Abstract
Fib420 is a recently identified subclass of normal human fibrinogen in which two extended α chain isoforms (αE ) replace the common α chains, yielding a molecule (ca. 420 kD) which is larger than the more abundant 340-kD form. Evidence for preservation of this subclass throughout vertebrate evolution suggests it performs some as yet unidentified vital function. A survey was undertaken to establish the range of plasma Fib420 levels in normal, healthy adults and in placental cord (fetal) blood. For measuring Fib420 , a quantitative Western blot assay was developed using monoclonal antibody against the exon-VI encoded C-terminus of the molecule's unique αE chain. This αE chain signal was normalized to that of the β chain, common to both fibrinogen forms. Analysis of plasma samples from the adult and newborn cohorts (n = 25 each; total fibrinogen ca. 2.6 mg/mL in both) revealed a statistically significant difference, with a mean level of 100 ± 28 μg/mL in the neonate compared to 34 ± 7 μg/mL in the adult. On average, 1 out of every 100 fibrinogen molecules in adult plasma belongs to the Fib420 subclass. Unlike in the newborn, adult Fib420 levels remained the same over a wide range of total plasma fibrinogen. The striking difference observed between these two cohorts suggests a changing developmental expression of the Fib420 subclass and a homeostatic control operating in later stages of life.
FIBRINOGEN, the blood protein that serves as substrate for the central reaction in clot formation, is a dimeric molecule composed of three pairs of nonidentical polypeptide chains (α, β, and γ). Fib420 is a recently identified subclass of normal human fibrinogen, so named because of its larger size (ca. 420 kD) relative to the more abundant 340-kD form, Fib340 .1 The molecule's larger size is due to replacement of both α subunits by extended α (αE ) subunits2 in the conventional fibrinogen structure (αβγ)2 to yield (αEβγ)2 . On the basis of αE mRNA levels, Fib420 has been estimated to represent only 1% to 2% of the fibrinogen molecules. Nevertheless this long overlooked Fib420 subclass is suspected of having a vital physiologic function based on its preservation throughout vertebrate evolution.3 4
Numerous studies, employing functional as well as immunological assays, have been conducted to assess total circulating fibrinogen levels in the human population (reviewed in ref. 5); however, to date no survey of fibrinogen subclass distribution has been attempted. In the current study, monoclonal antibodies (MoAbs) are employed to evaluate Fib420 levels relative to total fibrinogen in normal individuals. Comparison of samples from healthy adult blood donors with those from placental/umbilical cord blood, representing fetal circulation, provides evidence of a marked developmental change in Fib420 levels.
MATERIALS AND METHODS
Materials.Recombinant VI-domain2 was used to generate a monoclonal anti-VI antibody (MoAb 3-10); it recognizes an epitope located in the first third of the exon VI-encoded extended C-terminus of the Fib420-specific αE chain (Grieninger G. et al, in preparation). Monoclonal anti-β–chain antibody (MoAb Ea3, specific for an epitope in the C-terminal part of the β chain of fibrinogen) and monoclonal anti-α–chain antibody (MoAb 1D4, specific for an epitope in the center of the “αC” region of the α chain) have been described previously.6 7 Polyclonal rabbit antihuman fibrinogen was from Dako (Glostrup, Denmark).
Sample collection and storage.Samples of anticoagulated whole blood without personal identifiers were obtained within 24 hours of collection according to protocols approved by the Institutional Review Board of the New York Blood Center. Adult whole blood was drawn into tubes (lavender Vacutainer #6450, 7-mL draw) containing 0.07-mL EDTA anticoagulant. Placental/umbilical cord (fetal) blood samples,8 from delivered placenta, were taken from collection bags containing a reduced level (23 mL) of CPD A anticoagulant (citrate/phosphate/dextrose/adenine). Corresponding maternal blood samples were drawn within 24 hours after delivery into yellow Vacutainer tubes #4606, containing 1.5-mL anticoagulant (acid citrate dextrose solution [A]). Aliquots of all samples were centrifuged at 1,000g for 20 minutes at 4°C to remove blood cells. The final plasma supernatant was flash frozen in dry ice-ethanol and stored at −80°C. For assay, frozen samples were rapidly thawed in a water bath at 37°C. Neither freezing and thawing in this manner 10 times nor conditions for storage of anticoagulated whole blood before processing (room temperature or 4°C for up to 24 hours) affected analysis of samples by either of the assays, rocket immunoelectrophoresis or Western blot, used in this study.
Choice of anticoagulant did not affect the outcome of the assays as determined with blood from individual donors drawn into either EDTA or CPD A. In the case of fetal and maternal blood collection, where dilution of the plasma by anticoagulant was a significant factor, the dilution was calculated using standard hematocrit values of 61% for newborn infants at term and 36% for women post partum.
Determination of total fibrinogen.Immunoreactive fibrinogen was measured with polyclonal rabbit antibody to human fibrinogen in a rocket immunoelectrophoresis assay described previously.9 The assay was standardized with human fibrinogen from American Diagnostica Inc (Greenwich, CT). Plasma samples were diluted before assay in phosphate-buffered saline supplemented with Trasylol (30 KIU/mL) (aprotinin; Sigma, St Louis, MO) and EDTA (1 mmol/L). Each sample was analyzed on 4 separate gels; on average, variation by this method, expressed as standard deviation, was less than 20% of the mean.
Western blot analysis.Samples were prepared for electrophoresis in sample buffer in the presence or absence of 0.1 mol/L dithiothreitol,10 and separated on sodium dodecyl sulfate (SDS)/7.5% polyacrylamide gel electrophoresis (PAGE) (reducing conditions) or SDS/4% PAGE (nonreducing conditions) using a Mini-Protean II Electrophoresis Cell (Bio-Rad, Hercules, CA). Transfer onto 0.2-μm nitrocellulose membranes was performed with a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad). Membranes were incubated with primary MoAbs (either anti-VI, anti-β, or anti-α) followed by the secondary antibody, horseradish peroxidase (HRPO)-labeled sheep antimouse IgG (Amersham, Arlington Heights, IL). To visualize the enzyme activity, signals were developed by enhanced chemiluminescence (SuperSignal Substrate; Pierce, Rockford, IL), unless otherwise noted, and filmed.
Determination of Fib420 .A quantitative Western blot assay was developed for measuring Fib420 based on simultaneous evaluation of αE- and β-chain signals. For each determination, performed under reducing conditions, appropriate dilutions of a given plasma sample were applied to two lanes of a single SDS/7.5% PAGE (Ready Gels; Bio-Rad) and subjected to Western analysis as above. Signals from the two lanes, separated only during incubation with either anti-VI or anti-β MoAb at the primary antibody step, were developed in parallel and captured together on a single autoradiographic film. Scanning of films was performed on an Arcus II scanner (AGFA, Ridgefield Park, NY), and Intelligent Quantifier software (BioImage, Ann Arbor, MI) was used to evaluate the αE- and β-chain signal intensities.
MoAbs were used at dilutions that elicited comparable band signal intensities from equivalent moles of αE and β; this calibration was accomplished using purified Fib420 from human plasma, the isolation of which will be described elsewhere (D.E. Applegate and G. Grieninger, in preparation). The amount of plasma applied was adjusted separately for αE and β detection to yield signals within the narrow linear range of the assay for each chain. Scanned intensities were corrected for these plasma dilutions and a ratio of signal intensities was calculated, reflecting αE- to β-chain molar ratios and, by extension, the molar ratio of Fib420 to total fibrinogen (expressed as percent and referred to as molar %Fib420 ). Each specimen was analyzed on 4 separate blots; standard deviation of quadruplicate determinations was less than 25% of the mean. Plasma Fib420 levels were derived by multiplying these molar ratios with total fibrinogen levels as determined by rocket immunoelectrophoresis, taking into account molecular weight (a factor of 1.23 was used for all samples).
Statistical analysis.SigmaPlot and SigmaStat (SPSF Inc, San Rafael, CA) were used for statistical analyses. Results are expressed as mean and standard deviation. The Mann-Whitney Rank Sum Test was used to evaluate differences between cohorts. Correlation coefficients (r values) are calculated using the Pearson Product Moment Correlation.
RESULTS
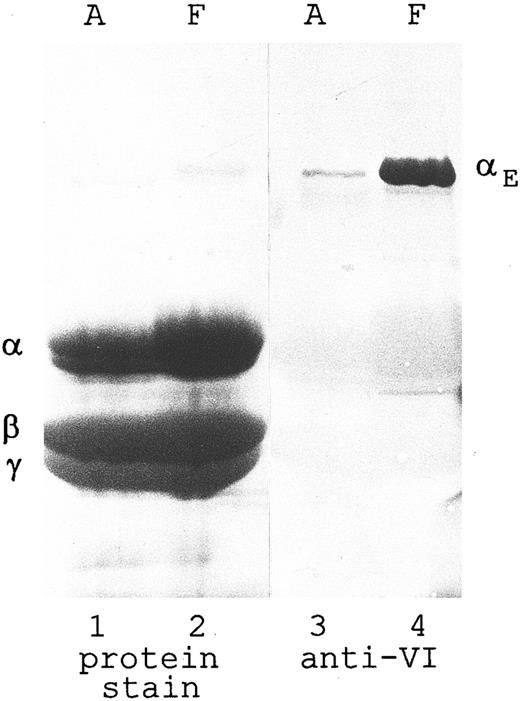
An MoAb against the exon VI-encoded C-terminus of the αE chain recognizes a single major band (Mr 110 kD) by Western blot analysis of reduced human fibrinogen, whether adult or fetal in origin (Fig 1, lanes 3 and 4). In this immunoblot of a heavily overloaded gel, no cross-reaction is detectable with either the predominant α chain or the β and γ chains (visualized by protein stain in lanes 1 and 2), even though the latter two share with the αE chains considerable C-terminal homology.2 There is strikingly more αE , unique to Fib420 , in the fetal preparation than in that of the adult, suggesting a greater preponderance of the Fib420 subclass earlier in human development.
Fibrinogen purified from adult and fetal sources: Disproportionately higher amount of αE chains in fetal material. Fibrinogen (I-2 fraction) was purified18 from plasma derived from normal adult blood (A) and umbilical cord (ie, fetal) blood (F ). The material was separated by SDS-PAGE (80 μg per lane) under reducing conditions and transferred to nitrocellulose. Lanes 1 and 2 were stained with Amido Black (Sigma); lanes 3 and 4 were treated with anti-VI, followed by HRPO-labeled antimouse IgG, and developed with chloronaphthol. Lanes 1 and 3, adult; and lanes 2 and 4, fetal. Positions of the individual chains are indicated. Overloading the gel enabled the Fib420-specific αE chain to be seen by protein staining as a faint band in lane 2 on the original blot.
Fibrinogen purified from adult and fetal sources: Disproportionately higher amount of αE chains in fetal material. Fibrinogen (I-2 fraction) was purified18 from plasma derived from normal adult blood (A) and umbilical cord (ie, fetal) blood (F ). The material was separated by SDS-PAGE (80 μg per lane) under reducing conditions and transferred to nitrocellulose. Lanes 1 and 2 were stained with Amido Black (Sigma); lanes 3 and 4 were treated with anti-VI, followed by HRPO-labeled antimouse IgG, and developed with chloronaphthol. Lanes 1 and 3, adult; and lanes 2 and 4, fetal. Positions of the individual chains are indicated. Overloading the gel enabled the Fib420-specific αE chain to be seen by protein staining as a faint band in lane 2 on the original blot.
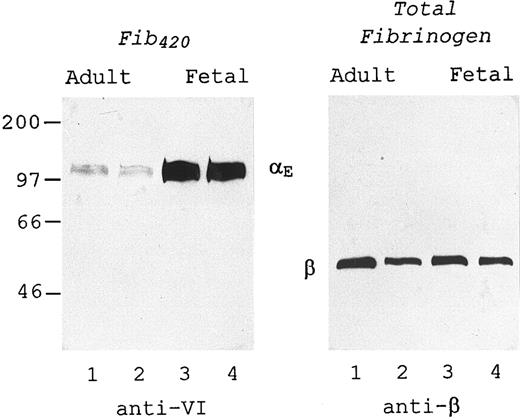
Analysis of whole plasma was undertaken to eliminate any influence of the process of fibrinogen isolation on this finding. Two distinct MoAbs were employed in Western blot analysis to evaluate the amount of αE chains, as a measure of Fib420 , relative to the amount of β chains, which are common to both Fib340 and Fib420 and therefore reflect total fibrinogen. For each specimen, anti-VI was used to detect αE in one lane, while anti-β was used to probe the more abundant β chains in a second lane. To accommodate the divergent molar amounts of these two subunits, the plasma samples were diluted proportionally more for the anti-β– than for the anti-VI–probed lanes to achieve comparable signals.
The immunoblot of Fig 2 shows this technique applied to two representative plasma samples from adult blood (lanes 1 and 2) and two from placental cord blood (lanes 3 and 4). The amount of αE in the fetal source was substantially higher than in that from the adult (left panel), in the presence of similar amounts of total fibrinogen (right panel), demonstrating in whole plasma the same developmental difference observed with isolated material in Fig 1. Of note, both antibodies recognized only one major protein band from among the multitude of proteins present in plasma, the power of the assay being such that an excellent signal to background ratio was maintained even when adding the larger amounts of plasma per lane required to detect αE (left panel).
Measurement in plasma: Fetal Fib420 level is higher than adult. Two representative plasma samples prepared from each source, adult blood (lanes 1 and 2) and umbilical cord blood (lanes 3 and 4), were subjected to SDS-PAGE under reducing conditions, transferred to nitrocellulose, and probed with either anti-VI (left panel) or anti-β (right panel) as described in Materials and Methods. The plasma samples in the right panel were diluted so that they contained approximately 25 ng total fibrinogen per lane. Lanes in the left panel contained 100 times more plasma than the corresponding lanes in the right panel.
Measurement in plasma: Fetal Fib420 level is higher than adult. Two representative plasma samples prepared from each source, adult blood (lanes 1 and 2) and umbilical cord blood (lanes 3 and 4), were subjected to SDS-PAGE under reducing conditions, transferred to nitrocellulose, and probed with either anti-VI (left panel) or anti-β (right panel) as described in Materials and Methods. The plasma samples in the right panel were diluted so that they contained approximately 25 ng total fibrinogen per lane. Lanes in the left panel contained 100 times more plasma than the corresponding lanes in the right panel.
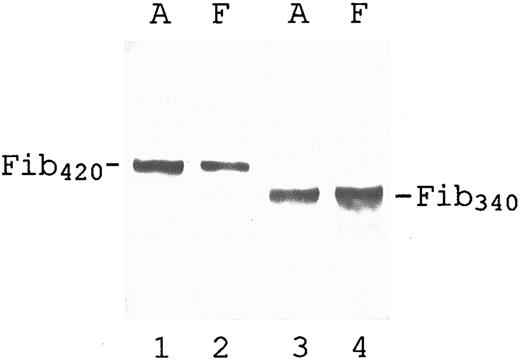
Underlying the use of αE to gauge Fib420 , as in Fig 2, is the assumption that this α-chain isoform is unique to Fib420 regardless of its source. This issue was explored by comparing the disulfide bonded molecular forms of fibrinogen in both the adult and newborn. The plasma samples were diluted so as to minimize intensity differences for better comparison, and SDS-PAGE was performed under nonreducing conditions. In both adult and fetal plasma, anti-VI recognized a single band (Fib420 ) with a molecular size significantly larger than that of the major band (Fib340 ) detected with anti-α (Fig 3) or anti-β (not shown), a result which validates use of the reduced αE-band signal to measure Fib420 in plasma.
Qualitative evaluation of intact, αE-containing fibrinogen in adult and fetal plasma. Plasma samples (same sources as used in lanes 2 and 3 of Fig 2) were subjected to SDS-PAGE under nonreducing conditions, transferred to nitrocellulose, and probed with either anti-VI (lanes 1 and 2) or anti-α (lanes 3 and 4) as described in Materials and Methods: lanes 1 and 3, adult (A); lanes 2 and 4, fetal (F ). For better comparison of their molecular species, plasma volumes analyzed in this Western blot were adjusted to minimize differences in band intensities. Lanes 3 and 4 each contained approximately 50 ng total fibrinogen. For lanes 1 and 2, more material was loaded (50 and 5 times, respectively) to obtain Fib420 bands of similar intensity. A faint Fib420 band can be seen above Fib340 in lane 4 on overexposure of the film.
Qualitative evaluation of intact, αE-containing fibrinogen in adult and fetal plasma. Plasma samples (same sources as used in lanes 2 and 3 of Fig 2) were subjected to SDS-PAGE under nonreducing conditions, transferred to nitrocellulose, and probed with either anti-VI (lanes 1 and 2) or anti-α (lanes 3 and 4) as described in Materials and Methods: lanes 1 and 3, adult (A); lanes 2 and 4, fetal (F ). For better comparison of their molecular species, plasma volumes analyzed in this Western blot were adjusted to minimize differences in band intensities. Lanes 3 and 4 each contained approximately 50 ng total fibrinogen. For lanes 1 and 2, more material was loaded (50 and 5 times, respectively) to obtain Fib420 bands of similar intensity. A faint Fib420 band can be seen above Fib340 in lane 4 on overexposure of the film.
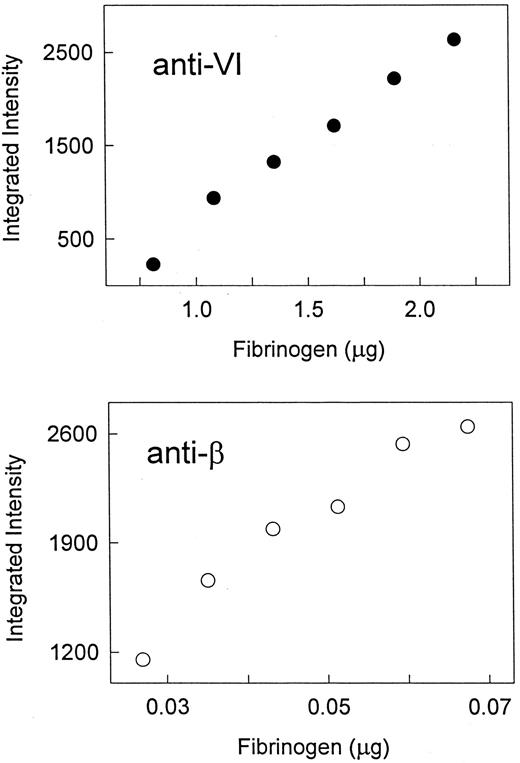
To extend our observations on the difference between adult and fetal Fib420 levels, we undertook a survey of the range of Fib420 levels in normal, healthy adults and in placental cord blood from normal deliveries. For this purpose, we developed a quantitative Western blot assay for the relative proportion of Fib420 using anti-VI and anti-β MoAbs as described in Materials and Methods. For each specimen, αE- and β-band intensities were determined concurrently on quadruplicate immunoblots that were processed together and captured on a single film, from which the molar ratio of the αE and β subunits (ie, the ratio of αE- to β-signal intensities, adjusted for dilution) was calculated. Fib420 as a percentage of total fibrinogen in a sample (molar %Fib420 ) was taken from this ratio. The amount of plasma applied to each lane of the Western blot was adjusted to obtain signals within the narrow (ca. twofold to threefold) linear range of the assay for each fibrinogen subunit (Fig 4). Although common applications of Western immunoblotting are qualitative, the method described here provided reproducibility of molar ratio determinations among four blots to within 25% of the mean. Plasma Fib420 levels were calculated, as described in Materials and Methods, from the molar %Fib420 and the total fibrinogen levels, measured independently by rocket immunoelectrophoresis.
Quantitative assay of αE and β subunits in plasma: linear range of Western blot analysis. A plasma sample (adult) was diluted to the micrograms of total fibrinogen per lane as indicated on the abscissa, and analyzed by Western immunoblotting with either anti-VI or anti-β, as indicated. Integrated intensity of the single band obtained with each antibody, as quantified by computer analysis, is plotted on the ordinate.
Quantitative assay of αE and β subunits in plasma: linear range of Western blot analysis. A plasma sample (adult) was diluted to the micrograms of total fibrinogen per lane as indicated on the abscissa, and analyzed by Western immunoblotting with either anti-VI or anti-β, as indicated. Integrated intensity of the single band obtained with each antibody, as quantified by computer analysis, is plotted on the ordinate.
For the adult survey, plasma was prepared and analyzed from 25 blood samples selected at random from among 128 samples being screened for apheresis donation at the New York Blood Center during a 2-week period in February 1996. The population of donors (76 males, 52 females) was characterized by a mean age of 44.8 years ± 13.4 (median 44), the individuals identifying themselves as either white (81%), black (4%), Hispanic (4%), Asian (0.8%), or other (10%). All met the general health and risk-free behavior requirements of blood donors at this facility. Among the adult samples analyzed, total fibrinogen ranged from 1.7 to 3.8 mg/mL plasma with a mean of 2.7 ± 0.6.
For the fetal survey, plasma was prepared and analyzed from 25 blood samples collected from the umbilical cords of 90 delivered placentas that were donated through The Placental Blood Program of the New York Blood Center in the first 3 weeks of February 1996. Selection of samples for analysis was random among those units containing at least 63 mL of collected blood. The entire group represented deliveries of babies (45% male, 55% female), with a mean physician-estimated gestational age of 39.0 ± 1.5 weeks and an average 5-minute Apgar score of 9.0 ± 0.3, born to mothers 25 to 43 years of age (mean 31.9 ± 6.2). The infants were born to couples describing themselves as white (70%), Hispanic (20%), and black/mixed (10%). Mean total fibrinogen level of the placental samples analyzed was 2.5 ± 0.4 mg/mL plasma (range: 1.8 to 3.4).
It is well known that fibrinogen levels increase during pregnancy. Indeed, for the mothers of our fetal cohort, from whom samples were also collected, the mean plasma fibrinogen level, 4.3 ± 0.8 mg/mL, was 1.6-fold higher than that of the adult blood donor cohort. Since the major regulator of fibrinogen synthesis, interleukin-6 (IL-6),11 exists in human plasma as a high molecular form that does not cross the placenta,12 it was not surprising that the mother-infant pairs demonstrated no significant correlation between their respective fibrinogen levels (r = −.08, P = .7, n = 25).
Means of quadruplicate Western blot assays, presented in Table 1, show the relative Fib420 levels to be substantially different between the newborns and normal adult donors (P < .001). In the fetal cohort, values ranged from 1.9% to 4.2% of total fibrinogen, with a mean of 3.2% ± 0.6, whereas in the adults, they ranged from 0.5% to 1.7%, with a mean of 1.1% ± 0.3. Applying these percentages to the total fibrinogen levels determined by rocket immunoelectrophoresis yielded average fetal and adult Fib420 levels of 100 ± 28 μg/mL and 34 ± 7 μg/mL, respectively, a threefold difference. Similar investigation of the proportion of Fib420 in cord plasma from a limited cohort (n = 12) of preterm infants, ranging from 29 to 34 (mean 32.2) weeks of gestation, indicated no significant difference from that of full-term infants (data not shown). This is in accord with the observation that there is no rapid change in the coagulation system between 30 and 40 weeks of gestational age.13
Survey of Fib420 Plasma Levels in Adults and Newborn Infants
| Adult . | Fetal . | ||||
|---|---|---|---|---|---|
| Fibrinogen . | Fib420 . | Molar . | Fibrinogen . | Fib420 . | Molar . |
| (mg/mL) . | (μg/mL) . | %Fib420 . | (mg/mL) . | (μg/mL) . | %Fib420 . |
| 2.7 | 33 | 1.0 | 2.5 | 120 | 3.9 |
| 2.7 | 26 | 0.8 | 1.9 | 81 | 3.4 |
| 2.7 | 35 | 1.0 | 2.6 | 120 | 3.7 |
| 2.8 | 30 | 0.9 | 2.6 | 99 | 3.1 |
| 2.7 | 33 | 1.0 | 2.4 | 58 | 1.9 |
| 3.7 | 23 | 0.5 | 2.5 | 121 | 3.9 |
| 2.5 | 31 | 1.0 | 2.6 | 119 | 3.7 |
| 2.3 | 37 | 1.3 | 2.6 | 101 | 3.2 |
| 1.9 | 24 | 1.1 | 1.8 | 52 | 2.4 |
| 2.4 | 35 | 1.2 | 2.8 | 100 | 2.8 |
| 1.7 | 21 | 1.0 | 2.8 | 124 | 3.6 |
| 3.1 | 47 | 1.2 | 2.1 | 70 | 2.6 |
| 2.6 | 31 | 1.0 | 2.6 | 136 | 4.2 |
| 2.9 | 40 | 1.1 | 2.1 | 80 | 3.1 |
| 1.8 | 28 | 1.2 | 2.2 | 67 | 2.5 |
| 2.7 | 29 | 0.9 | 2.0 | 53 | 2.2 |
| 3.2 | 31 | 0.8 | 3.3 | 150 | 3.7 |
| 2.2 | 37 | 1.3 | 2.6 | 126 | 3.9 |
| 3.5 | 39 | 0.9 | 2.4 | 93 | 3.2 |
| 2.0 | 33 | 1.3 | 2.2 | 102 | 3.8 |
| 3.4 | 41 | 1.0 | 2.2 | 87 | 3.2 |
| 2.4 | 49 | 1.7 | 3.2 | 133 | 3.4 |
| 2.3 | 42 | 1.5 | 1.8 | 92 | 4.2 |
| 2.7 | 24 | 0.7 | 3.4 | 129 | 3.1 |
| 3.8 | 43 | 0.9 | 2.9 | 82 | 2.3 |
| Mean: | |||||
| 2.7 | 34 | 1.1 | 2.5 | 100 | 3.2 |
| SD: | |||||
| 0.6 | 7 | 0.3 | 0.4 | 28 | 0.6 |
| Adult . | Fetal . | ||||
|---|---|---|---|---|---|
| Fibrinogen . | Fib420 . | Molar . | Fibrinogen . | Fib420 . | Molar . |
| (mg/mL) . | (μg/mL) . | %Fib420 . | (mg/mL) . | (μg/mL) . | %Fib420 . |
| 2.7 | 33 | 1.0 | 2.5 | 120 | 3.9 |
| 2.7 | 26 | 0.8 | 1.9 | 81 | 3.4 |
| 2.7 | 35 | 1.0 | 2.6 | 120 | 3.7 |
| 2.8 | 30 | 0.9 | 2.6 | 99 | 3.1 |
| 2.7 | 33 | 1.0 | 2.4 | 58 | 1.9 |
| 3.7 | 23 | 0.5 | 2.5 | 121 | 3.9 |
| 2.5 | 31 | 1.0 | 2.6 | 119 | 3.7 |
| 2.3 | 37 | 1.3 | 2.6 | 101 | 3.2 |
| 1.9 | 24 | 1.1 | 1.8 | 52 | 2.4 |
| 2.4 | 35 | 1.2 | 2.8 | 100 | 2.8 |
| 1.7 | 21 | 1.0 | 2.8 | 124 | 3.6 |
| 3.1 | 47 | 1.2 | 2.1 | 70 | 2.6 |
| 2.6 | 31 | 1.0 | 2.6 | 136 | 4.2 |
| 2.9 | 40 | 1.1 | 2.1 | 80 | 3.1 |
| 1.8 | 28 | 1.2 | 2.2 | 67 | 2.5 |
| 2.7 | 29 | 0.9 | 2.0 | 53 | 2.2 |
| 3.2 | 31 | 0.8 | 3.3 | 150 | 3.7 |
| 2.2 | 37 | 1.3 | 2.6 | 126 | 3.9 |
| 3.5 | 39 | 0.9 | 2.4 | 93 | 3.2 |
| 2.0 | 33 | 1.3 | 2.2 | 102 | 3.8 |
| 3.4 | 41 | 1.0 | 2.2 | 87 | 3.2 |
| 2.4 | 49 | 1.7 | 3.2 | 133 | 3.4 |
| 2.3 | 42 | 1.5 | 1.8 | 92 | 4.2 |
| 2.7 | 24 | 0.7 | 3.4 | 129 | 3.1 |
| 3.8 | 43 | 0.9 | 2.9 | 82 | 2.3 |
| Mean: | |||||
| 2.7 | 34 | 1.1 | 2.5 | 100 | 3.2 |
| SD: | |||||
| 0.6 | 7 | 0.3 | 0.4 | 28 | 0.6 |
Plasma samples were analyzed from an adult and a fetal cohort representing 25 normal blood donations and 25 placental/umbilical cord blood donations, respectively. Fibrinogen, expressed in mg per mL plasma and rounded to the nearest tenth, represents the mean of quadruplicate determinations by rocket immunoelectrophoresis. Molar %Fib420 equals the mean of quadruplicate molar ratio determinations by quantitative Western blot assay and is presented to the nearest tenth of a percent. Levels of Fib420, in μg/mL plasma and rounded to the nearest whole number, were calculated from these values for fibrinogen and molar %Fib420 as described in Materials and Methods.
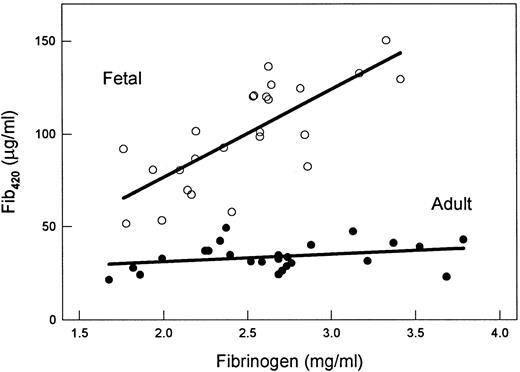
Since the adult and full-term infant have similar average fibrinogen levels (ca. 2.6 mg/mL; Table 1 and ref 13), we have superimposed a comparison of each sample's Fib420 and total fibrinogen levels to reveal a further unexpected difference between these two surveyed groups (Fig 5). Whereas the fetal Fib420 levels were positively correlated with total fibrinogen (r = .74, P < .001), adult Fib420 levels were remarkably consistent across the spectrum of fibrinogen values in the study population (r = .29, P = .15). This phenomenon suggests a Fib420-specific regulation.
Fib420 versus total fibrinogen: Fib420 levels in the adult but not fetal population remain constant. Values plotted are taken from Table 1. Total immunoreactive plasma fibrinogen as determined by rocket immunoelectrophoresis is plotted along the abscissa. Plasma Fib420 concentration (ordinate) was derived from the latter using the molar proportion of Fib420 as determined by quantitative Western blot assay. First-order linear regression analyses are shown. Adult plasma (•); fetal plasma (○).
Fib420 versus total fibrinogen: Fib420 levels in the adult but not fetal population remain constant. Values plotted are taken from Table 1. Total immunoreactive plasma fibrinogen as determined by rocket immunoelectrophoresis is plotted along the abscissa. Plasma Fib420 concentration (ordinate) was derived from the latter using the molar proportion of Fib420 as determined by quantitative Western blot assay. First-order linear regression analyses are shown. Adult plasma (•); fetal plasma (○).
Determination of Fib420 on a random subset of 10 maternal samples indicated that maternal molar ratios (mean 1.3% ± 0.2) were comparable to those of normal adult blood donors and markedly below those of their infants (Table 2). Although the analyzed subset was small, maternal Fib420 and total fibrinogen were positively correlated (r = .73, P = .017) in a manner that was intermediate between that of the fetal and adult cohorts described in Fig 5 above. This may reflect override of the mechanism governing low Fib420 levels shared by normal healthy individuals in adulthood.
Fib420 Levels of Newborn Infants and Their Mothers
| Fetal . | Maternal . | ||||
|---|---|---|---|---|---|
| Fibrinogen . | Fib420 . | Molar . | Fibrinogen . | Fib420 . | Molar . |
| (mg/mL) . | (μg/mL) . | %Fib420 . | (mg/mL) . | (μg/mL) . | %Fib420 . |
| 2.5 | 120 | 3.9 | 3.3 | 64 | 1.5 |
| 2.6 | 120 | 3.7 | 5.2 | 58 | 0.9 |
| 1.8 | 52 | 2.4 | 4.8 | 84 | 1.4 |
| 2.8 | 100 | 2.8 | 4.7 | 96 | 1.6 |
| 2.2 | 67 | 2.5 | 4.5 | 89 | 1.6 |
| 3.3 | 150 | 3.7 | 4.3 | 62 | 1.1 |
| 2.6 | 126 | 3.9 | 5.3 | 96 | 1.4 |
| 2.2 | 102 | 3.8 | 3.5 | 63 | 1.4 |
| 2.2 | 87 | 3.2 | 2.7 | 39 | 1.1 |
| 1.8 | 92 | 4.2 | 5.7 | 91 | 1.3 |
| Mean: | |||||
| 2.4 | 102 | 3.4 | 4.4 | 74 | 1.3 |
| SD: | |||||
| 0.5 | 29 | 0.6 | 1.0 | 20 | 0.2 |
| Fetal . | Maternal . | ||||
|---|---|---|---|---|---|
| Fibrinogen . | Fib420 . | Molar . | Fibrinogen . | Fib420 . | Molar . |
| (mg/mL) . | (μg/mL) . | %Fib420 . | (mg/mL) . | (μg/mL) . | %Fib420 . |
| 2.5 | 120 | 3.9 | 3.3 | 64 | 1.5 |
| 2.6 | 120 | 3.7 | 5.2 | 58 | 0.9 |
| 1.8 | 52 | 2.4 | 4.8 | 84 | 1.4 |
| 2.8 | 100 | 2.8 | 4.7 | 96 | 1.6 |
| 2.2 | 67 | 2.5 | 4.5 | 89 | 1.6 |
| 3.3 | 150 | 3.7 | 4.3 | 62 | 1.1 |
| 2.6 | 126 | 3.9 | 5.3 | 96 | 1.4 |
| 2.2 | 102 | 3.8 | 3.5 | 63 | 1.4 |
| 2.2 | 87 | 3.2 | 2.7 | 39 | 1.1 |
| 1.8 | 92 | 4.2 | 5.7 | 91 | 1.3 |
| Mean: | |||||
| 2.4 | 102 | 3.4 | 4.4 | 74 | 1.3 |
| SD: | |||||
| 0.5 | 29 | 0.6 | 1.0 | 20 | 0.2 |
Data from a subset (n = 10) of our survey of fetal plasmas (Table 1) is compared with that obtained on plasmas of their mothers by rocket immunoelectrophoresis (for total fibrinogen) and by quantitative Western blot assay (for %Fib420). Means of quadruplicate fibrinogen determinations and calculated Fib420 levels are presented in the units indicated, rounded to the nearest tenth and whole number, respectively. Molar %Fib420, the mean of quadruplicate molar ratio determinations, is presented to the nearest tenth of a percent. Each row corresponds to a separate mother-infant pair.
DISCUSSION
This study shows that, on average, one of every 100 fibrinogen molecules in the blood of a healthy adult belongs to the subclass Fib420 , ie, it contains the isoform αE in place of the conventional α subunits. The finding is in close agreement with our earlier estimate of αE mRNA in adult human liver.2 With a Fib420 concentration of only 34 ± 7 μg/mL in adult plasma, or less than 0.1 μmol/L, it is understandable that this subclass escaped notice in previous decades of fibrinogen research.
In an extension of the survey to umbilical cord blood, fetal Fib420 levels, 100 ± 28 μg/mL plasma, were shown to average 3 times higher than those of adults. There are conflicting reports in the literature regarding the behavior of fibrinogen in the fetal circulation,14,15 and speculation regarding Fib420's contribution is premature. It remains to be seen whether the infant's Fib420 drops by 6 months after birth, at a time when other components of the coagulation system mature.16
Fib420 , it should be emphasized, is not a fetal form of fibrinogen since it persists through adult life, albeit at a lower concentration; the same set of fibrinogen genes remains active throughout, with the α gene giving rise to both isoforms α and αE . In contrast, hemoglobin expression involves deactivation of distinct fetal genes resulting in replacement of the fetal protein (α2γ2 ) with an adult version (α2β2 ) within 3 to 6 months of birth.
For this study, a quantitative Western blot assay was developed permitting measurement of Fib420 relative to total fibrinogen in plasma. Conditions were established to control for variabilities inherent in this type of analysis so that, from a determination of αE- and β-chain signal intensities, Fib420 could be determined as a percentage of all the fibrinogen molecules present. The assay's underlying premise is based on the demonstration (Fig 3) that the subunit chains are confined primarily to hexameric (ie, fully assembled) fibrinogen forms: αE to Fib420 , and β to both Fib420 and the more abundant Fib340 . It is interesting to note that in plasma each subclass (Fib420 as well as Fib340 ) represents a rather homogeneous population of molecules, whether derived from infant or adult.
In addition to documenting a sizable difference between fetal and adult Fib420 levels, the data presented in this report uncover a further differential regulation. Only in the fetal cohort did individuals exhibiting high fibrinogen levels have relatively high Fib420 levels, as might be expected, given that both αE- and α-subunit isoforms have the same promotor (ie they are generated from the same gene by alternative splicing). Among normal adults, circulating Fib420 was maintained at the same low level, independent of the widely varying total fibrinogen concentration in this study population. This differential regulation and the widespread distribution of homologues to Fib420's αE chain throughout the vertebrate kingdom4 17 support the notion that the Fib420 subclass may have function(s) distinct from those of the abundant Fib340 subclass.
What mechanism maintains this pattern of universally low levels of Fib420 among healthy adults (Fig 5) but not among newborns and their mothers? Differential control over Fib420 and Fib340 levels is not likely to be exerted at initiation of fibrinogen gene transcription, since stimulation by IL-6 leads to a proportional increase in hepatocellular synthesis of both αE and α chains.2 Although modulation of αE synthesis by regulated termination of transcription and/or efficiency of splicing are a priori possible, differential Fib420 levels more likely arise from posthepatic mechanisms involving Fib420 stability or sequestration into an extravascular compartment(s).
ACKNOWLEDGMENT
The authors acknowledge with gratitude Pablo Rubinstein, Ludy Dobrila, Jay Valinsky, and Donna Strauss for their cooperation in making samples available, and Zaher Ahadi and Steve Callender for their expert technical assistance. We are particularly grateful to Dianne Applegate and Mary Ann Chiasson for many helpful discussions.
Supported in part by grants from the National Institutes of Health (Bethesda, MD) (HL 51050), the American Heart Association (New York, NY), and the Hugoton Foundation (New York, NY).
Address reprint requests to Gerd Grieninger, PhD, 310 E 67th St, New York, NY 10021.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal