduce the Ca2+ signals (Fig 6), and abolish bleb formation and PS exposure (Fig 7). Surprisingly, endogenous thromboxane formation contributes only little to the collagen-evoked Ca2+ and procoagulant response, since treatment of the platelets with aspirin inhibited these reactions only partially (Fig 7).
We used three nonfluorescent inhibitors of tyrosine kinases, genistein, U73343 and wortmannin, to determine the involvement of tyrosine phosphorylation in the platelet responses evoked by collagen adhesion. In the presence of these inhibitors, in 61%, 89%, and 70% of the platelets, the prolongedly high Ca2+ signals were reduced to spiking or low increases in [Ca2+]i (see Fig 6), in agreement with earlier reported effects of genistein.31 After 15 minutes of adhesion, U73343 and wortmannin had a larger effect on the fractions of platelets with elevated [Ca2+]i than genistein, whereas all three compounds reduced PS exposure to the same, low level (Fig 7). Thus, tyrosine kinases appear to control both the shape of the [Ca2+]i transients and the procoagulant response. On the other hand, no significant effect of genistein treatment could be measured on PS exposure of platelets that were stimulated with A23187, showing continous elevation in [Ca2+]i . Taken together, these results suggest that the modulating effect of tyrosine kinase inhibitors on the shape of the collagen-induced Ca2+ signal can, at least partially, account for the decreased PS exposure. However, we cannot yet rule out the possibility that tyrosine kinases may influence other relevant activation steps as well. Development of the procoagulant response may thus need a sustained increase in [Ca2+]i and not an oscillatory or spiking Ca2+ signal, such as detected in the presence of tyrosine kinase inhibitors. For instance, the Ca2+-dependent scramblase and calpain protease may require a sustained Ca2+ signal of long duration, before sufficient activation is reached for PS exposure and bleb formation, respectively.
At least three different protein tyrosine kinases, ie, p72syk,22,24 p125FAK,14 and pp60c-src,43 are known to be involved in collagen-dependent tyrosine phosphorylation. Although the precise contributions of these kinases are unknown, tyrosine phosphorylation appears to control Ca2+ signaling with collagen in at least two ways: (1) phosphorylation and stimulation of phospholipase C-γ2,29,30 and (2) stimulation of collagen-induced entry of external Ca2+ through the so-called store-regulated Ca2+ influx pathway (ref. 37 and references therein). Taking into account the present data, continuous formation of inositol 1,4,5-trisphosphate by activated phospholipase C-γ2 and unreduced Ca2+ entry may be necessary to reach sustained rises in [Ca2+]i and procoagulant activity. From the present literature data, it is difficult to speculate on the identity of the collagen receptor and the tyrosine kinase(s) involved in these responses. For instance, the three kinases known to be activated by collagen, p72syk p125FAK and pp60c-src, are also activated by thrombin and fibrinogen,15,23,25 which combination of agonists gives only spiking Ca2+ signals and little or no bleb formation and PS exposure. However, an interesting possibility, which is under current investigation, is that the recently suggested activation of p72syk and phospholipase C-γ2 by glycoprotein VI receptor occupancy43 plays a role in these collagen-induced responses.
Conclusions.We have shown that the two physiological substrates, collagen and fibrinogen, evoke in platelets completely different Ca2+ response patterns, which are dissociated from the adhesion and spreading events. Platelet interaction with collagen, but not fibrinogen even in combination with thrombin as a coagonist, results in a high and sustained Ca2+ signal that is partially dependent on tyrosine kinase activation and is accompanied by exocytosis, PS exposure, prothrombin activation, and bleb formation. Collagen appears to be an agonist capable of evoking the procoagulant reaction with similar potency as the Ca2+ ionophores, A23187 and ionomycin. Fibrinogen and collagen may thus have distinct functions in the hemostatic process, by providing structures on which platelets can spread and cluster into aggregates, and by offering surfaces where platelets can develop membrane sites for propagation of the coagulation process, respectively.
ACKNOWLEDGMENT
We thank D. Billy for participation in some of the experiments.
Supported by Grants No. 900-526-192 and 902-68-241 from the Netherlands Organization for Scientific Research (The Hague) and Grant No. 93.166 from the Netherlands Heart Foundation (The Hague).
An abstract describing part of this work is presented at the XVIth ISTH Congress in Florence, Italy (June 10, 1997).
Address reprint requests to Johan W.M. Heemskerk, PhD, Departments of Biochemistry/Human Biology, University of Maastricht, PO Box 616, 6200 MD Maastricht, The Netherlands.

![Fig. 3. Exposure of P-selectin on the surface of single platelets during adhesion to fibrinogen or collagen matrices. Fura-2–loaded platelets were allowed to adhere to immobilized fibrinogen (A) or collagen (B) in the presence of 2 mmol/L CaCl2 and FITC-labeled AK-6 antibody against P-selectin. Arrows indicate timepoint of addition of 2 nmol/L thrombin or timepoint of attachment to collagen. Fluorescence images were taken during 5 to 10 minutes, and traces represent changes in FITC fluorescence of individual platelets. These platelets were high in [Ca2+]i at the end of the FITC fluorescence recordings. Traces are representative of >35 platelets out of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2615/3/m_bl_0025f3.jpeg?Expires=1767934950&Signature=HOJfu54W8X~ezMY5bV9b6CHHZqRpresvG9KsGDbwc~LZFrvdBPw6FD8d10kvpT5GDgv6HpGodZr5MC0Mi1ijTZd8wOf4OoZ0pnedzimw14dSUztB1Fa7X45KgnpNsSeOYrajSww8cc4Gtphz8vqYZsAOfySumBbFU1IG~U4DFmwD2u-Ch1aLNErCMAkae~BkVRfhlBe61Y4GiyhyPgwLZDkNb4YmXI9a7ifPVSP1zaybYhb8V50IeUJt-K4dZ1pYcU-fUDFXxbyo24M16315THviQzcOpDW8zDeH6q-orkg4EJwXNZ-cI3QwlcoluF~VUC2GP3RrdgnDDGpPHnHQIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Pseudo-color fluorescence video images of fura-2–loaded platelets adhering to fibrinogen or collagen matrices in the presence of 2 mmol/L CaCl2 and FITC-labeled annexin V. (A) Fura-2 ratio image of platelets adhering to fibrinogen during 20 minutes. (B) FITC-annexin V intensity image of platelets adhering to fibrinogen during 20 minutes and subsequent stimulation with 10 μmol/L ionomycin. (C and D) Corresponding fura-2 ratio image (C) and FITC-annexin V intensity image (D) from one microscopic field of platelets adhering to fibrinogen during 20 minutes in the presence of 2 nmol/L α-thrombin. (E and F ) Corresponding fura-2 ratio image (E) and FITC-annexin V intensity image (F ) from one microscopic field with platelets adhering to collagen during 20 minutes. The false-color rainbow of fura-2 fluorescence ratio was set such that dark blue indicates basal levels of [Ca2+]i and that color changes from light blue, green, yellow to red point to increasing levels of [Ca2+]i . Color changes from blue to red also indicate increasing intensities of FITC fluorescence.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2615/3/m_bl_0025f4.jpeg?Expires=1767934950&Signature=tV75o0KbeqdqBRyJyMkGv0DgFOqyf3UxFPQJDfvL72L3eZh6OJALp6PqvLbf2KhW13V8zGUcS~dk6LgCUcNA0XmlLFb5W0RKPIpfvkhD23-v97Kzfey1LnQ2OnELWur~-dp-GFm-I5ch6lIKH7zGe2BY~1WO2CDqqZw7UbBAtY8R4aOF8N8k80X-vPGGABAIIGTboP-pN~ihfA4LTOKuou-TYLgO8~IdSyDbVBHb0i2R1wMZ2Dk8~suxq38A7oap1YiEgiXSC38k0-SBeA7lGVWVwHCRaY3UEBW2LvcEinc-NJmWvdOqEyRhQbRCzBfasXYbpiGZKUniX4GGRlhIkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Effects of activation inhibitors on Ca2+ responses and annexin V binding capacity of platelets adhering to collagen. Fura-2–loaded platelets were treated with various drugs and allowed to adhere to collagen in the presence of 2 mmol/L CaCl2 and FITC-annexin V, exactly as described for Fig 6. Given are the percentages of platelets that were elevated in [Ca2+]i (A), and stained positively with FITC-annexin V (B) after 15 minutes of adhesion. Data are averaged values ± SEM from three to five independent experiments (350 to 600 cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2615/3/m_bl_0025f7.jpeg?Expires=1767934950&Signature=Hex3dkC04~6cg5NMbftloHutkg5qpKW8IfN5jFsAoxkjX1RAs6e8SRqeqMQoeqJMW-9HfeaDeCmb4nirO0xl2GVK~TBoEcyuALBVYv4haX9EEqi0ZE6fewzBQET3w7HNAQM3tuT8d3~ij2JvQQlJux8rfcIJYxhmF~Jzws9dpPNoEir8-xWpYfL~d643uw9L9EPGJs6R-Vgpu4P1nhMo3-YM-qXtv~MOYXhgxc4k3YClRzOVXEh3cKN4qo6Uw2rxcl3mQYPS8BPnVQ-cBgONW8IFvw~8t2SKXbnAZ1G8bUTbDzdgF~7m2Rre5YytwULWl4R0Ba4TX09JA7mNT~oq8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal