Abstract
In an increasing number of hematopoietic cytokine receptor systems (T-cell receptor, B-cell receptor, and macrophage colony-stimulating factor, stem cell factor, interleukin-3, and erythropoietin [EPO] receptors), inhibitory roles for the protein tyrosine phosphatase hematopoietic cell phosphatase (HCP; SHPTP1, PTP1C, and SHP1) have been defined in proliferative signaling. However, evidence exists to suggest that HCP also may exert important effects on blood cell differentiation. To investigate possible roles for HCP during late erythroid differentiation, effects of manipulating HCP expression or recruitment on EPO-induced hemoglobinization in erythroleukemic SKT6 cells have been investigated. No effects of EPO on levels of HCP, Syp, Stat5, the EPO receptor, or GATA-1 expression were observed during induced differentiation. However, the tyrosine phosphorylation of JAK2, the EPO receptor, and Stat5 was efficiently activated, and HCP was observed to associate constitutively with the EPO receptor in this differentiation-specific system. In studies of HCP function, inhibition of HCP expression by antisense oligonucleotides enhanced hemoglobinization, whereas the enforced ectopic expression of wild-type (wt) HCP markedly inhibited EPO-induced globin expression and Stat5 activation. Based on these findings, epidermal growth factor (EGF) receptor/EPO receptor chimeras containing either the wt EPO receptor cytoplasmic domain (EECA) or a derived HCP binding site mutant (EECA-Y429,431F ) were expressed in SKT6 cells, and their abilities to mediate differentiation were assayed. Each chimera supported EGF-induced hemoglobinization, but efficiencies for EECA-Y429,431F were enhanced 400% to 500%. Thus, these studies show a novel role for HCP as a negative regulator of EPO-induced erythroid differentiation. In normal erythroid progenitor cells, HCP may act to prevent premature commitment to terminal differentiation. In erythroleukemic SKT6 cells, this action also may enforce mitogenesis.
FOR THE MAJORITY OF hematopoietic growth factor (HGF ) receptors studied to date, the ligand-induced activation of receptor-encoded or receptor-associated protein tyrosine kinases (PTKs) constitutes a primary signaling event. HGF receptors that directly encode PTKs include c-fms,1 c-kit,2 flt-3,3 and Ron,4 whereas those that function through associated PTKs include the T-cell receptor (TCR),5 B-cell receptor (BCR),6 natural killer (NK) cell inhibitory receptor p58,7 and receptors of the type 1 and 2 cytokine superfamilies.8 In the BCR and TCR complexes, Src-like kinases and zap70 function as upstream effectors, whereas type 1 and 2 cytokine receptors directly engage Janus PTKs. Despite this divergence in PTK signaling, in each of these receptor systems a common SH2 domain-encoding effector, the protein tyrosine phosphatase (PTP) hematopoietic cell phosphatase (HCP) has been suggested to act as an important regulatory component. Specifically, within the BCR,9 TCR,10 NK cell inhibitory receptor p58,7 and several type 1 cytokine receptor systems,11,12 HCP has been shown to function as negative regulator of HGF-induced mitogenesis.7 9-12 However, several lines of emerging evidence suggest that, in addition to this well-established role, HCP also may exert significant effects on blood cell differentiation. In the present study, investigations focus on HCP function in this latter context, and experiments have been performed to test possible roles for HCP in mediating the EPO-dependent induction of hemoglobinization.
HCP (SHPTP1, PTP1C, SHP1) is a nontransmembrane, SH2 domain-encoding PTP that originally was discovered by low stringency screening of a human breast carcinoma cDNA library with a probe derived from the transmembrane PTP, leukocyte antigen-related receptor.13 Subsequently, HCP was cloned from murine spleen14 and from rat megakaryocytic and murine bone marrow monocytic cDNA libraries15,16 and was shown to be expressed primarily in hematopoietic tissues. Structurally, HCP contains two functional SH2 domains within its amino terminus, a central PTP domain, and a short carboxyl terminal region that contains putative regulatory tyrosine residues.13,17,18 With regard to its association with HGF receptors, HCP first was demonstrated to be recruited via its SH2 domains to c-kit19 and since has been reported to associate with CD22 (BCR),9 the TCR,10 and the receptors for FcγIII,20 interleukin-3 (IL-3),11 and erythropoietin (EPO).12 Within the EPO receptor system, HCP has been shown to associate with (phospho)tyrosine residues 429 and 431 of the EPO receptor cytoplasmic domain after EPO stimulation.12 Furthermore, in 32D cells stably expressing an EPO receptor form mutated at these residues, HCP binding is inhibited and levels of EPO-induced activation of JAK2 and mitogenesis are enhanced.12
Additional insight into functional roles played by HCP has been provided by in vivo and ex vivo studies of blood cell development in motheatenviable (mev/mev) mice.21-23 Autosomal recessive mutations at the motheaten locus have been shown to correspond to loss-of-function mutations in HCP,24-26 and mev/mev mice exhibit multiple hematopoietic abnormalities. This includes perturbations of lymphocyte and macrophage development27 and an overexpansion of cells within the erythroid lineage.28 For colony-forming units-erythrocyte (CFU-e), this expansion largely is limited to a splenic population, and in these mev/mev splenic CFU-e, levels of EPO-dependent 59Fe incorporation into heme are markedly enhanced. Thus, the question is raised as to whether HCP possibly might also act to modulate effects of EPO on red blood cell differentiation. To directly test possible roles for HCP during EPO-induced erythrocyte maturation, we have adopted murine erythroleukemic SKT6 cells as a model system29 30 and have investigated effects on hemoglobinization of manipulating HCP expression and recruitment. This included experiments in which HCP expression was inhibited using antisense oligonucleotides, HCP expression was enforced via ectopic expression, or HCP recruitment was blocked via the mutation of the above-noted HCP binding sites (P)Y 429 and 431 in a chimeric EGF receptor/EPO receptor construct. Because HCP acts as a negative effector of EPO-induced mitogenesis, HCP might be predicted to act as a positive regulator of EPO-induced differentiation. Alternatively (and as in mitogenesis) HCP might act as a negative effector of EPO-induced differentiation, possibly to prevent premature commitment to this terminal pathway. Interestingly, the results of the above-mentioned experiments in SKT6 cells uniformly provide evidence that HCP functions to inhibit EPO-induced differentiation.
MATERIALS AND METHODS
HCP and chimeric receptor constructs. HCP and chimeric receptor constructs were prepared as follows. For HCP, a murine wild-type (wt) cDNA was isolated from pGEX2TwtHCP15 and was cloned into the expression vector pCINeo (Promega, Madison, WI). For the chimeric constructs EECA and EECA-Y429, 431F, the extracellular domain is that of the human EGF receptor, whereas the transmembrane and cytoplasmic domains are derived from the murine EPO receptor.31 The construction of pCINeo D Bgl II-EECA has been described previously.30 EECA-Y429, 431F is a derived chimera in which codons encoding Y429 and Y431 of the EPO receptor were mutated to codons for phenylalanine. The vector pCINeoEECA-Y429, 431F was constructed by substituting a point-mutated 686-bp 5′ Bgl II → 3′ Sal I EPO receptor cDNA fragment into pCINeo D Bgl II-EECA. Constructs were confirmed by sequencing. For pCINeowtHCP, expression also confirmed by the transient transfection of Cos cells and Western blotting.
SKT6 cell culture and electrotransfections. SKT6 cells29 30 were maintained at 3 to 9 × 105 cells/mL in Optimem I medium (Life Technologies, Gaithersburg, MD) containing 4% fetal bovine serum (FBS; Hyclone Labs, Logan, UT), 10 μmol/L β-mercaptoethanol, penicillin (100 U/mL), streptomycin (0.1 mg/mL), and amphotericin (0.25 mg/mL). Electrotransfections were performed as follows. SKT6 cells in exponential growth phase (7 × 105 cells/mL) were washed in CaCl2 -deficient Optimem I medium at 4°C and were resuspended at 1.1 × 107 cells/mL. Cells then were electroporated with HCP or EPO receptor constructs in pCINeo-derived expression vectors (750 V, 25 μF, 4 mm electroporation cuvettes, 60 μg pDNA; Gene Pulser II; BioRad, Hercules, CA). Electroporated cells were incubated sequentially at 0°C (10 minutes) and 37°C (10 minutes) in pre-equilibrated Optimem I medium containing 33% dialyzed FBS. Cells then were diluted into 6-well plates (3-fold serial dilutions) and, after 48 hours of culture, were cultured for ≥18 days in the presence of G418 (1.1 mg/mL; Geneticin; Life Technologies).
Immunoprecipitations and Western blotting of HCP and EPO receptor. In immunoprecipitation experiments, SKT6 cells (7 × 105 cells/mL) were exposed to EPO as indicated and were collected, washed at 4°C in Optimem I medium, and lysed in 150 mmol/L NaCl, 1 mmol/L EGTA, 1 mmol/L NaF, 50 mmol/L Tris-HCl, (pH 7.4) 1% NP-40, 0.1 mmol/L Na3VO4 , 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, 50 μg/mL phenylmethylsulfonyl fluoride (PMSF ), and 2.2 μg/mL aprotinin (2 × 107 cells per 0.25 mL per sample). Lysates then were cleared by microcentrifugation (5 minutes at 500g and 5 minutes at 4,500g ) and were preadsorbed with protein A Sepharose CL4B (15 μL per sample). Supernatants then were incubated with antibodies to HCP (1 μg) or EPOR (4 μL; UBI, Lake Placid, NY) and immune complexes were adsorbed to 30 μL of washed protein A Sepharose CL4B (1 hour at 4°C). Gels were washed four times in 1 mL of 10-fold diluted lysis buffer, and immunoprecipitated proteins were eluted in sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (3.4% SDS, 0.2 mol/L dithiothreitol [DTT], 0.05 mmol/L bromophenyl blue, 10% glycerol, and 0.12 mol/L Tris-HCl, pH 6.8) and were analyzed by enhanced chemiluminescence (ECL) Western blotting (Amersham, Arlington Heights, IL).
Assays of JAK2, EPO receptor, and Stat5 tyrosine phosphorylation. In assays of JAK2, EPO receptor, and Stat5 tyrosine phosphorylation, SKT6 cultures were initiated at 3 × 105 cells/mL. Expanded cultures (7 × 105 cells/mL) then were stimulated with EPO (±50 U/mL for 7.5 minutes) and cells were collected, washed at 0°C in Optimem I medium, and lysed at 4°C in 1% NP-40, 50 mmol/L NaCl, 30 mmol/L Na4P2O4 , 50 mmol/L NaF, 0.1 mmol/L Na3VO4 , 10 mmol/L Tris-HCl, pH 7.5, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, 50 μg/mL PMSF, 2.2 μg/mL aprotinin (2 × 107 cells per mL per sample). Antibodies to JAK2 (5 μL), EPO receptor (4 μg) or Stat5 (5 μL; UBI) were then added to cleared supernatants. After incubation for 4 hours at 4°C, samples were incubated for 1 hour at 4°C with 30 μL of protein A Sepharose CL4B and were washed four times in 10-fold diluted lysis buffer. Proteins were eluted in sample buffer for SDS-PAGE and were analyzed by Western blotting with phosphotyrosine antibody 4G10 (UBI) and ECL.
Treatment of SKT6 cells with HCP antisense oligonucleotides. An HCP antisense oligonucleotide (5′-A*C*C*T*CACCATCCTGG*G*G*T*-3′; Operon Technologies, Inc, Alameda, CA; *denotes thiolated nucleotides) was designed to target the AUG translational start codon of murine HCP.18 A scrambled (S) oligonucleotide of an equivalent basepair composition also was synthesized (5′-*T*C*T*AGCCCAGCTGC*T*A*G*-3′; Midland Certified Reagent Co, Midland, TX) and was used in parallel control experiments. In oligonucleotide transfections, cells were washed and resuspended in Optimem I medium at 1 × 106 cells/mL. Oligonucleotides in Optimem I medium and 1% FBS (20 or 40 μmol/L) were incubated with DMRIE-C (15 μg; Life Technologies) for 30 minutes at 23°C. Liposome-oligonucleotide complexes then were incubated for 5 hours at 37°C with 7.5 × 105 cells (total volume, 1.5 mL). An equal volume of Optimem I medium containing 8% FBS was added, and cultures were exposed to EPO (±10 U/mL). Subsequently, growth medium (1 mL) was added at 24 hours of culture, and at 48 hours hemoglobinization was assayed by staining with 2,7 diaminofluorene (DAF ) as described.30 In assays of HCP expression, cells were collected, washed at 0°C in Optimem I medium, and lysed at 4°C in 1% NP-40, 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EGTA, 1 mmol/L NaF (pH 7.4), 0.1 mmol/L Na3VO4 , 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, 50 μg/mL PMSF, and 2.2 μg/mL aprotinin (3.0 × 106 cells per 0.25 mL per sample). Antibodies to HCP (1 μg; UBI) were incubated with cleared supernatants (16 hours at 4°C) and immune complexes were adsorbed to 30 μL of washed protein A Sepharose CL4B (1 hour at 4°C). Gels were washed four times in 10-fold diluted lysis buffer, and HCP was eluted in SDS-PAGE sample buffer and assayed by ECL Western blotting.
Assays of EPO-induced SKT6 cell hemoglobinization and globin expression. In assays of EPO-induced SKT6 cell hemoglobinization, cultures (2 mL) were initiated at 2.5 × 105 cells/mL and were exposed to EPO typically at 2 U/mL. At 24 hours, 1 mL of growth medium was added, and at 24, 48, and 72 hours, hemoglobin-positive cells were assayed by staining with DAF. In assays of globin expression by Western blotting, cells were washed in Optimem I medium and lysates were prepared by incubation in RIPA buffer (1% NP-40, 0.4% SDS, 0.5% sodium deoxycholate, 0.5 μg/mL leupeptin, 0.7 mg/mL pepstatin A, 50 mg/mL PMSF and 2.2 μg/mL aprotinin in phosphate-buffered saline [PBS], 0.27 mmol/L KCl, 0.15 mmol/L KH2PO4 , 13.7 mmol/L NaCl, 0.81 mmol/L Na2HPO4⋅7H2O, pH 7.4). Samples (2 × 107 cells per 1 mL of RIPA buffer) were vortexed for 3 minutes at 4°C and were microcentrifuged for 6 minutes at 4,500g. SDS sample buffer was added to cleared supernatants and heat-denatured samples were electrophoresed in 15% polyacrylamide gels. Globins were detected by Western blotting using affinity-purified antibodies to murine hemoglobin (Cappel, Durham, NC) and ECL.
FDCW2 cell culture, electrotransfection, and assays of mitogenesis. Murine myeloid FDCW2 cells32 and derived cell lines were maintained in Optimem I medium supplemented with 8% FBS, 10−5 mol/L 2-mercaptoethanol, penicillin (100 U/mL), streptomycin (0.1 mg/mL), amphotericin (0.25 mg/mL), and 8% conditioned medium from WEHI-3B cells (WEHI-3CM) as a source of IL-3. The constructs pCINeo D Bgl II-EECA and pCINeo D Bgl II-EECAY429,431F were transfected into FDCW2 cells (1 × 107 cells/mL in Optimem I medium) by electroporation (250 V, 950 μF; GeneZapper; IBI, New Haven, CT). Stably transfected lines were obtained through sequential selection in G418 (1.1 mg/mL for 14 days) and recombinant human (rh) EGF (50 ng/mL for 7 days). Cytokine-induced proliferation of derived FDCW2-EECA and FDCW2-EECAY429,431F cell lines was assayed as follows. Cells in exponential growth phase (7.0 × 105 cells/mL) were plated at 2 × 105 cells/mL in Optimem I, 8% FBS, and 10 μmol/L 2-mercaptoethanol (0.1 mL per well in a 96-well plate) and were exposed to rhEGF or IL-3 at increasing concentrations. Cells were then incubated for 2 hours with [methyl-3H] thymidine (74 Gbq/mmol; 10 μCi/mL) before harvesting and scintillation counting (1205 Betaplate counter; KBL Pharmacia, Gaithersburg, MD).
Stat5 activation assays. Stat5 activation was assayed based on binding to a biotinylated prolactin response element (PRE), 5′-XGATTTGAATTCCTAGAAATCT-3′ (X denotes biotin).30 SKT6 sublines in exponential growth (7.0 × 105 cells/mL) were exposed to EPO (±20 U/mL), washed, lysed in 10 mmol/L CHAPS, 2 mmol/L Na2 EDTA, 0.1 mmol/L Na3VO4 , 5 mmol/L NaF, 1 mmol/L DTT, 50 mmol/L PMSF, 0.5 mg/mL leupeptin, 0.7 μg/mL pepstatin A, 2.2 μg/mL aprotinin, 50 mmol/L Tris, pH 8.0. Cell lysates were sonicated (setting 4, pulse 50% for 30 seconds) and then clarified by microcentrifugation (10,000g for 5 minutes). Supernatants (175 μL), poly dIdC (10 μg), PRE cassette (250 μmol/L), and binding buffer (60 mmol/L KCl, 0.5 mmol/L Na2EDTA, 1 mmol/L DTT, 2% glycerol, 4 mmol/L Tris-HCl, 12 mmol/L HEPES, pH 7.9) were combined to yield 300 mL. Samples were incubated for 20 minutes at 4°C and 25°C and complexes then were adsorbed to streptavidin agarose CL4B (Sigma, St Louis, MO) for 45 minutes at 4°C. Samples were washed four times in binding buffer at 4°C and bound Stat5 then was eluted in 50 μL SDS sample buffer and was assayed by ECL Western blotting.
Northern blotting and ligand binding assays. Expression of the above-mentioned chimeric receptor constructs in SKT6 and FDCW2 cell derived lines was assayed by Northern blotting. Total RNA was isolated using Trizol reagent (107 cells/mL Trizol; Life Technologies), was electrophoresed in 1.5% agarose gels containing 5% formaldehyde, and was blotted to Nytran membranes (Schleicher and Schuell, Keene, NH). Heat-fixed filters were preincubated with QuikHyb solution (Stratagene, La Jolla, CA) and were hybridized to [32P]-labeled cDNA probes for 2 hours at 68°C. For ectopic EGF receptor/EPO receptor chimera transcripts, a 1,700-bp EcoRI to Bgl II fragment isolated from EECA was used in hybridizations. For GAPDH, a 550-bp Kpn I to Xho I cDNA fragment was used. Filters were washed in 2× SSC (0.3 mol/L sodium chloride, 0.03 mol/L sodium citrate, pH 7.0), 0.1% SDS at 50°C for 1 hour and exposed to film. In assays of 125I ligand binding to SKT6, SKT6-EECA, and SKT6-EECA-Y429, 431F cells, 125I-EGF (750 Ci/mmol; Amersham) at 0.5 nmol/L was incubated with cells under equilibrium conditions in the presence, or absence, of a 50-fold molar excess of unlabeled ligand. Levels of specifically bound ligand then were assayed as described previously.29
RESULTS
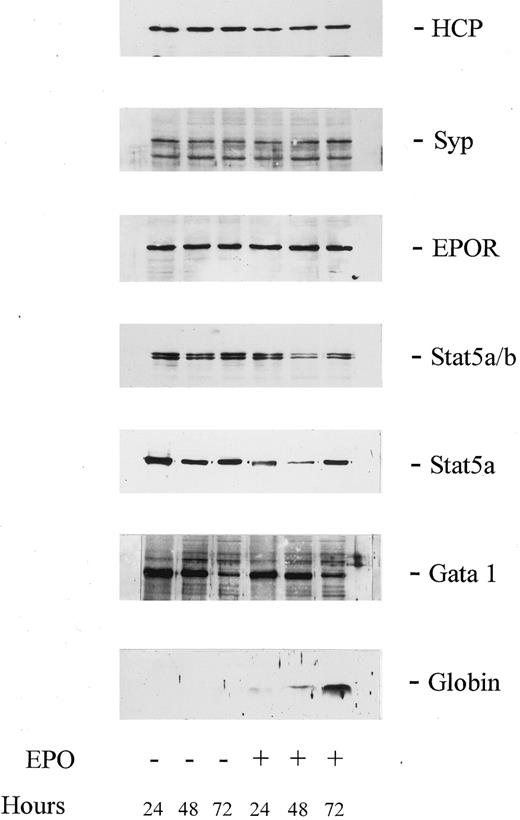
EPO activation of JAK2, EPO receptor, and STAT5 tyrosine phosphorylation and association of HCP with the EPO receptor in SKT6 cells. As introduced above, HCP recently has been shown to function in the EPO receptor system as a negative regulator of mitogenesis.12 33 However, little is known concerning possible roles for HCP during late erythroid differentiation and we presently have used SKT6 cells to investigate this prospect. Possible effects of EPO on HCP expression in SKT6 cells first were assessed, because in SKT6 cells (and CFU-e) induction of hemoglobinization requires approximately 2 days, and sufficient time exists for modulation of HCP at this level. However, as assayed by Western blotting of total cell lysates, EPO did not detectably modulate HCP expression over this time interval (Fig 1). Syp, STAT5, and EPO receptor expression levels also were assayed, with little if any modulation in expression observed. Among signaling factors analyzed, varied expression was detected only for GATA-1, with a limited yet reproducible dimunition in protein expression levels observed at 72 hours of culture. However, this latter effect occurred both in the presence and absence of EPO, and its possible significance therefore is unclear. Thus, these primary analyses discount EPO-modulation of HCP expression as a candidate regulatory mechanism.
Levels of expression of HCP, Syp, EPOR, Stat5, and GATA-1 are not modulated during EPO-induced globin expression in SKT6 cells. SKT6 cells (2.5 × 105 cells/mL) were cultured in the presence or absence of EPO (±10 U/mL) for the indicated intervals. Lysates then were prepared and were analyzed by Western blotting using antibodies to HCP, Syp, EPOR, Stat5a/b, Stat5a, GATA-1, and murine globins (as indexed in the right margin). For each lane, lysate from 2 × 105 cells was analyzed. For Syp, its correspondence to the upper protein of the two antigens detected was confirmed by the reprobing of stripped blots with a distinct Syp antibody (E.R.S., data not shown).
Levels of expression of HCP, Syp, EPOR, Stat5, and GATA-1 are not modulated during EPO-induced globin expression in SKT6 cells. SKT6 cells (2.5 × 105 cells/mL) were cultured in the presence or absence of EPO (±10 U/mL) for the indicated intervals. Lysates then were prepared and were analyzed by Western blotting using antibodies to HCP, Syp, EPOR, Stat5a/b, Stat5a, GATA-1, and murine globins (as indexed in the right margin). For each lane, lysate from 2 × 105 cells was analyzed. For Syp, its correspondence to the upper protein of the two antigens detected was confirmed by the reprobing of stripped blots with a distinct Syp antibody (E.R.S., data not shown).
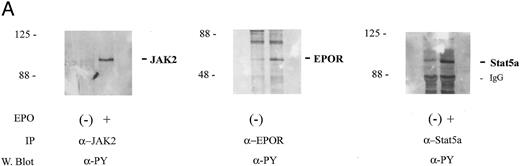
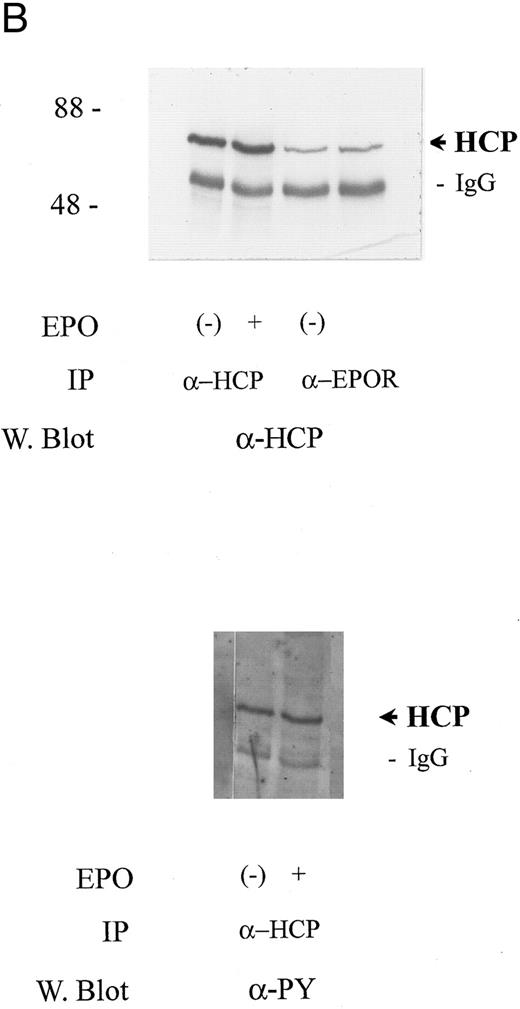
In at least certain cytokine receptor systems (c-fms, c-mpl, and c-kit), cytokine-induced tyrosine phosphorylation of HCP has been shown19,22,34 and in insulin-induced human lymphoblastic IM-9 cells this has been suggested to enhance phosphatase activity.17 Based on these reports, the possibility that EPO might regulate HCP tyrosine phosphorylation in SKT6 cells next was tested. However, in this context, it is important to consider that SKT6 cells are an erythroleukemic line derived from mice infected with the anemia-inducing strain of Friend virus (FV-A).29 In the related polycythemia-inducing Friend virus strain (FV-P), a gag-env fusion protein gp55 has been shown to constitutively activate the EPO receptor.35 FV-A likewise expresses a highly related gp55 protein,36 but this product apparently does not affect EPO signaling.37 Nonetheless, to account for possible effects of transformation on signaling in SKT6 cells, experiments were performed to establish the intactness of an EPO-activatable JAK2, EPO receptor, Stat5 signaling pathway. In EPO-stimulated cells, the induced tyrosine phosphorylation of JAK2, the EPO receptor, and Stat5a proved to be activated efficiently (Fig 2A). However, for HCP, tyrosine phosphorylation was detected but was not stimulated further upon EPO exposure (Fig 2B). This finding is consistent with the results of assays of HCP phosphorylation in mitogenic models.12,33 In addition, in at least certain mitogenic models,12 EPO has been shown to stimulate the recruitment of HCP to the EPO receptor at (P)Y429 and possibly (P)Y431 sites. However, in SKT6 cells, this effect on recruitment was not observed. Rather, HCP was observed to associate constitutively with EPO receptor complexes as demonstrated via its co-immunoprecipitation in both the absence and presence of EPO (Fig 2B, upper panel). These analyses show an intactness of a JAK2, EPO receptor, STAT5 signaling pathway in SKT6 cells and biochemically establish a association between HCP and the EPO receptor in this model system.
HCP associates constitutively with the EPO receptor in SKT6 cells but is not tyrosine phosphorylated in response to EPO. (A) SKT6 cells were exposed to EPO at the concentrations and intervals described in the Materials and Methods and Results. JAK2, EPO receptors, and Stat5a then were immunoprecipitated from Triton-X 100 lysates, and levels of tyrosine phosphorylation of these factors were assayed by Western blotting with antibodies to phosphotyrosine (α-PY). (B) SKT6 cells were exposed to EPO (±50 U/mL) for 7.5 minutes and cell lysates were prepared as detailed in the Materials and Methods. HCP and EPO receptor complexes then were isolated by immunoprecipitation (IP) and were detected by Western blotting (W. blot). In the upper panel, the coimmunoprecipitation of HCP with EPO receptor complexes is shown. This association was constitutive, was observed in three independent experiments, and was not detectably modulated by EPO exposure. In the lower panel, levels of tyrosine phosphorylation of HCP in control versus EPO-stimulated cells were compared via the Western blotting of HCP immunoprecipitates with the α-PY antibody, 4G10.
HCP associates constitutively with the EPO receptor in SKT6 cells but is not tyrosine phosphorylated in response to EPO. (A) SKT6 cells were exposed to EPO at the concentrations and intervals described in the Materials and Methods and Results. JAK2, EPO receptors, and Stat5a then were immunoprecipitated from Triton-X 100 lysates, and levels of tyrosine phosphorylation of these factors were assayed by Western blotting with antibodies to phosphotyrosine (α-PY). (B) SKT6 cells were exposed to EPO (±50 U/mL) for 7.5 minutes and cell lysates were prepared as detailed in the Materials and Methods. HCP and EPO receptor complexes then were isolated by immunoprecipitation (IP) and were detected by Western blotting (W. blot). In the upper panel, the coimmunoprecipitation of HCP with EPO receptor complexes is shown. This association was constitutive, was observed in three independent experiments, and was not detectably modulated by EPO exposure. In the lower panel, levels of tyrosine phosphorylation of HCP in control versus EPO-stimulated cells were compared via the Western blotting of HCP immunoprecipitates with the α-PY antibody, 4G10.
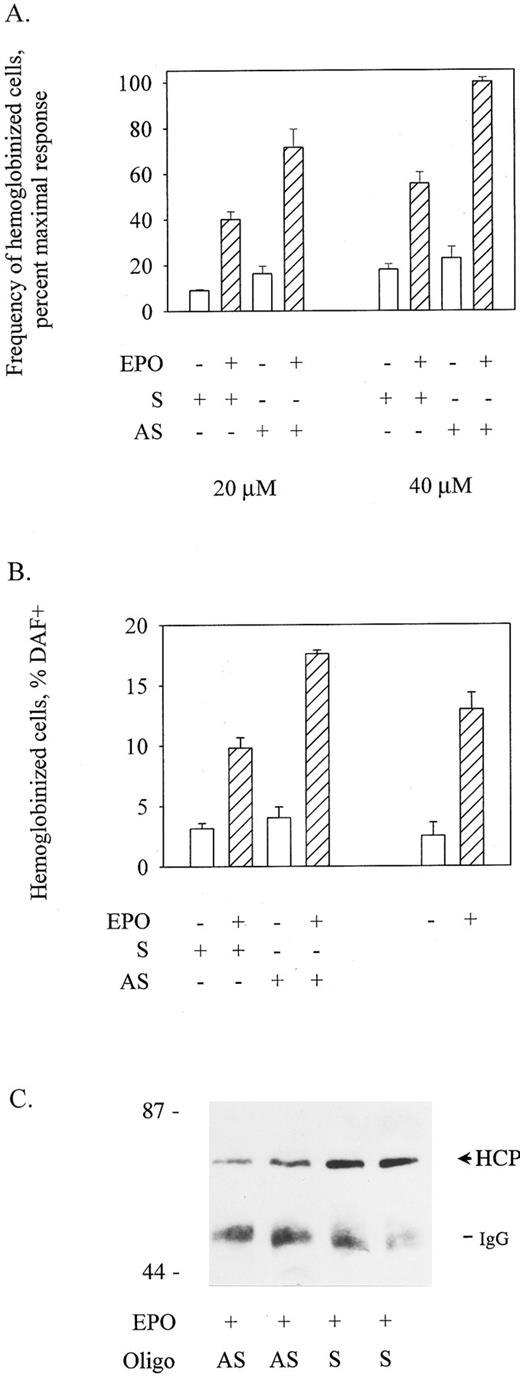
EPO-induced hemoglobinization of SKT6 cells is potentiated by antisense oligonucleotide inhibition of HCP expression and is attenuated by the enforced expression of HCP. To functionally address roles for HCP in the EPO-induced hemoglobinization of SKT6 cells, effects of the inhibited and enforced expression of HCP on this response pathway next were tested. Effects of inhibited expression were assessed using an antisense thio-oligonucleotide approach. Here, AUG-targeted and control oligonucleotides were transfected into SKT6 cells using DMRIE-C. In primary experiments, oligonucleotide concentrations of ≥60 mmol/L were observed to detectably inhibit cell growth and viability, whereas concentrations of ≤10 mmol/L had little if any reproducible effect on HCP expression. Therefore, possible effects on differentiation of exposing SKT6 cells to oligonucleotides at 20 and 40 mmol/L were investigated. At each concentration, antisense oligonucleotides (AS) but not control oligonucleotides (S; scrambled) enhanced the ability of EPO to promote hemoglobinization (Fig 3A and B). Specifically, AS oligonucleotides at 40 mmol/L increased frequencies of hemoglobinized cells to 133% of control values (P < .005; Fig 3B). By comparison, control oligonucleotides at this concentration slightly inhibited hemoglobinization (and this control effect underlines the significance of the enhancement of hemoglobinization induced by antisense oligonucleotides). Levels of HCP expression also were assayed in EPO-exposed SKT6 cells after treatment with antisense oligonucleotides. As assayed by immunoprecipitation and immunoblotting of HCP at 48 hours of EPO exposure, a twofold to threefold specific inhibition of HCP expression was affected by antisense versus control oligonucleotides at 40 mmol/L concentrations (Fig 3C). In experiments using oligonucleotides at concentrations of ≤40 μmol/L, little effect on SKT6 cell proliferation was observed. However, in this context, it is notable that EPO in this erythroleukemic model has little if any significant effect on proliferation during the presently studied time-course of induced differentiation. Thus, the above-mentioned antisense oligonucleotide experiments provided initial evidence that HCP may act to specifically downmodulate EPO signaling of hemoglobinization.
Antisense oligonucleotide-inhibition of HCP expression enhances EPO-induced SKT6 cell hemoglobinization. (A) SKT6 cells were transfected with HCP antisense (AS) or control (S) oligonucleotides at 20 μmol/L and 40 μmol/L concentrations using DMRIE-C as a liposomal agent (see the Materials and Methods). At 48 hours posttransfection, frequencies of hemoglobinized cells in EPO-exposed versus control cultures were assayed by staining with DAF. Values are means ± standard deviations for three replicate cultures and are expressed as the percentage of maximal response to EPO. (B) To further illustrate effects of the treatment of SKT6 cells with HCP antisense oligonucleotides, data from above (A; 40 μmol/L oligonucleotide exposure) are expressed directly as frequencies of hemoglobinized cells, and SKT6 cells treated with oligonucleotides are compared with cells treated in parallel with DMRIE-c alone (right histograms). (C) In experiments in which SKT6 cells were transfected with antisense (AS) versus control (S) oligonucleotides at 40 μmol/L, the specific inhibition of HCP expression was assessed. After stimulation with EPO (+10 U/mL for 48 hours) cell lysates were prepared, and levels of HCP expression were assayed by immunoprecipitation and Western blotting. Based on densitometry, an estimated twofold inhibition of HCP expression was achieved by antisense oligonucleotide treatment.
Antisense oligonucleotide-inhibition of HCP expression enhances EPO-induced SKT6 cell hemoglobinization. (A) SKT6 cells were transfected with HCP antisense (AS) or control (S) oligonucleotides at 20 μmol/L and 40 μmol/L concentrations using DMRIE-C as a liposomal agent (see the Materials and Methods). At 48 hours posttransfection, frequencies of hemoglobinized cells in EPO-exposed versus control cultures were assayed by staining with DAF. Values are means ± standard deviations for three replicate cultures and are expressed as the percentage of maximal response to EPO. (B) To further illustrate effects of the treatment of SKT6 cells with HCP antisense oligonucleotides, data from above (A; 40 μmol/L oligonucleotide exposure) are expressed directly as frequencies of hemoglobinized cells, and SKT6 cells treated with oligonucleotides are compared with cells treated in parallel with DMRIE-c alone (right histograms). (C) In experiments in which SKT6 cells were transfected with antisense (AS) versus control (S) oligonucleotides at 40 μmol/L, the specific inhibition of HCP expression was assessed. After stimulation with EPO (+10 U/mL for 48 hours) cell lysates were prepared, and levels of HCP expression were assayed by immunoprecipitation and Western blotting. Based on densitometry, an estimated twofold inhibition of HCP expression was achieved by antisense oligonucleotide treatment.
In related experiments, possible effects of the enforced ectopic expression of wt murine HCP on EPO-induced SKT6 cell hemoglobinization were investigated. Here, SKT6 cells were transfected stably with an HCP expression vector (pCINeo-wtHCP) or with an empty vector (pCINeo) as a control. G418-resistant sublines then were isolated, and the ability of EPO to induce hemoglobinization in these lines was assayed by in situ staining with diaminofluorene. To confirm any observed effects, total cell lysates also were prepared and were analyzed for globin levels by Western blotting. As shown in Fig 4 (left panels), the enforced expression of wt HCP in SKT6 cells resulted in a sixfold to sevenfold inhibition of EPO-induced hemoglobinization as assayed by DAF staining. A marked inhibition in levels of EPO-induced globin expression also was observed (Fig 4, right panels), with little if any effect exerted on cell growth (E.R.S., data not shown). Thus, the results of these experiments are consistent with the above-mentioned antisense HCP inhibition experiments and provide independent evidence that HCP may act to downmodulate EPO-induced differentiation.
The enforced expression of wt HCP in SKT6 cells inhibits EPO-induced globin expression, and hemoglobinization. (A through C) SKT6 cells were electrotransfected with pCINeowtHCP or pCINeo expression vectors, and stably transfected lines were isolated by selection in G418 (see the Materials and Methods). In assays of EPO-induced hemoglobinization and globin expression, cell lines were cultured at 2.5 × 105 cells/mL in the presence or absence of EPO (±2 U/mL). At 24, 48, and 72 hours of EPO exposure, frequencies of hemoglobinized cells were assayed by staining with DAF. Values are the means ± standard deviations of three replicate cultures. At each time interval, lysates also were prepared for assays of globin expression levels via Western blotting. For each lane, lysate from 2 × 105 cells was analyzed. For SKT6-wtHCP cells, data are for cell lines prepared from two independent transfections.
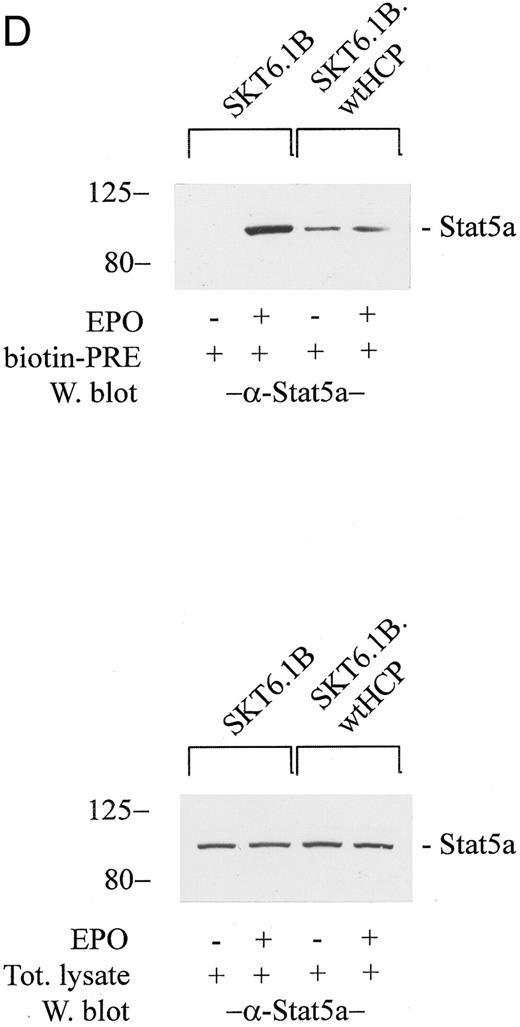
(D) In assays of EPO-induced Stat5a activation, SKT6 cell lines (parental SKT6.1B cells, and stably transfected SKT6.1B-wtHCP cells) were exposed to EPO (±50 U/mL) for 15 minutes. Lysates then were prepared and were incubated with a biotinylated PRE cassette. Levels of activated Stat5a were assayed by adsorption of Stat5a-DNA complexes to streptavidin agarose CL4B and ECL Western blotting after the elution of bound factor from washed gels (upper gels). As a control, levels of Stat5a in total lysates also were assayed by Western blotting (lower panel).
The enforced expression of wt HCP in SKT6 cells inhibits EPO-induced globin expression, and hemoglobinization. (A through C) SKT6 cells were electrotransfected with pCINeowtHCP or pCINeo expression vectors, and stably transfected lines were isolated by selection in G418 (see the Materials and Methods). In assays of EPO-induced hemoglobinization and globin expression, cell lines were cultured at 2.5 × 105 cells/mL in the presence or absence of EPO (±2 U/mL). At 24, 48, and 72 hours of EPO exposure, frequencies of hemoglobinized cells were assayed by staining with DAF. Values are the means ± standard deviations of three replicate cultures. At each time interval, lysates also were prepared for assays of globin expression levels via Western blotting. For each lane, lysate from 2 × 105 cells was analyzed. For SKT6-wtHCP cells, data are for cell lines prepared from two independent transfections.
(D) In assays of EPO-induced Stat5a activation, SKT6 cell lines (parental SKT6.1B cells, and stably transfected SKT6.1B-wtHCP cells) were exposed to EPO (±50 U/mL) for 15 minutes. Lysates then were prepared and were incubated with a biotinylated PRE cassette. Levels of activated Stat5a were assayed by adsorption of Stat5a-DNA complexes to streptavidin agarose CL4B and ECL Western blotting after the elution of bound factor from washed gels (upper gels). As a control, levels of Stat5a in total lysates also were assayed by Western blotting (lower panel).
In SKT6-wtHCP cells, experiments also were performed to test for possible effects of the forced expression of HCP on the EPO-dependent activation of Stat5. Specifically, this involved the assay of levels of Stat5 response element (PRE) binding activity in SKT6-wtHCP versus control SKT6 cells. Cells were exposed to EPO, and total cell lysates were incubated with a biotinylated PRE cassette and subsequently with streptavidin agarose. Bound, activated Stat5 then was eluted from washed gels and was assayed by ECL Western blotting (Fig 4D). In SKT6-wtHCP cells, the ability of EPO to activate Stat5 was inhibited markedly. Interestingly, an increase in basal levels of constitutively active Stat5 in SKT6-wtHCP cells also was reproducibly observed. Mechanisms underlying this latter effect presently are unclear. The results of these experiments likewise suggest that HCP acts to inhibit EPO-stimulated globin expression and at least suggest a mechanism involving HCP-inhibition of JAK2-Stat5 signaling.
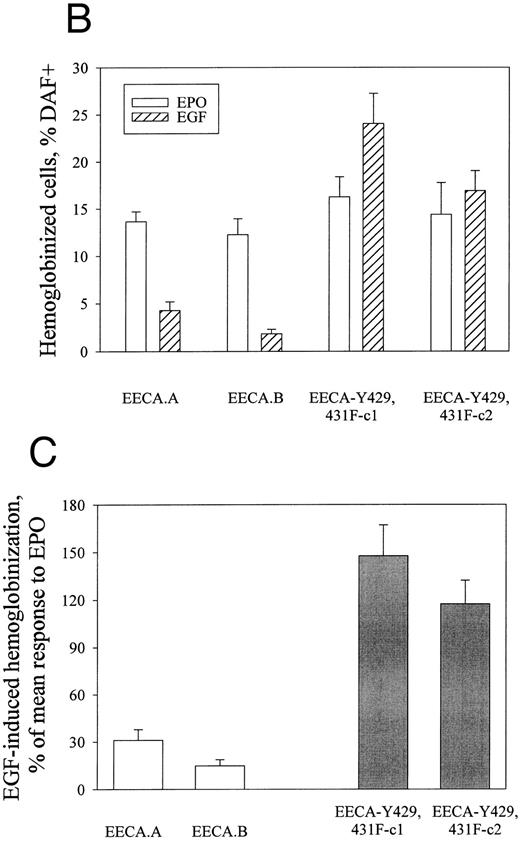
The EGF receptor/EPO receptor chimera EECA-Y429, 431F mediates enhanced hemoglobinization in SKT6 cells. To further investigate the above-indicated role for HCP in EPO-induced SKT6 cell differentiation, a chimeric receptor approach next was used. In recent studies,37 our laboratory has developed an EGF receptor/EPO receptor chimera (EECA) that signals mitogenesis in FDCW2 cells and mediates EGF-induced hemoglobinization in SKT6 cells. In the present study, a derived chimeric construct (EECA-Y429, 431F ) was prepared in which codons for residues Y429 and Y431 of the EPO receptor were mutated to encode phenylalanine (Fig 5A). In the wt EPO receptor, these (phospho)tyrosine sites have been shown to mediate the recruitment of HCP and their mutation has been shown to lead to increased activity in EPO-induced mitogenic signaling in 32D cells.12 Based on these reports, primary experiments served to compare the mitogenic activities of the chimeric receptor forms EECA and EECA-Y429, 431F using myeloid FDCW2 cells used as a proliferative model.32 FDCW2 cells were transfected stably with EECA or the point-mutated EECA-Y429, 431F constructs and mitogenic signaling via these chimeras was assayed based on EGF-stimulated rates of 3H-thymidine incorporation (Fig 5C). As predicted by studies in 32D cells,12 the mutation of HCP recruitment sites in EECA-Y429, 431F increased the mitogenic activity (at low ligand concentrations) of this chimera fourfold to fivefold as compared with the activity of wt chimera EECA.
Construction of the EGF receptor/EPO receptor chimera EECA-Y429, 431DF, and confirmation of its enhanced mitogenic activity in myeloid FDC2 cells. (A) For use in experiments designed to determine the effect of HCP recruitment in ligand-mediated SKT6 cell hemoglobinization, two EGF receptor/EPO receptor chimeras were constructed, EECA and EECA-Y429, 431F. In EECA, the extracellular domain is that of the human EGF receptor, and the transmembrane and cytoplasmic domains are those of the murine EPO receptor. In EECA-Y429, 431F, codons for tyrosine residues 429 and 431 of EECA are mutated to encode phenylalanine. (B) To confirm and to initially compare the activities of the above EECA and EECA-Y429, 431F chimeric receptors, constructs were expressed stably in myeloid FDC2 cells and their abilities to mediate mitogenesis were assayed based on rates of EGF-stimulated [methyl-3H] thymidine incorporation. Values are means (±standard deviations) for triplicate cultures.
Construction of the EGF receptor/EPO receptor chimera EECA-Y429, 431DF, and confirmation of its enhanced mitogenic activity in myeloid FDC2 cells. (A) For use in experiments designed to determine the effect of HCP recruitment in ligand-mediated SKT6 cell hemoglobinization, two EGF receptor/EPO receptor chimeras were constructed, EECA and EECA-Y429, 431F. In EECA, the extracellular domain is that of the human EGF receptor, and the transmembrane and cytoplasmic domains are those of the murine EPO receptor. In EECA-Y429, 431F, codons for tyrosine residues 429 and 431 of EECA are mutated to encode phenylalanine. (B) To confirm and to initially compare the activities of the above EECA and EECA-Y429, 431F chimeric receptors, constructs were expressed stably in myeloid FDC2 cells and their abilities to mediate mitogenesis were assayed based on rates of EGF-stimulated [methyl-3H] thymidine incorporation. Values are means (±standard deviations) for triplicate cultures.
Next, the chimeric constructs EECA and EECA-Y429, 431F were transfected stably into SKT6 cells to directly test roles for HCP in regulating erythroid differentiation. For derived SKT6-EECA and SKT6-EECA-Y429, 431F sublines, expression of chimeric constructs was assayed by Northern blotting. Expression of each construct first was confirmed using a probe derived from an EGF receptor cDNA (Fig 6A). Expression levels for EECA were estimated to be approximately twofold greater than EECA-Y429, 431F, whereas no EGF receptor expression was detected in parental SKT6 cells. Second, levels of expression of these chimeras were compared with these of endogenous EPO receptors using a probe derived from the 3′ domain of the EPO receptor. In these analyses, endogenous EPO receptor expression levels were shown to be threefold greater than levels of ectopically expressed chimeric receptor transcripts (E.R.S., data not shown). Finally, for SKT6-EECA versus SKT6-EECA-Y429, 431F cells, equilibrium binding assays using 125I-EGF confirmed approximate equivalence in the cell surface expression of these chimeric receptor forms (300 to 400 receptors per cell v approximately 800 receptors per cell for parental SKT6 cells).29
The EGF receptor/EPO receptor chimera EECA-Y429, 431F mediates SKT6 cell hemoglobinization at enhanced levels (versus the control chimeric construct EECA). (A) Levels of expression of EECA and EECA-Y429, 431F receptor forms in stably transfected SKT6 cell lines were assayed by Northern blotting. Hybridization was performed using a 1,700-bp EcoRI → Bgl II cDNA fragment of ECCA (see the Materials and Methods). (B) SKT6 cells were electrotransfected with pCINeo vectors encoding the chimeric receptors EECA or EECA-Y429, 431F, and stably transfected cell lines (SKT6-EECA and SKT6-EECA-Y429, 431F ) were isolated by culture in G418 (see the Materials and Methods). In these derived lines, the ability of these chimeric receptors to mediate EGF-induced hemoglobinization then was compared. Briefly, cells (2.5 × 105 cells/mL) were exposed to EGF (±25 ng/mL) or EPO (±2 U/mL) for 72 hours and the frequencies of ligand-induced hemoglobinization were assayed by in situ staining with DAF. Values are means ± standard deviations for three replicate cultures and are corrected for background (ie, low levels of DAF-positive cells observed in the absence of EGF or EPO are subtracted). Two clones from each electrotransfection are represented. (C) To provide for a more direct comparison of the activities of EECA and EECA-Y429, 431DF receptor forms, data from above (A) for SKT6-EECA and SKT6-EECA-Y429, 431DF cell lines are compared directly with frequencies of hemoglobinization in these lines as induced by EPO in parallel cultures. (D) To confirm results obtained via the staining of hemoglobinized SKT6 cells with DAF, levels of globin in lysates from the above cultures also were assayed by Western blotting.
The EGF receptor/EPO receptor chimera EECA-Y429, 431F mediates SKT6 cell hemoglobinization at enhanced levels (versus the control chimeric construct EECA). (A) Levels of expression of EECA and EECA-Y429, 431F receptor forms in stably transfected SKT6 cell lines were assayed by Northern blotting. Hybridization was performed using a 1,700-bp EcoRI → Bgl II cDNA fragment of ECCA (see the Materials and Methods). (B) SKT6 cells were electrotransfected with pCINeo vectors encoding the chimeric receptors EECA or EECA-Y429, 431F, and stably transfected cell lines (SKT6-EECA and SKT6-EECA-Y429, 431F ) were isolated by culture in G418 (see the Materials and Methods). In these derived lines, the ability of these chimeric receptors to mediate EGF-induced hemoglobinization then was compared. Briefly, cells (2.5 × 105 cells/mL) were exposed to EGF (±25 ng/mL) or EPO (±2 U/mL) for 72 hours and the frequencies of ligand-induced hemoglobinization were assayed by in situ staining with DAF. Values are means ± standard deviations for three replicate cultures and are corrected for background (ie, low levels of DAF-positive cells observed in the absence of EGF or EPO are subtracted). Two clones from each electrotransfection are represented. (C) To provide for a more direct comparison of the activities of EECA and EECA-Y429, 431DF receptor forms, data from above (A) for SKT6-EECA and SKT6-EECA-Y429, 431DF cell lines are compared directly with frequencies of hemoglobinization in these lines as induced by EPO in parallel cultures. (D) To confirm results obtained via the staining of hemoglobinized SKT6 cells with DAF, levels of globin in lysates from the above cultures also were assayed by Western blotting.
Subsequent experiments tested the abilities of EECA and EECA-Y429, 431F receptors to mediate ligand-induced SKT6 cell hemoglobinization and globin expression. Low yet reproducible activity was observed for EECA (Fig 6B), and this is consistent with the activity levels for this control chimera as observed in independently transfected SKT6 sublines.30 By comparison, in SKT6-EECA-Y429, 431F cells, hemoglobinization was induced by EGF at frequencies 500% to 600% greater than in control SKT6-EECA cells, and this effect was confirmed in Western blot analyses of globin levels in these cells (Fig 6C). Thus, the results of these experiments provide further direct evidence that HCP plays an important inhibitory role in modulating EPO-induced globin expression.
DISCUSSION
In the present study, murine erythroleukemic SKT6 cells have been used as a model to investigate possible regulatory roles for HCP during EPO-induced late erythroid differentiation. No significant effects of EPO on the expression or tyrosine phosphorylation of HCP were observed. However, HCP was shown to associate constitutively with the EPO receptor in this system. To test possible functional roles for HCP in EPO-induced differentiation, three approaches were applied. First, the specific inhibition of HCP expression by antisense oligonucleotides was observed to result in the enhanced hemoglobinization of EPO-exposed SKT6 cells. Second, upon its enforced ectopic expression in stably transfected SKT6 cells, wt HCP was shown to efficiently inhibit the ability of EPO to promote globin expression, and hemoglobinization. Third, the ligand-induced hemoglobinization of SKT6 cells was observed to be mediated at fivefold to sixfold enhanced levels by an EGF receptor/EPO receptor chimera in which HCP recruitment sites Y429 and Y431 were mutated (EECA-Y429, 431F ). Thus, these studies in SKT6 cells show a novel role for HCP in downmodulating the inductive effects of EPO on globin expression during late erythroid differentiation.
As introduced above, the majority of studies of HCP function to date have focused on its established role as a negative regulator of HGF-induced mitogenesis. This includes effects exerted by HCP on mitogenic signaling in the EPO receptor system as studied in vitro12 and in erythroid splenocytes derived from mev/mev mice.27 In vitro studies have provided insight into molecular mechanisms of HCP action, and evidence has been generated to suggest that HCP may at least in part act by dephosphorylating and inactivating of JAK2.12,38 In 32D cells stably expressing a Y429, 431 EPO receptor mutant that is unable to bind HCP, the EPO-induced tyrosine phosphorylation of JAK2 was shown to be sustained and proliferation was supported by lowered concentrations of EPO.12 In addition, upon their overexpression in Cos cells, HCP has been shown to mediate the dephosphorylation of EPO receptor-JAK2 complexes.38 However, in these systems, differentiation is not induced by EPO and no opportunity is provided to consider possible roles that HCP might play in this response pathway. By comparison, our recent establishment of stable SKT6 cell sublines that efficiently hemoglobinize in response to EPO30 offers a unique opportunity to investigate the possibility that HCP might act as an important modulator of late erythroid differentiation. As summarized above, the experiments presently reported point to a previous unidentified role for HCP as a negative regulator of EPO-induced globin expression and SKT6 cell hemoglobinization. Possible mechanisms that may mediate this action of HCP are considered below.
Based on the apparent negative regulation exerted by HCP on JAK2 during EPO-induced mitogenesis in 32D cells,12 JAK2-dependent pathways comprise one candidate target for HCP during EPO-induced differentiation in SKT6 cells. To date, we have not quantitatively compared profiles of ligand-induced JAK2 tyrosine phosphorylation this model. However, the observed inhibition of the ability of EPO to stimulate the DNA binding of Stat5 in SKT6-wtHCP cells (Fig 4D) is consistent with an HCP-JAK2–dependent mechanism of HCP action. In addition, this HCP-dependent inhibition of Stat5 activation in SKT6 cells also at least suggests that Stat5 might play an integral role during EPO-induced globin expression in this model. Independent support for this prospective role for Stat5 is provided by two recent studies. First, we recently have shown that high differentiation signaling activity in SKT6 cells retained by an ectopically expressed truncated EGF receptor-EPO receptor chimera that lacks seven of eight EPO receptor cytoplasmic (P)Y sites, yet retains (P)Y343 as a singular site for Stat5 binding.30 Second, we also recently have shown that EPO-dependent signaling of globin expression and hemoglobinization in SKT6 cells is inhibited markedly upon the forced expression of a dominant negative form of Stat5a.39 Thus, with regards to HCP action, these findings and the present study uniformly point to a mechanism whereby this HCP acts to inhibit differentiation signaling in SKT6 cells by targeting a JAK2/Stat5-driven pathway. However, it is important to note that this has not yet been shown directly and that other important targets for HCP may exist. Specifically, in thymocytes derived from me/me mice, increased tyrosine phosphorylation and activity of the Src kinases Lck21 and Fyn21 have been reported, and in erythroleukemic J2E cells an apparent role for Lyn in EPO-induced globin expression recently has been described.40 Thus, Lyn in particular comprises one alternate potential target for HCP that merits investigation.
Mechanisms that regulate the recruitment and activity of HCP within the EPO receptor and related HGF receptor systems also are of interest to consider. In the EPO receptor system, recruitment of HCP in mitogenic models (including 32D, DA-3, and BaF3 cells)12,33 has been shown to be stimulated by EPO, and this appears to depend on JAK2-mediated phosphorylation of receptor sites Y429 and Y431.12 In contrast, in erythroid SKT6 cells, HCP presently is shown to occur in constitutive association with EPO receptor complexes (see Results and Fig 2). This observed difference in recruitment mechanisms might be explained by one of two alternate explanations. First, HCP may exist in constitutive association with EPO receptor complexes selectively in erythroid cells or selectively during differentiation programming. The constitutive association of HCP with HGF receptor complexes is not uncommon and is observed in the KIR, somatostatin, and interferon α/β receptor systems.7,23,41 In addition, the direct binding of HCP to JAK2 in Cos cells also has been reported.38 Alternatively, the constitutive binding of HCP to EPO receptors in erythroleukemic SKT6 cells somehow may be associated with transforming events, including events affected by FV-A infection, and attenuation of differentiation by HCP in this system, in fact, may contribute to sustained proliferation.
Finally, it is noteworthy that apparent roles for HCP recently have been described in several additional hematopoietic differentiation response pathways. These include an induction of HCP phosphatase activity in HL-60 cells upon phorbol myristate acetate (PMA)-induced monocytic differentiation42 and a demonstrated role for HCP (via the expression of a dominant-negative HCP mutant) in NK-92 cells in NK cell inhibitory receptor p58-dependent target cell lysis.7 In these response pathways, the nature of substrates for HCP are largely undefined and, as shown in the CSF-1 receptor system HCP action, may be affected by several additional associated cofactors, including Grb2 and p130.22 Whether such cofactors and/or alternate substrates might likewise mediate HCP action during the EPO-induced differentiation of SKT6 cells is the subject of ongoing investigations.
ACKNOWLEDGMENT
The authors thank Tamara T. Reese and Richard C. Gregory for the construction of pCINeo D BglIIEECA and FDCW2-EECA cells, respectively. We also thank Dr Benjamin Neel (Beth Israel Hospital, Boston, MA) for the generous provision of murine HCP cDNA clones and Amgen (Thousand Oaks, CA) for the generous provision of rhEPO.
Supported by Grants No. NIH DK 40242 and RCDA HL 03042 to D.M.W.
Address reprint requests to D.M. Wojchowski, PhD, 115 William L. Henning Bldg, The Pennsylvania State University, University Park, PA 16802.


![Fig. 5. Construction of the EGF receptor/EPO receptor chimera EECA-Y429, 431DF, and confirmation of its enhanced mitogenic activity in myeloid FDC2 cells. (A) For use in experiments designed to determine the effect of HCP recruitment in ligand-mediated SKT6 cell hemoglobinization, two EGF receptor/EPO receptor chimeras were constructed, EECA and EECA-Y429, 431F. In EECA, the extracellular domain is that of the human EGF receptor, and the transmembrane and cytoplasmic domains are those of the murine EPO receptor. In EECA-Y429, 431F, codons for tyrosine residues 429 and 431 of EECA are mutated to encode phenylalanine. (B) To confirm and to initially compare the activities of the above EECA and EECA-Y429, 431F chimeric receptors, constructs were expressed stably in myeloid FDC2 cells and their abilities to mediate mitogenesis were assayed based on rates of EGF-stimulated [methyl-3H] thymidine incorporation. Values are means (±standard deviations) for triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2175/3/m_bl_0047f5a.jpeg?Expires=1763469152&Signature=Ir5WTVsDq8WgbP2xmC6VOS0KTFO6NEZ4n~O3hFogx89Cs6ii4zzF5OADqV47kfKmtSYVs7rFZeRfDg-U7rV~T3UK08SRHpUWeXKyxKaO~MOLI7EFedF8L7VMH7TWAsG8QPeB0oC0UNtY86g7l9Yq~1LdWkr~35yQj9TPz7-sz~t6uXBzAW5d-GFvK4JI-FyrQ3wYtfJ6C-DEjAl6iQiMQB3MENjrjHbhyao2ipO6yBOvGvLFiO9~nfBNJYHIhC2wy3NuZj5xOpmPOKyh4kcr3LxNhCbKllXIRYvpEWQhnz1zBtGxf-YOhzAR7PHQVVCMbH717PobHgFEAtPQu~0HwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Construction of the EGF receptor/EPO receptor chimera EECA-Y429, 431DF, and confirmation of its enhanced mitogenic activity in myeloid FDC2 cells. (A) For use in experiments designed to determine the effect of HCP recruitment in ligand-mediated SKT6 cell hemoglobinization, two EGF receptor/EPO receptor chimeras were constructed, EECA and EECA-Y429, 431F. In EECA, the extracellular domain is that of the human EGF receptor, and the transmembrane and cytoplasmic domains are those of the murine EPO receptor. In EECA-Y429, 431F, codons for tyrosine residues 429 and 431 of EECA are mutated to encode phenylalanine. (B) To confirm and to initially compare the activities of the above EECA and EECA-Y429, 431F chimeric receptors, constructs were expressed stably in myeloid FDC2 cells and their abilities to mediate mitogenesis were assayed based on rates of EGF-stimulated [methyl-3H] thymidine incorporation. Values are means (±standard deviations) for triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2175/3/m_bl_0047f5b.jpeg?Expires=1763469152&Signature=DPmcbrldFlOCLo2OzdQlEclVR7CBRWeuP5pE~hKlQs7vvrxwz1Cax6~zJoSty35Z4v2YAtWFFJmZK4b2Px0e2MKETqpEUNRWHl2qQ6d8F5nvu0wDdE7rRCQ~mEZ0VBbuXPAPJUcNNvGuRLIgEUb2dU3lEozA7TbM2XEawGKyntmEiGpYx~1NCG~f216SGChYwOWyOeSsdhxwyffPidYYzB8Gx0-N5ZF9OpiKY7GtmxMAbUzKy1p2YIY9-Q6REpq9cCDAYNlcz6hCB8fy0oD63VlPBRkHIpmY-rE2dqtlxL2v-j564BwutgL3QZ7VtdU39HKQZW9FBEpzNTI9aFslSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal