Abstract
CD19 (B4) is a signal transduction molecule restricted to the B-cell lineage and the target of the immunotoxin anti-B4–blocked ricin (anti-B4–bR), which is composed of the monoclonal antibody (MoAb) anti-B4 and the modified plant toxin blocked ricin. To explore the influence of conjugation of blocked ricin to anti-B4 on functional activation of CD19, we investigated the effects of anti-B4–bR, and that of unconjugated anti-B4, on intracellular calcium mobilization and ligand/receptor internalization. The data showed that anti-B4–bR was more potent than anti-B4 in triggering cell calcium mobilization. Two other immunotoxins that bind to the B-cell surface, anti-CD20–bR and anti-CD38–bR, were devoid of the calcium increasing effect of anti-B4–bR. Furthermore, anti-B4 conjugated to ricin A-chain was also without effect in Namalwa cells, indicating that the ricin B-chain component was required for anti-B4–bR effect. Anti-B4–bR-induced calcium mobilization was inhibited in the presence of lactose, yet the calcium response induced by cross-linking anti-B4–bR with a second step antibody was not affected. The extent of CD19 modulation induced by anti-B4–bR was higher than that induced by anti-B4, and lactose dampened the effect of the immunotoxin down to that of the MoAb. Moreover, the number of internalized immunotoxin molecules was higher than that of unconjugated MoAb. Although a mechanism involving dimerization of the immunotoxin cannot be excluded, our findings suggest that the residual binding activity of the blocked ricin B-chain to cell surface molecules plays an important role in the greater calcium fluxes and greater internalization rate of anti-B4–bR, and is of functional significance in the mechanism of intoxication of cells by the immunotoxin.
CD19 (B4) IS A 95-kD B-lineage–specific membrane glycoprotein and a member of the Ig supergene family. It can be detected on the B-cell surface at approximately the same time as Ig heavy-chain rearrangement, but it is lost during terminal B-cell differentiation into plasma cells.1-3 CD19 is part of a multimolecular signal transduction complex, which includes the complement receptor type 2 (CR2; CD21), TAPA-1 (CD81), and Leu 13, as well as a number of unidentified proteins.4-6 Although the physiologic ligand of CD19 has not been identified to date, recent experiments with CD19-deficient mice and mice overexpressing the CD19 antigen have demonstrated its major role in normal B-cell development, activation, proliferation, and differentiation.7-9 The signaling pathways that follow the triggering of CD19 antigens have been the subject of several in vitro studies with anti-CD19 monoclonal antibodies (MoAb), early mobilization of intracellular calcium ([Ca2+]i ) being one of the better understood.10 Binding of CD19 to the Src-family protein-tyrosine kinases (PTKs) p53/p56 Lyn, p56 Lck, and p59 Fyn, followed by tyrosine phosphorylation of phospholipase C-γ1/2 and subsequent generation of inositol-1,4,5-trisphosphate are responsible for the CD19-mediated [Ca2+]i increases.11-14 Studies with deletion mutants showed that the CD19 cytoplasmic domain is required for optimal antibody-induced increases in [Ca2+]i , while the transmembrane domain is essential for complex formation with TAPA-1 and the induction of homotypic adhesion and growth arrest.15 In addition, coexpression of CD21 or Leu 13 is not necessary for anti-CD19 MoAb-induced [Ca2+]i increases, and antibody binding to CD21, TAPA-1, and Leu 13 individually induces only minimal [Ca2+]i changes,6,14 15 indicating that among the identified members of the CD19 complex, CD19 is uniquely responsible for the induction of [Ca2+]i mobilization in B cells.
The expression of CD19 on nearly all malignant B lymphocytes16,17 and its ability to undergo a certain degree of internalization on antibody binding16,18-21 make this antigen a particularly appealing target for immunotoxins (ITs). Anti-B4–blocked ricin (anti-B4–bR) is an IT consisting of the anti-CD19 MoAb anti-B4, and a modified form of the plant toxin ricin that has the galactose-binding sites of the B-chain blocked by covalently linked affinity ligands.22,23 The blocked B-chain retains some residual low-affinity for cell surface galactose residues that seems to be necessary for efficient translocation of the ribosome-inactivating ricin A-chain into the cytoplasm.24,25 Anti-B4–bR has shown safety and efficacy in phase I/II trials of in vivo administration26-28 and ex vivo purging of autologous bone marrow29 30 in patients with B-cell acute lymphoblastic leukemia and non-Hodgkin's lymphoma. This IT is currently being tested in several phase II/III clinical trials.
This study was undertaken to investigate the effect of conjugating anti-B4 to blocked ricin on cellular functions that may be modulated on binding of anti-B4 to the CD19 surface antigen. The results showed that, in the absence of a second step antibody, anti-B4–bR triggers greater CD19-mediated [Ca2+]i increases and greater CD19 internalization than the unconjugated anti-B4. We also found that this property of anti-B4–bR depends on the presence of the B-chain component of the blocked ricin moiety.
MATERIALS AND METHODS
Cells.The human Burkitt lymphoma cell lines Namalwa and Daudi, and the human acute T-lymphoblastic leukemia cell line, Molt-4, were maintained in culture by serial passages in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT), 2 mmol/L L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin, and 1 mmol/L sodium pyruvate (GIBCO). Cells were grown at 37°C in a water saturated 5% carbon dioxide/95% air atmosphere.
Antibodies and immunotoxins.Mouse IgG1 MoAb specific for the CD19 antigen (anti-B4)3 was prepared and supplied by ImmunoGen, Inc (Cambridge, MA). A sample of HB7 (anti-CD38 MoAb) was also provided by ImmunoGen, Inc. Fluorescein isothiocyanate (FITC)-conjugated anti-CD19 MoAb (B4-FITC; IgG1), the purified isotype-matched MsIgG1 and MsIgG1-FITC, as well as anti-CD20, anti-CD21, and rat antihuman CD77 MoAbs were purchased from Coulter Immunology (Hialeah, FL). Goat F(ab′)2 antimouse-Ig (G + L) (GAM), goat F(ab′)2 antimouse-Ig (G + L)-FITC (GAM-FITC), and goat F(ab′)2 antirat-Ig (G + L)-FITC (GAR-FITC), were purchased from Tago, Inc (Burlingame, CA). The ITs anti-B4–bR, anti-B4–ricin A-chain, B1-bR (anti-CD20-bR), HB7-bR (anti-CD38–bR), and anti-MY9-bR (anti-CD33–bR), were manufactured at ImmunoGen, Inc. They were formulated in phosphate-buffered saline (PBS) containing 1 mg/mL of human serum albumin. For preparations of anti-B4–bR containing predominantly dimeric (noncovalently linked) or monomeric IT, nonformulated anti-B4–bR was fractionated by gel filtration on a column of Sephacryl S-300 HR equilibrated in PBS at 4°C. Peak fractions corresponding to dimer and monomer were resubmitted to gel filtration under the same conditions. The purified peak fractions from this second gel filtration were stored sterile (0.2 μm filtration) at 4°C, conditions under which the rate of re-equilibration into dimer/monomer mixtures was negligible.
Chemicals.Native ricin D and purified ricin A-chain was purchased from Inland Chemical Laboratories (Austin, TX). The blocked ricin was obtained from ImmunoGen, Inc. The PTK inhibitor tyrphostin A25 was purchased from Calbiochem (La Jolla, CA). Ethylene glycol-bis (b-aminoethyl ether)-N, N, N′, N′ -tetraacetic acid (EGTA), lactose, maltose, galactose, and DNAse I type IV were purchased from Sigma Chemicals Co. (St Louis, MO). Fluo-3 acetoxymethyl ester (Fluo-3 AM), and Pluronic F-127 were from Molecular Probes (Eugene, OR).
Fluo-3 loading protocol and [Ca2+]i -dependent fluorescence processing.Cells (5 × 106) under exponential growth conditions were harvested by centrifugation, washed twice, and resuspended at 1 × 106/mL in RPMI 1640 medium containing 20 mmol/L HEPES buffer, pH 7.0. They were then exposed to the Ca2+-sensitive dye Fluo-3 AM31 at a final concentration of 1 μmol/L together with 5 μmol/L of the nonionic detergent Pluronic F-127 for 45 minutes while incubating in the dark at 37°C in a shaker bath. Loaded cells were then brought up to 10 mL with RPMI 1640 medium, 5% FBS, and 20 mmol/L HEPES buffer, pH 7.4, and kept loading for an additional 15 minutes. After that loading procedure, the cells were washed twice and resuspended in RPMI 1640 medium, 5% FBS, 20 mmol/L HEPES buffer, pH 7.2, containing 10 μg/mL of DNAse I type IV. They were then stored in the dark at 5 × 105/mL at room temperature until assay. The measurement of [Ca2+]i was performed by flow cytometry on a linear scale. In brief, samples of 0.5 or 1.0 mL of cell suspension were transferred to 37°C, and continuously stirred throughout the experiment. Fluo-3 green fluorescence emission was detected with a band-pass filter (530 ± 15 nm) on a FACStar-Plus flow cytometer (Becton Dickinson, Mountain View, CA) equipped with an argon ion laser (200 mW at 488 nm), and a modified sample station constructed as previously described32 to provide on-line reagent addition and reduced sample transit time. Forward and right angle light scatter gated cells were analyzed at a typical flow rate of 200 cells per second, and fluorescence from single cells was collected approximately every second for a period up to 512 to 1,024 seconds, using Lysis II software and subsequently analyzed by Chronys software (Becton Dickinson). Mean flourescence intensity (MFI) values were calculated with cumulative fluorescence data obtained over periods of 25 seconds, on a total number of approximately 5,000 cells per time point. Basal fluorescence values and percentage of responding cells were measured during the first 125 seconds of the data collection period and were always stable and followed by the addition of [Ca2+]i mobilizing agents to cell suspension. In some experiments, a second step GAM antibody was added to cells pretreated with anti-B4 or anti-B4–bR. The inorganic salts contained in the RPMI 1640 medium used for cell suspension during [Ca2+]i measurement were 0.42 mmol/L Ca(NO3 )2 .4H2O, 0.41 mmol/L MgSO4 , 102.65 mmol/L NaCl, 5.37 mmol/L KCl, 23.81 mmol/L NaHCO3 , and 5.64 mmol/L Na2HPO4 , except for experiments where extracellular Ca2+ was depleted by addition of 0.5 mmol/L EGTA. In inhibition experiments with sugars, Fluo-3-loaded cells were resuspended in RPMI medium containing 50 mmol/L of either lactose, maltose, or galactose immediately before collection of fluorescence.
CD19 modulation by anti-B4 and anti-B4–bR in Namalwa cells.Modulation of CD19 induced by anti-B4 or anti-B4–bR was evaluated by indirect immunofluoresecence and flow cytometry. Namalwa cells in RPMI 1640 medium supplemented with 10% FCS and 10 mmol/L HEPES buffer, pH 7.4, were mixed at 0°C with 5 nmol/L of either anti-B4 or anti-B4–bR. Some samples were treated with anti-B4–bR in the presence of lactose (50 to 100 mmol/L), and others with N901-bR (anti-CD56–bR), which was used as a negative control. The suspensions were then incubated at 37°C for 2.5 hours in the continuous presence of MoAb or IT, so that the dissociation rate was negligible, and that the antigen was always saturated. Other samples treated with MoAb or IT were kept at 0°C in the presence of 0.01% sodium azide to evaluate the level of antigen expression on unmodulated cells. After three washings with cold medium, cells were stained with GAM-FITC for 30 minutes at 4°C, washed again, and fixed to measure the amount of surface-bound IT or MoAb remaining on the cell. Ten thousand events were analyzed on a FACScan flow cytometer (Becton Dickinson). The extent of modulation at 37°C was calculated for each sample as the percentage of MFI in relation to unmodulated cells according to the equation: Modulation (%) = 100 − 100 × (MFI [sample] − MFI [negative control])/(MFI [unmodulated cells] − MFI [negative control]).
Binding and internalization of radioiodinated anti-B4 and anti-B4–bR by Namalwa cells.Samples of anti-B4 and anti-B4–bR were radioiodinated by the Bolton-Hunter procedure. The specific radioactivity of anti-B4 was 3.6 × 1011 cpm/μmol, and that of anti-B4–bR was 2.7 × 1011 cpm/μmol. Namalwa cells in RPMI 1640 medium supplemented with 10% FCS and 10 mmol/L HEPES buffer, pH 7.4, were mixed at 0°C with 0.1 nmol/L of either 125I-anti–B4 or 125I-anti–B4-bR. The suspensions were then incubated at 37°C with occasional gentle shaking for 2.5 hours. Samples (2 × 106 cells/sample) were then taken for measurement of bound and/or internalized radiolabel. Some samples were stripped of the surface-bound radioactive material by washing the cells for 5 minutes at 0°C with 150 μL of 0.1 mol/L acetic acid containing 0.145 mol/L NaCl. Samples of cells either surface-stripped (ie, with only internalized material) or not (ie, surface-bound and internalized material) were separated from liquid by layering onto a mixture of silicone and paraffin oils (10:3.1 vol/vol) and centrifuging for 1 minute to separate the cells from the medium containing nonbound material, as described previously.15 Control experiments, with incubation at 0°C where endocytosis is inhibited, showed that less than 3% of the material initially bound at 0°C was left after such treatment. Other control experiments showed that incubation of 125I-anti–B4 or 125I-anti–B4-bR under these conditions in the presence of 1 μmol/L nonradiolabeled anti-B4 either at 0°C or 37°C resulted in accumulation of ≤ 2% of the amount of radiolabel measured without the blocking Ab.
Statistical analysis.Statistical significance of results was determined using the unpaired Student's t-test. P values of ≤.05 were considered significant.
RESULTS
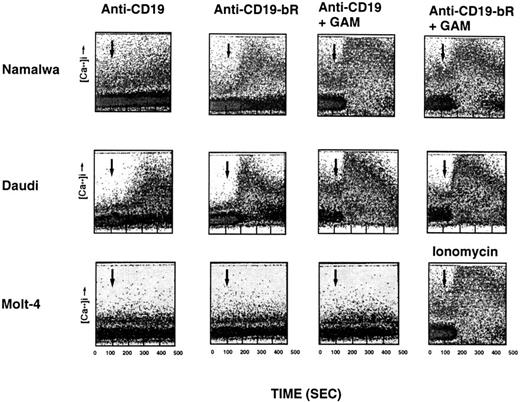
Characteristics of [Ca2+]i mobilization induced by anti-B4–bR IT and unconjugated anti-B4 MoAb.In preliminary dose-response studies, we showed that the effects of anti-B4–bR and that of anti-B4 on the calcium response were optimal at 3 to 7.5 nmol/L, while a measurable effect occurred at a concentration of 1 nmol/L. The concentration of 5 nmol/L was retained for most of the following experiments. We first investigated the kinetics of anti-B4–bR and anti-B4 on [Ca2+]i mobilization in the CD19-positive Burkitt lymphoma cells, Namalwa and Daudi. As shown in Fig 1, anti-B4–bR (5 nmol/L) was able to release [Ca2+]i in Namalwa cells in a time-dependent manner. The increase in intensity of fluorescence, which corresponded to an increase in [Ca2+]i , began rapidly, as early as 30 seconds after addition of the IT, and peaked at about 3 minutes. Therafter, the fluorescence intensity decreased slowly toward the level of unstimulated cells. No significant increase of fluorescence was observed with anti-B4 MoAb (5 nmol/L). With Daudi cells, both anti-B4–bR and anti-B4 MoAb could induce an increase in fluorescence, but with different magnitude and kinetics (Fig 1). Maximum intensity was about two times more pronounced with anti-B4–bR than with unconjugated anti-B4. In addition, the peak of anti-B4–bR effect was observed at 2 to 2.5 minutes after addition of the IT and decreased thereafter, while that of anti-B4 was observed only after 3.5 minutes and remained stable for the remaining 2.5 minutes of the data collection period. The maximal percentages of cells responding to anti-B4 and anti-B4–bR were 0% and 30%, and 34% and 55%, in Namalwa and Daudi cells, respectively.
Time-dependent and cell-specific effects of anti-B4–bR and anti-B4 on intracellular calcium mobilization. Namalwa, Daudi, and Molt-4 cells were loaded with the Ca2+ indicator Fluo-3-AM and then stimulated with a concentration of 5 nmol/L of unconjugated anti-B4 MoAb (anti-CD19), or anti-B4–bR (anti-CD19-bR) followed by GAM. In some experiments, Molt-4 cells were stimulated with ionomycin (3 μmol/L). Agents were added at 125 seconds as indicated by the arrows, and fluorescence was measured every second with no interruption of sample flow during the data collection period (from 0 to 512 seconds). Data are expressed as dot plots of fluorescence versus time. Traces are representative of eight, five, and two separate experiments with Namalwa, Daudi, and Molt-4 cells, respectively.
Time-dependent and cell-specific effects of anti-B4–bR and anti-B4 on intracellular calcium mobilization. Namalwa, Daudi, and Molt-4 cells were loaded with the Ca2+ indicator Fluo-3-AM and then stimulated with a concentration of 5 nmol/L of unconjugated anti-B4 MoAb (anti-CD19), or anti-B4–bR (anti-CD19-bR) followed by GAM. In some experiments, Molt-4 cells were stimulated with ionomycin (3 μmol/L). Agents were added at 125 seconds as indicated by the arrows, and fluorescence was measured every second with no interruption of sample flow during the data collection period (from 0 to 512 seconds). Data are expressed as dot plots of fluorescence versus time. Traces are representative of eight, five, and two separate experiments with Namalwa, Daudi, and Molt-4 cells, respectively.
To determine the influence of cross-linking with a second step antibody on anti-B4-bR– and anti-B4–induced calcium mobilization, Namalwa and Daudi cells were preincubated with these reagents at a concentration of 5 nmol/L and subsequently stimulated with a saturating concentration of GAM (17 μg/mL). In Namalwa, GAM induced a major increase in fluorescence (126% and 148%) in a majority of cells (55% and 59%) preincubated with anti-B4–bR, and anti-B4, respectively (Fig 1). In Daudi, GAM induced similarly important increases in fluorescence in 76% of cells preincubated with anti-B4–bR, and 73% of cells preincubated with anti-B4 (Fig 1). These results indicate that cross-linking with GAM augmented the effects of anti-B4–bR and anti-B4, and led to similar maximum increases in [Ca2+]i mobilization in cells pretreated with either reagent.
We speculated that the induction of a [Ca2+]i response in Daudi, but not in Namalwa cells, by unconjugated anti-B4 in the absence of cross-linking reagent (Fig 1) could be explained if there were a higher density of CD19 antigens on the membrane surface of Daudi, that would increase the chance of bivalent binding of anti-B4 to two CD19 antigens. We therefore analyzed the immunophenotype of Namalwa and Daudi cells. As shown in Table 1, the MFI values corresponding to CD19 expression were 58 and 132 in Namalwa and Daudi cells, respectively, indicating that the level of CD19 expressed on Daudi cells was twofold to threefold higher than on Namalwa cells. The other maturation antigens, CD20, CD21, and surface IgM (sIgM), were also expressed on Daudi cells at levels fivefold, 1.5-fold, and threefold higher than on Namalwa (Table 1). In comparison, the Molt-4 T-lymphoma cell line did not express any of the B-cell markers except for CD21, which was weakly expressed with a MFI of 26 on 34% of cells. Both Namalwa, Daudi, and Molt-4 expressed CD38, while only Daudi expressed CD77.
Immunophenotype of Namalwa, Daudi, and Molt-4 Cells
| Antigen . | Namalwa . | Daudi . | Molt-4 . | |||
|---|---|---|---|---|---|---|
| . | % Positive Cells . | MFI . | % Positive Cells . | MFI . | % Positive Cells . | MFI . |
| CD19 | 100 | 58 | 99 | 132 | 0.3 | 4 |
| CD20 | 57 | 314 | 98 | 1486 | 0.1 | 5 |
| CD21 | 77 | 89 | 98 | 133 | 34 | 26 |
| CD38 | 99 | 526 | 100 | 858 | 99 | 303 |
| CD77 | 0,3 | 4 | 98 | 88 | 0.3 | 4 |
| sIgM | 100 | 1413 | 96 | 4455 | 0.7 | 4 |
| Antigen . | Namalwa . | Daudi . | Molt-4 . | |||
|---|---|---|---|---|---|---|
| . | % Positive Cells . | MFI . | % Positive Cells . | MFI . | % Positive Cells . | MFI . |
| CD19 | 100 | 58 | 99 | 132 | 0.3 | 4 |
| CD20 | 57 | 314 | 98 | 1486 | 0.1 | 5 |
| CD21 | 77 | 89 | 98 | 133 | 34 | 26 |
| CD38 | 99 | 526 | 100 | 858 | 99 | 303 |
| CD77 | 0,3 | 4 | 98 | 88 | 0.3 | 4 |
| sIgM | 100 | 1413 | 96 | 4455 | 0.7 | 4 |
Cell surface antigens were measured either by direct immunofluorescence (CD19, CD20, CD21, and sIgM) or indirect immunofluorescence (CD38, and CD77), followed by flow cytometric determination.
Abbreviation: MFI, mean fluorescence intensity (arbitrary units).
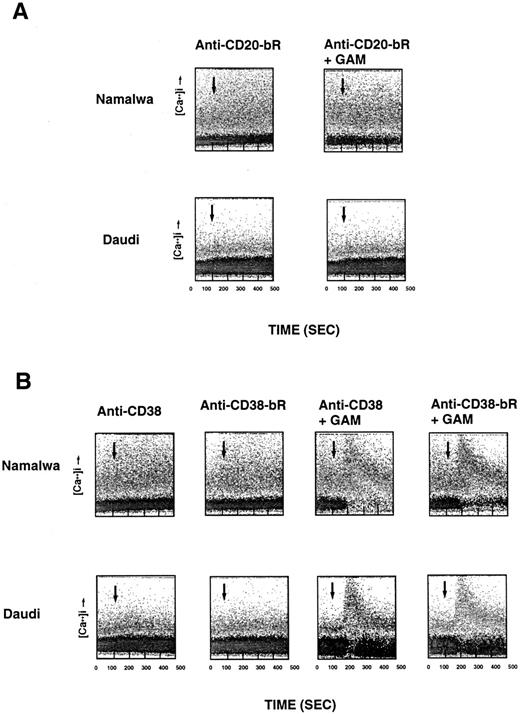
Specificity of anti-B4–bR-induced [Ca2+]i release.Several control experiments were performed in attempts to confirm the CD19 specificity of anti-B4–bR induced [Ca2+]i release. We first used the CD19-negative T-lymphoma cell line, Molt-4. Neither anti-B4–bR nor anti-B4 could induce any [Ca2+]i mobilization on Molt-4 cells, which responded well to the calcium ionophore ionomycin (Fig 1). Anti-B4–bR [Ca2+]i signal was abrogated by preincubating Namalwa cells with unconjugated anti-B4 (5 nmol/L), but not by increasing concentrations (1 to 100 nmol/L) of the isotype control MsIgG1 (data not shown), thus showing the requirement for specific binding of anti-B4–bR to cell surface CD19 for induction of the calcium response, and excluding potential contribution of Fc receptors. No [Ca2+]i increase was observed when Namalwa cells were treated with either unconjugated blocked ricin, blocked ricin conjugated to irrelevant anti-MY9 (anti-CD33) MoAb, mixtures of unconjugated blocked ricin with unconjugated anti-B4, whole native ricin, ricin A-chain, or anti-B4-ricin A-chain IT. In addition, preincubation of Namalwa cells with increasing concentrations (1 to 100 nmol/L) of free ricin or free blocked ricin did not affect the calcium response to anti-B4–bR (data not shown). Taken together, these results indicate that a link between ricin B-chain and anti-B4 MoAb was required for anti-B4–bR-induced [Ca2+]i mobilization in CD19-expressing cells. Next, we tested the effect of two other immunotoxins that bind to the B-cell surface, ie, anti-CD20-bR (Fig 2A), and anti-CD38-bR (Fig 2B). None of those ITs could induce any detectable calcium response neither in Namalwa nor in Daudi cells, although cross-linking of anti-CD38 MoAb or anti-CD38–bR could increase cell calcium levels. In other experiments (not shown) anti-CD38 MoAb did not affect anti-B4–bR-induced calcium responses in Namalwa and Daudi cells.
Lack of effect of anti-CD20–bR (A) and anti-CD–38-bR (B) on calcium mobilization in Namalwa and Daudi cells. The experimental conditions are similar to those described in Fig 1.
Lack of effect of anti-CD20–bR (A) and anti-CD–38-bR (B) on calcium mobilization in Namalwa and Daudi cells. The experimental conditions are similar to those described in Fig 1.
Partial inhibition of anti-B4–bR-induced [Ca2+]i increase by chelation of extracellular Ca2+.Namalwa cells responded efficiently to the [Ca2+]i -increasing effect of anti-B4–bR, while unconjugated anti-B4 and unconjugated blocked ricin were ineffective. Thus, we chose these cells for further characterization. To determine whether extracellular Ca2+ influx was involved in the [Ca2+]i response to anti-B4–bR, we measured the intensity of the [Ca2+]i increase after depletion of extracellular Ca2+ with 0.5 mmol/L EGTA. As shown in Fig 3A, anti-B4–bR was still able to ellicit a [Ca2+]i signal in the presence of 0.5 mmol/L EGTA. However, the maximum intensity of the signal was diminished by 21% compared with cells stimulated with anti-B4–bR in the absence of EGTA. The subsequent reintroduction of extracellular Ca2+ led to an instantaneous and sustained increase in [Ca2+]i , indicating that extracellular Ca2+ enters the cell following stimulation by anti-B4-bR (Fig 3A). The addition of extracellular Ca2+ also resulted in a sustained [Ca2+]i mobilization with anti-B4–bR in cells, which were not previously chelated with EGTA.
Effect of EGTA and tyrphostin on [Ca2+]i mobilization induced by anti-B4–bR in Namalwa cells. (A) Anti-B4–bR induces both mobilization of intracellular Ca2+ and extracellular Ca2+ influx. Namalwa cells were pretreated with EGTA (0.5 mmol/L; Δ) at 60 seconds to chelate extracellular Ca2+ and were stimulated with the IT anti-B4–bR (5 nmol/L) at 125 seconds. CaCl2 (0.5 mmol/L) was then added at 662 seconds to restore extracellular Ca2+. Response of control cells without pretreatment with EGTA, but with stimulation with IT and addition of Ca2+ are included (•). Data are expressed as HMFI versus time. (B) Dose-dependent effect of tyrphostin on [Ca2+]i response induced by anti-B4–bR. Namalwa cells were pretreated for 18 hours at 37°C with increasing concentrations of tyrphostin (0 μmol/L; •); (10 μmol/L; □); (30 μmol/L; ▴); (50 μmol/L; ▵), (100 μmol/L; ▪), and (200 μmol/L; ○). Cells were then loaded with Fluo3-AM and analyzed by flow cytometry for IT-induced calcium mobilization. Data are expressed as HMFI versus time, and traces are representative of three experiments.
Effect of EGTA and tyrphostin on [Ca2+]i mobilization induced by anti-B4–bR in Namalwa cells. (A) Anti-B4–bR induces both mobilization of intracellular Ca2+ and extracellular Ca2+ influx. Namalwa cells were pretreated with EGTA (0.5 mmol/L; Δ) at 60 seconds to chelate extracellular Ca2+ and were stimulated with the IT anti-B4–bR (5 nmol/L) at 125 seconds. CaCl2 (0.5 mmol/L) was then added at 662 seconds to restore extracellular Ca2+. Response of control cells without pretreatment with EGTA, but with stimulation with IT and addition of Ca2+ are included (•). Data are expressed as HMFI versus time. (B) Dose-dependent effect of tyrphostin on [Ca2+]i response induced by anti-B4–bR. Namalwa cells were pretreated for 18 hours at 37°C with increasing concentrations of tyrphostin (0 μmol/L; •); (10 μmol/L; □); (30 μmol/L; ▴); (50 μmol/L; ▵), (100 μmol/L; ▪), and (200 μmol/L; ○). Cells were then loaded with Fluo3-AM and analyzed by flow cytometry for IT-induced calcium mobilization. Data are expressed as HMFI versus time, and traces are representative of three experiments.
Inhibition of anti-B4–bR-induced [Ca2+]i increase by a protein-tyrosine kinase inhibitor.The signal transduction pathways responsible for anti-B4–bR-induced [Ca2+]i elevation were further investigated by evaluating the effect of the PTK inhibitor tyrphostin on the induction of [Ca2+]i mobilization in Namalwa cells. Anti-B4–bR-induced [Ca2+]i mobilization gradually decreased (18%, 34%, 54%, 77%, and 94% inhibition) in cells pretreated with increasing concentrations (10 μmol/L, 30 μmol/L, 50 μmol/L, 100 μmol/L, and 200 μmol/L, respectively) of tyrphostin (Fig 3B). Propidium iodide staining and flow cytometric determination of CD19 expression were used to ensure that concentrations of tyrphostin up to 200 μmol/L were not toxic to Namalwa cells and did not affect significantly the level of CD19 expression (data not shown).
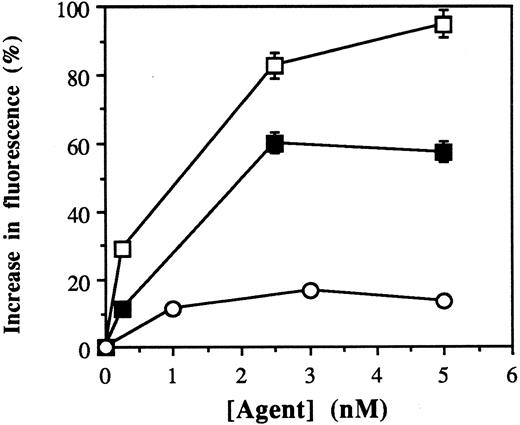
[Ca2+]i mobilization by purified dimeric and monomeric fractions of anti-B4–bR.Blocked ricin exists as an equilibrium mixture of monomeric and dimeric forms.33 Anti-B4–bR likewise exists as a mixture of monomeric and dimeric forms (ratio about 1:3) at neutral pH. Because the rate of re-equilibration is negligible at 4°C, purified dimeric and monomeric species can be separated by gel filtration at 4°C. The effect of increasing concentrations of such purified dimeric and monomeric fractions of anti-B4–bR were tested on Namalwa cells to evaluate the influence of dimerization of IT blocked ricin moiety on IT-induced cell calcium mobilization. We found that the effects of both IT fractions were dose-dependent, with optimal concentrations of dimeric IT (5 nmol/L) inducing a 95% increase, and optimal concentrations of monomeric IT (2.5 nmol/L) inducing a 60% increase in fluorescence over basal level (Fig 4A). Higher concentrations of monomeric IT (up to 45 nmol/L) or dimeric IT (up to 22.5 nmol/L) did not increase fluorescence further (data not shown). These results suggest that while dimerization of anti-B4–bR molecules can amplify the [Ca2+]i response to the IT, most of the increase can be effected by the monomeric form.
Dose-dependent effects of dimeric and monomeric fractions of anti-B4–bR on calcium mobilization. Dimers and monomers of IT were purified by gel filtration and tested for calcium mobilization in Namalwa cells. Data are expressed as the peak values of percent increase in fluorescence over basal level in cells treated with increasing concentrations of dimeric IT (□), monomeric IT (▪), or unconjugated anti-B4 (○).
Dose-dependent effects of dimeric and monomeric fractions of anti-B4–bR on calcium mobilization. Dimers and monomers of IT were purified by gel filtration and tested for calcium mobilization in Namalwa cells. Data are expressed as the peak values of percent increase in fluorescence over basal level in cells treated with increasing concentrations of dimeric IT (□), monomeric IT (▪), or unconjugated anti-B4 (○).
Inhibition of anti-B4–bR-induced [Ca2+]i increase by lactose.Lactose (50 mmol/L) caused a 30% to 40% inhibition of anti-B4–bR-induced [C2+]i mobilization, while the [Ca2+]i response induced by cross-linking of anti-B4–bR with GAM was not affected (Table 2). Another disaccharide, maltose (50 mmol/L), was less effective than lactose in inhibiting anti-B4–bR effect, while the monosaccharide galactose (50 mmol/L) had no inhibiting effect (Table 2).
Effects of Sugars on Calcium Responses in Namalwa Cells
| Experiment . | Sugars . | MFI (arbitrary units) . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | . | Basal Level . | Anti-B4–bR . | Anti-B4–bR + GAM . | . | . | ||
| . | . | . | . | . | . | . | . | . |
| 1 | No sugar | 215 | 371 | 522 | ||||
| Lactose (50 mmol/L) | 225 | 328 | (−34%)* | 521 | (−4%) | |||
| Maltose (50 mmol/L) | 229 | 345 | (−26%) | 548 | (+4%) | |||
| 2 | No sugar | 230 | 412 | 696 | ||||
| Lactose (50 mmol/L) | 222 | 324 | (−44%) | 637 | (−11%) | |||
| Maltose (50 mmol/L) | 212 | 353 | (−23%) | 678 | (0) | |||
| 3 | No sugar | 223 | 385 | 703 | ||||
| Lactose (50 mmol/L) | 224 | 314 | (−44%) | 640 | (−13%) | |||
| Galactose (50 mmol/L) | 212 | 366 | (−5%) | 720 | (+6%) | |||
| Experiment . | Sugars . | MFI (arbitrary units) . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | . | Basal Level . | Anti-B4–bR . | Anti-B4–bR + GAM . | . | . | ||
| . | . | . | . | . | . | . | . | . |
| 1 | No sugar | 215 | 371 | 522 | ||||
| Lactose (50 mmol/L) | 225 | 328 | (−34%)* | 521 | (−4%) | |||
| Maltose (50 mmol/L) | 229 | 345 | (−26%) | 548 | (+4%) | |||
| 2 | No sugar | 230 | 412 | 696 | ||||
| Lactose (50 mmol/L) | 222 | 324 | (−44%) | 637 | (−11%) | |||
| Maltose (50 mmol/L) | 212 | 353 | (−23%) | 678 | (0) | |||
| 3 | No sugar | 223 | 385 | 703 | ||||
| Lactose (50 mmol/L) | 224 | 314 | (−44%) | 640 | (−13%) | |||
| Galactose (50 mmol/L) | 212 | 366 | (−5%) | 720 | (+6%) | |||
Data are expressed as the peak values of the MFI of Fluo-3-loaded cells before (basal level) and after addition of anti-B4–bR (anti-B4–bR) followed by GAM (anti-B4–bR + GAM).
Percent change of MFI by sugar compared with control without sugar, after substraction of corresponding basal levels.
Antigenic modulation and internalization of anti-B4 and anti-B4–bR.Modulation of CD19 expression on Namalwa cells by anti-B4 and anti-B4–bR was measured by indirect immunofluorescence and flow cytometry. Cells were saturated with MoAb or IT at 0°C, and then incubated for 2.5 hours at 37°C to allow for antigenic modulation. As shown in Table 3, the extent of modulation was 36% with anti-B4–bR, versus only 14% in the presence of anti-B4 MoAb. Moreover, lactose (50 to 100 mmol/L) dampened the effect of anti-B4–bR down to the level of unconjugated anti-B4. Binding and internalization were studied in Namalwa cells using radioiodinated anti-B4 and anti-B4–bR, and saturation and incubation conditions similar to those for modulation experiments. As shown in Table 4, the number of total cell-associated molecules was 2.1 × 104, and 1.8 × 104 for anti-B4–bR and anti-B4, respectively. Although the number of surface-bound anti-B4–bR and anti-B4 molecules was similar, the number of internalized IT molecules was more than threefold higher than unconjugated anti-B4, indicating that conujugation of blocked ricin to anti-B4 MoAb potentiates the internalization process.
CD19 Modulation with Anti-B4 and Anti-B4–bR in Namalwa Cells
| Treatment . | MFI . | Modulation (%) . | |
|---|---|---|---|
| . | O°C . | 37°C . | . |
| N901-bR | 4 | 4 | — |
| Anti-B4 | 138 | 119 | 14 |
| Anti-B4–bR | 142 | 93 | 36 |
| Anti-B4–bR + lactose (50 mmol/L) | 141 | 119 | 16 |
| Anti-B4–bR + lactose (100 mmol/L) | 145 | 130 | 11 |
| Treatment . | MFI . | Modulation (%) . | |
|---|---|---|---|
| . | O°C . | 37°C . | . |
| N901-bR | 4 | 4 | — |
| Anti-B4 | 138 | 119 | 14 |
| Anti-B4–bR | 142 | 93 | 36 |
| Anti-B4–bR + lactose (50 mmol/L) | 141 | 119 | 16 |
| Anti-B4–bR + lactose (100 mmol/L) | 145 | 130 | 11 |
Cells were mixed with anti-B4, anti-B4–bR, or anti-B4–bR plus lactose at 0°C and incubated for 2.5 hours at 37°C in the continuous presence of the reagents. Control cultures were incubated with N901-bR. After washings with cold medium, cells were stained with GAM-FITC to detect MoAb or IT remaining on the cell surface.
Binding and Uptake of Radiolabeled Anti-B4 and Anti-B4–bR by Namalwa Cells
| Material . | Molecules/Cell . | Ratio of Molecules Inside/Molecules Outside . | ||
|---|---|---|---|---|
| . | Total Cell-Associated Molecules . | Internalized Molecules . | Surface-Bound Molecules4-150 . | . |
| Anti-B4–bR | 2.1 ± 0.3 × 104 | 5.8 ± 0.4 × 103 | 1.5 ± 0.3 × 104 | 0.39 ± 0.09 |
| Anti-B4 | 1.8 ± 0.3 × 104 | 1.8 ± 0.3 × 103 | 1.6 ± 0.3 × 104 | 0.11 ± 0.03 |
| Material . | Molecules/Cell . | Ratio of Molecules Inside/Molecules Outside . | ||
|---|---|---|---|---|
| . | Total Cell-Associated Molecules . | Internalized Molecules . | Surface-Bound Molecules4-150 . | . |
| Anti-B4–bR | 2.1 ± 0.3 × 104 | 5.8 ± 0.4 × 103 | 1.5 ± 0.3 × 104 | 0.39 ± 0.09 |
| Anti-B4 | 1.8 ± 0.3 × 104 | 1.8 ± 0.3 × 103 | 1.6 ± 0.3 × 104 | 0.11 ± 0.03 |
Values are the mean ± SEM of two independent experiments, each performed in duplicate.
Cell surface-bound molecules are derived from total number of cell-associated molecules minus the number of internalized molecules.
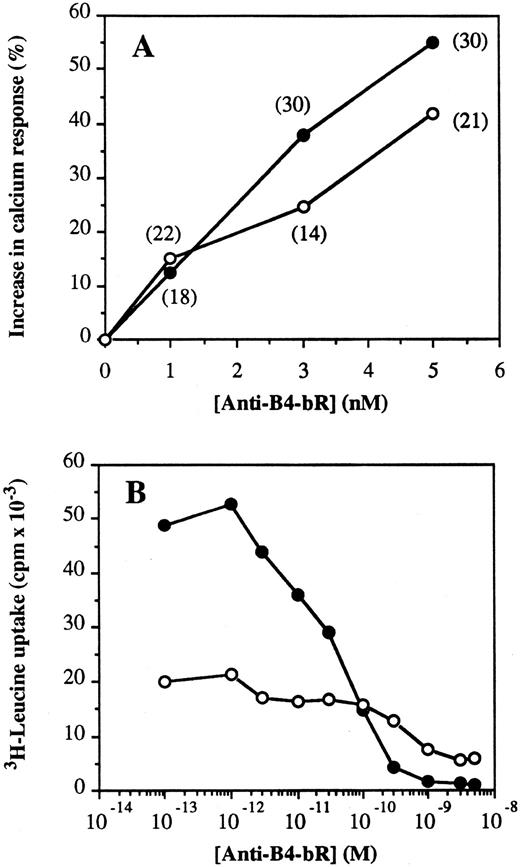
Relation between calcium flux augmentation and protein synthesis inhibition by anti-B4–bR.The effect of increasing concentrations of anti-B4–bR was tested on calcium mobilization and protein synthesis inhibition in Namalwa and Daudi cells as an approach to evaluate the importance of the early calcium response for long-term cytotoxic activity of the IT. Although the absolute effects of anti-B4 and anti-B4–bR on calcium fluxes were more important in Daudi than in Namalwa cells (Fig 1), the increasing effect of anti-B4–bR over that of anti-B4 was 10% to 15% higher in the latter, both in terms of intensity of fluorescence and in terms of frequency of responding cells (Fig 5A). Similarly, cell sensitivity to protein synthesis inhibition by the immunotoxin was higher in Namalwa than Daudi cells, with IC50 values of 5 × 10-11 mol/L and 7 × 10−10 mol/L, respectively (Fig 5B). In the absence of anti-B4–bR treatment, leucine uptake was 2.5-fold lower in Daudi than in Namalwa cells, indicating an intrinsic lower protein synthesis rate in the former.
Association between increases in calcium response and sensitivity to protein synthesis inhibition by anti-B4–bR in Namalwa (•) and Daudi cells (○). (A) Increase in calcium response was expressed as the peak values (achieved at 2 to 3 minutes after IT addition) of percent increase in fluorescence over the level induced by unconjugated anti-B4 MoAb. Numbers in parentheses indicate the peak values of percentage of responding cells to anti-B4–bR over the effect of anti-B4. (B) Inhibition of protein synthesis was monitored by incorporation of 3H-leucine. Cells were incubated for 18 hours at 37°C with IT, washed twice and pulsed for 4 hours at 37°C with 1 μCi of 3H-leucine. Results are expressed as the mean cpm × 10−3 of two separate experiments, each performed in triplicate. The standard error of the mean (SEM) of each determination did not exceed 10%.
Association between increases in calcium response and sensitivity to protein synthesis inhibition by anti-B4–bR in Namalwa (•) and Daudi cells (○). (A) Increase in calcium response was expressed as the peak values (achieved at 2 to 3 minutes after IT addition) of percent increase in fluorescence over the level induced by unconjugated anti-B4 MoAb. Numbers in parentheses indicate the peak values of percentage of responding cells to anti-B4–bR over the effect of anti-B4. (B) Inhibition of protein synthesis was monitored by incorporation of 3H-leucine. Cells were incubated for 18 hours at 37°C with IT, washed twice and pulsed for 4 hours at 37°C with 1 μCi of 3H-leucine. Results are expressed as the mean cpm × 10−3 of two separate experiments, each performed in triplicate. The standard error of the mean (SEM) of each determination did not exceed 10%.
DISCUSSION
Antibody binding to CD19 initiates multiple intracellular signal transduction cascades and modulates several B-cell functions.4,10,34 The work presented in this report focuses on exploring the influence of blocked ricin conjugation on antibody-induced [Ca2+]i mobilization and ligand/receptor internalization. We found that in the absence of cross-linking antibody, the IT was remarkably superior to its MoAb moiety in inducing [Ca2+]i mobilization. Indeed, anti-B4–bR, but not anti-B4, could trigger a rapid and transient [Ca2+]i signal in Namalwa cells. In Daudi cells, anti-B4–bR calcium increasing effect was twofold greater in intensity than that of anti-B4 alone, and the former was extremely more rapid than the latter. Anti-B4–bR calcium mobilizing effect, in the absence of secondary cross-linking antibody, was 50% and 60% as potent as that observed after cross-linking with GAM, in Namalwa and Daudi cells, respectively. The effect of Anti-B4–bR was CD19-mediated because it could be blocked by preincubation with unconjugated anti-B4 on Namalwa cells. In addition, the IT induced no calcium mobilization in the CD19-negative Molt-4 cells. By modulating extracellular calcium concentrations, we showed that anti-B4–bR-mediated increases in [Ca2+]i involved both the release of intracellular stores, and transmembrane influx from the extracellular milieu. Furthermore, anti-B4–bR effect was sensitive to the PTK inhibitor, tyrphostin. These results indicate that anti-B4–bR-induced calcium mobilization shares most of the characteristcs and the signaling pathways of cross-linking of anti-CD19 antibodies with a second step antimouse Ig antibody,14 and yet with the important difference that anti-B4–bR effect could be achieved in the absence of any secondary antibody.
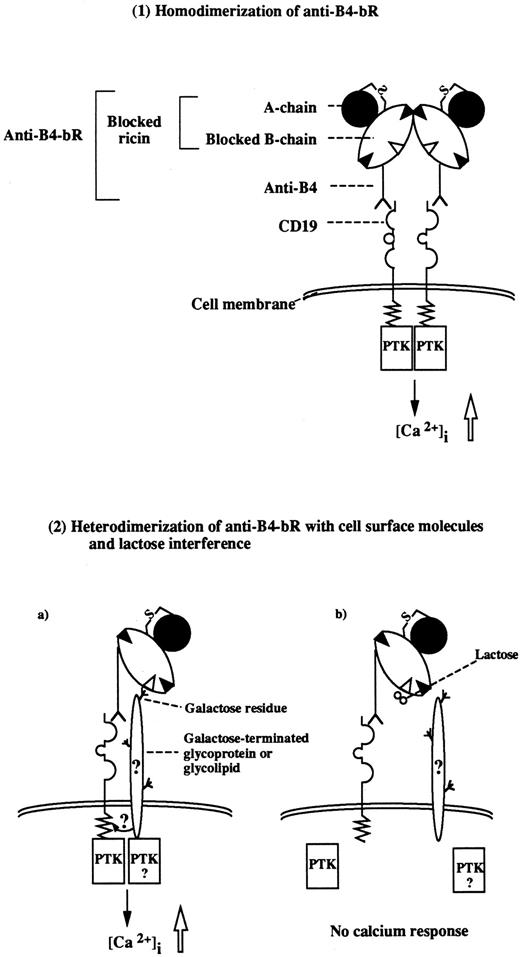
We further examined the mechanisms of anti-B4–bR effect on [Ca2+]i mobilization by analyzing in detail the role of the IT blocked ricin moiety. Blocked ricin conjugated to MoAbs that bind to other B-cell surface molecules (ie, CD20, and CD38) did not increase calcium fluxes in the B-lymphoma cells. The results clearly indicate that any immunotoxin prepared with blocked ricin and binding to the B-cell surface might not induce calcium responses. Because blocked ricin is composed of a modified B chain with covalently linked ligands and an unmodified A chain, we then sought to determine the respective importance of the two subunits in anti-B4–bR effect. The observation that anti-B4–ricin A chain IT, devoid of blocked ricin B-chain, was ineffective in increasing [Ca2+]i in Namalwa cells while the IT containing the whole blocked ricin had an intrinsic calcium mobilizing effect suggested that the B chain was responsible for this effect. Therefore, the increased calcium flux induced by anti-B4–bR could be due to the blocked ricin B chain acting as a cross-linking agent to either other blocked ricin-conjugated anti-B4 molecules (homodimerization), or to other molecules on the B-cell surface (heterodimerization), as illustrated in Fig 6. The evidence supporting each of those mechanisms is the following.
Proposed mechanisms of increased calcium mobilization induced by anti-B4–bR. (1). Homodimerization of monomeric anti-B4–bR occurs either in solution or on cell surface, leading to cross-linking of two CD19 molecules. (2a). The MoAb moiety of anti-B4–bR binds to one CD19 antigen, while a putative residual galactose binding site within the blocked ricin moiety binds to cell surface molecules. (2b). The latter interaction is specifically inhibitable with lactose. It remains to be established whether the surface molecules, which bind to blocked ricin, are CD19 or other molecules that synergize with CD19 signaling. In both models, the downstream signaling pathways may include promotion of physical interaction between CD19 and receptor-associated PTK, followed by phosphorylation of PLCγ, generation of IP3, release of calcium from intracellular stores, and influx from the extracellular milieu. The two models proposed are nonexclusive and could be complementary.
Proposed mechanisms of increased calcium mobilization induced by anti-B4–bR. (1). Homodimerization of monomeric anti-B4–bR occurs either in solution or on cell surface, leading to cross-linking of two CD19 molecules. (2a). The MoAb moiety of anti-B4–bR binds to one CD19 antigen, while a putative residual galactose binding site within the blocked ricin moiety binds to cell surface molecules. (2b). The latter interaction is specifically inhibitable with lactose. It remains to be established whether the surface molecules, which bind to blocked ricin, are CD19 or other molecules that synergize with CD19 signaling. In both models, the downstream signaling pathways may include promotion of physical interaction between CD19 and receptor-associated PTK, followed by phosphorylation of PLCγ, generation of IP3, release of calcium from intracellular stores, and influx from the extracellular milieu. The two models proposed are nonexclusive and could be complementary.
Homodimerization of monomeric anti-B4–bR molecules is supported by recent analysis of blocked ricin by gel filtration and ultracentrifugation, which have shown that unlike ricin, blocked ricin has a tendency to self-associate in solution, existing predominantly in a dimeric form at neutral pH. The association-dissociation of blocked ricin, which appears to be mediated by the blocked B-chain, is a noncovalent interaction that is readily reversible and is insensitive to lactose treatment. The equilibrium ratio between monomeric and dimeric forms can be altered by changing pH; at acidic pH (pH < 5.0) the monomeric form predominates.33 Conjugation of blocked ricin does not alter its propensity for noncovalent reversible self-association (JML, unpublished results). Thus, the noncovalent self-association of anti-B4–bR mediated through its blocked ricin component may provide a certain degree of association of the target antigen/IT complex at the cell surface, which could, like the cross-linking of such complexes via secondary antibody, promote cell signaling. Our present results show that purified monomeric anti-B4–bR was capable of inducing most of the calcium mobilizing activity of purified dimeric anti-B4–bR. One could hypothesize that, at neutral pH, the purified monomeric immunotoxins rapidly dimerize either in solution or on the cell surface, leading to generation of a calcium response. The data support the notion, therefore, that the putative natural CD19 ligand activates CD19 at the cell surface by inducing dimerization of the monomeric receptor. However, this would not explain the absence of calcium mobilization with the anti-CD38–bR IT, especially since GAM cross-linking of anti-CD38 MoAb generates a calcium signal.
The heterodimerization mechanism of blocked ricin with cell surface molecules that contain terminal galactose moieties or other complex oligosaccharides is supported by our findings that lactose could specifically inhibit anti-B4–bR increasing effect on calcium response. It has been shown previously that blocked ricin retains a residual sugar-binding activity22,23 that is sensitive to lactose treatment and is important both for binding to cell membrane24 and for cytotoxicity22-25 of anti-B4–bR. Indeed, under certain conditions, ricin can be modified on the B chain with a third affinity ligand, which both abrogates the residual sugar-binding of the blocked ricin and greatly reduces its potency as an effector moiety of anti-B4–bR.22-24 Other recent studies using different approaches35,36 also indicate that ricin has a third galactose-binding site, albeit of lower affinity (in the millimolar range) than the two known binding sites that is important for the full cytotoxic potency of ricin. Collectively, the data suggest that the residual sugar-binding capacity of blocked ricin plays an important role in the increasing effect of anti-B4–bR on calcium responses over its unconjugated anti-B4 counterpart. The requirement of high lactose doses for inhibition of anti-B4–bR effect is consistent with the weak affinity of the sugar-binding capacity of blocked ricin, while evidence for specificity of lactose sensitivity is provided by the lack of alteration of calcium fluxes induced by cross-linking anti-B4–bR with GAM. Our findings that free ricin and free blocked ricin could not block anti-B4–bR increasing effect are not against the heterodimerization mechanism of blocked ricin with cell surface molecules, but are consistent with the fact that it is impossible to saturate ricin binding sites on cell surface.37
Potential B-cell surface carbohydrate structures, which bind to the blocked ricin moiety of anti-B4–bR and are responsible for the increased CD19 signaling capacity of the immunotoxin, may include CD19 itself, as the latter is highly glycosylated.1 Other candidates, which have been shown previously to be physically and/or functionally associated with CD19, are surface IgM molecules,14,38-41 the glycoprotein CD38,42 and the glycosphingolipid CD77.43 On the basis of our results, the contribution of CD77 could be excluded because it was not expressed on Namalwa cells, which responded to the calcium increasing effect of anti-B4–bR. In contrast, CD38 was expressed both on Namalwa and Daudi cells, and the possibility that blocked ricin could modify CD38 that in turn alter CD19 signaling was appealing for the following reasons: (1) CD38 is a bifunctional enzyme that catalyzes at its ectocellular domain both the synthesis and hydrolysis of the calcium-mobilizing metabolite cyclic adenosine diphosphate-ribose44; and (2) incubation of Namalwa cells with external NAD+ can elicit modulation of cell surface CD38 and its internalization in nonclathrin-coated vesicles, and the internalized CD38 remains enzymatically active.45 Therefore, we examined the potential contribution of CD38 in anti-B4–bR effect by using an anti-CD38 MoAb, but we found no inhibitory effect of the MoAb on the calcium responses to the IT. These results do not exclude, however, any potential role of CD38 in anti-B4–bR effect, as anti-CD38 MoAb could bind to an epitope (perhaps a peptide) distinct from that recognized by blocked ricin (sugar residues). Determination of the exact nature of cell surface molecules that are involved in binding of blocked ricin and in augmentation of CD19-mediated calcium signaling deserves further investigation.
Another interesting finding of the present studies is that, in contrast to Namalwa cells, Daudi cells do not require cross-linking of anti-CD19 antibodies by a second antibody for detectable calcium signaling. This is consistent with the observations of a previous study using another anti-CD19 MoAb (CLB-CD19) on Daudi cells.46 The higher sensitivity of Daudi cells to anti-CD19 antibodies compared with most other malignant or normal B-lineage cells,14,16,47 probably reflects the higher maturation stage of the former, as indicated by the level of expression of CD20, CD21, and sIgM on these cells, and/or the greater density of CD19 antigens on Daudi compared with Namalwa cells. The latter hypothesis seems most likely because transfection of the human Rex T-cell line, which lacks CD20, CD21, and sIgM, with CD19 cDNA results in high levels of expression of CD19 and mobilization of [Ca2+]i in response to anti-CD19 MoAb without additional cross-linking antibody.15 Together, these findings suggest that high antigen density may increase the possibility of bivalent binding of anti-B4 to two CD19 antigens on the cell surface, leading to [Ca2+]i mobilization. Nevertheless, high level of CD19 expression by itself was not sufficient to induce maximum calcium mobilization with anti-B4, at levels that could be reached with anti-B4–bR.
We showed here that anti-B4–bR is more potent than unconjugated anti-B4 MoAb in inducing CD19-mediated modulation and internalization, and the greater antigenic modulation could be blocked by lactose. Therefore, we suggest that the greater uptake of immunotoxin molecules may rely on mechanisms similar to those operating for the greater calcium fluxes (Fig 6), although at the intracellular level, CD19 internalization may be independent of calcium levels.15 The extent of internalization of antigen/IT complexes is a factor of prime importance for cytotoxicity of the IT.19 Also, most of first generation ITs made from ricin A-chain have lower cytotoxicity than those in which the B chain is conserved,48 suggesting that the greater internalization rate of anti-B4–bR is important for its potent cytotoxic activity.
Early changes in intracellular calcium levels have been found to be important for a variety of long-term biological responses, although the molecular mechanisms involved are not completely known in all cases.49-52 It is noteworthy that the presence of calcium ions in the medium and possibly calcium influx into the cells are required for the entry of and cell sensitivity to ricin and other toxic proteins, while calcium deprivation has little effect on the binding and endocytosis of the toxins.53 We demonstrated that the calcium mobilizing activity of anti-B4–bR was sensitive to lactose treatment, but it was insensitive to galactose; similarly, the cytotoxic activity of the IT was reported to be highly sensitive to lactose25 and quite insensitive to galactose,29 thus suggesting that the early calcium signal and the long-term cytotoxic effect may be associated. Also, comparing Namalwa and Daudi cells, we showed that greater calcium fluxes induced by anti-B4–bR over the effect of unconjugated anti-B4 was associated with greater sensitivity to protein synthesis inhibition. However, the lower sensitivity of Daudi cells could be explained by other mechanisms. Moreover, the observation that anti-CD38-bR was unable to increase calcium levels in antigen-bearing cells, while it has been previously shown to be highly cytotoxic to those cells,54 seems to be against a general requirement of calcium fluxes for activity of immunotoxins prepared with blocked ricin. Further studies will be necessary to elucidate the importance of the greater calcium response for the long-term biological effects of anti-B4–bR.
To our knowledge, this is the first report showing that an IT displays a greater capacity of triggering intracellular calcium mobilization, antigenic modulation, and antigen internalization than its corresponding MoAb alone. The effect could be explained by two complementary mechanisms both depending on the blocked ricin B chain component of the blocked ricin moiety within the IT molecule, ie, its propensity to dimerize at neutral pH, and its ability to bind to galactose-terminated cell membrane oligosaccharides. Thus, our findings support the notion that the residual galactose-binding activity of blocked ricin is of functional significance.
ACKNOWLEDGMENT
We thank Dr F. Leblond for construction of the modified sample station adapted to our FACStar-Plus flow cytometer to provide accurate kinetic determinations of calcium mobilization.
Supported in part by an operating Grant No. MT-13259 from the Medical Research Council of Canada (MRCC), by the Fonds de la Recherche en Santé du Québec (FRSQ), and by the Cancer Research Society of Canada. E.K. is a recipient of a Research Career Award in Health Sciences from the Pharmaceutical Manufacturers' Association of Canada-Health Research Foundation/MRCC, and D.C.R. is a scholar of the FRSQ.
Address reprint requests to Edouard Kouassi, PhD, Maisonneuve-Rosemont Hospital Research Center, 5415 boulevard l'Assomption, Montreal, Quebec, H1T 2M4, Canada.

![Fig. 3. Effect of EGTA and tyrphostin on [Ca2+]i mobilization induced by anti-B4–bR in Namalwa cells. (A) Anti-B4–bR induces both mobilization of intracellular Ca2+ and extracellular Ca2+ influx. Namalwa cells were pretreated with EGTA (0.5 mmol/L; Δ) at 60 seconds to chelate extracellular Ca2+ and were stimulated with the IT anti-B4–bR (5 nmol/L) at 125 seconds. CaCl2 (0.5 mmol/L) was then added at 662 seconds to restore extracellular Ca2+. Response of control cells without pretreatment with EGTA, but with stimulation with IT and addition of Ca2+ are included (•). Data are expressed as HMFI versus time. (B) Dose-dependent effect of tyrphostin on [Ca2+]i response induced by anti-B4–bR. Namalwa cells were pretreated for 18 hours at 37°C with increasing concentrations of tyrphostin (0 μmol/L; •); (10 μmol/L; □); (30 μmol/L; ▴); (50 μmol/L; ▵), (100 μmol/L; ▪), and (200 μmol/L; ○). Cells were then loaded with Fluo3-AM and analyzed by flow cytometry for IT-induced calcium mobilization. Data are expressed as HMFI versus time, and traces are representative of three experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2364/3/m_bl_0003f3.jpeg?Expires=1769324362&Signature=3MDvzFhP9YncvNrrp4hk6D7SkYxDBIiakr7Lgz5z~eqFwy-jQJnTldnewnk3h7TzPFlbC4WRjgtU21IlnkZ9OdwqGKzBcf9OKQPn9-x80xq7-joqJrGKZ17daHnT92pE0U7ZF8soTmgb5eC5ajgIwEsTwMFyO20wCo7vm4piPOQ72aHXBYph-9fbi7dkWdBG-LSsWtN2GTpD4~ypLccLmevP3AGzJl0UunwmA91zM-7Il7vRD-ZTlmAwL3l1GgfWeZFEooywLPYUyMPGIFjZm0YXiXwxreXhLJbI98mL4ByKuMh1BPyycvnLO-2OO-ndW8zViBGhD0OOFF52V1jy5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal