Abstract
Bone marrow (BM) trephine biopsies from 15 pediatric patients with acute lymphoid (ALL) or myeloid (AML) leukemia were engrafted subcutaneously into severe combined immunodeficiency (SCID) mice conditioned by 200 cGy total-body irradiation. Implants were harvested 5 to 19 weeks later for histologic, cytologic, and/or flow cytometric analysis of the residing marrow. Eighteen of 19 grafts contained viable human leukemic cells to various extents as assessed by one or more of these methods. Thirteen of 14 implants analyzed by flow cytometry included high numbers of tumor cells, accounting for 85% to 100% of the total nucleated cells in seven of them. Histologically, engrafted marrow samples exhibited areas of blastic infiltration, and tumor-specific gene rearrangements were retrieved in long-term engrafted biopsies. Importantly, engrafted mice remained perfectly healthy even 5 months posttransplantation, and no human tumor cell dissemination was detected in the hematolymphoid and nonhematopoietic tissues at the time of autopsy. These results demonstrate that human malignant hematopoiesis can be sustained long-term in its original, intact marrow stromal environment transplanted in appropriately conditioned immunodeficient mice.
EXPERIMENTAL MODELS of human hematopoiesis in tolerant laboratory animals have multiplied since the transplantation of human hematopoietic cell suspensions1,2 and solid blood-forming tissues3 into severely immunodeficient mice was reported in 1988. These two distinct engraftment strategies for human hematopoietic cells, ie, in the presence or absence of the natural stromal microenvironment, be it thymus or bone marrow (BM) have been used in generally distinct areas of research.
Severe combined immunodeficiency (SCID)-hu chimeras are made by surgical implantation in SCID mice4 of human fetal thymus,3 to which fetal liver can be added as a source of precursor cells to ensure long-term thymopoiesis.5 Human fetal BM also remains hematopoietic over extended periods of engraftment in SCID mice.6,7 Such experimental systems in which human hematogenous tissues are maintained in the mouse host in their intact three-dimensional architecture have been used to address several aspects of human intrathymic development,8 thymus infection by HIV,9 and bone marrow physiology.10 Human fetal hematopoietic stromas implanted in SCID mice have also yielded assay systems for human hematopoietic stem cells.6 11-14
Human hematopoietic cells of blood or BM origin also engraft immunodeficient mice on intravenous (IV) or intraperitoneal (IP) injection,1,2 although only low numbers of human cells can be later retrieved from the mouse marrow and spleen and human clonogenic progenitors are only transiently detected. Treatment of recipient mice with the Pixy 321 IL-3/GM-CSF fusion protein dramatically improved the model in terms of the number of human cells present, whereas all categories of colony-forming progenitors were detected over extended periods of engraftment.15 Several facets of the healthy or diseased human hematopoietic system have been examined in mouse xenochimeras harboring human hematolymphoid cells in their own blood-forming tissues, including stem cell engraftment and differentiation,16,17 immune responses,18,19 and autoimmune diseases.20
Several groups have also reported the inoculation of dispersed human hematopoietic tumor cells into SCID mice.21-23 In the case of acute lymphoid leukemias (ALLs), T-ALL and pre-B-ALL blasts broadly disseminated in SCID mice on IV injection along a highly reproducible pattern mimicking disease aggressiveness in the donor patient, and often causing the death of the mouse host.24,25 Some myelogenous leukemia cell samples also engrafted when injected into SCID mice, although the success rate was much lower than with lymphoid leukemias. A refined human/SCID mouse model of acute myelogenous leukemia (AML) was devised by injecting high numbers of blasts into Pixy 321–treated SCID mice, which dramatically improved the engraftment rate.26 Advantage was taken of that experiment to identify putative leukemic “stem” cells inside the CD34+ CD38− cell population, since cells displaying this early immature phenotype sorted from AML marrow reinitiated tumor development when injected into SCID mice.26 In the same line, CD34+CD38− stem cells for the rare but aggressive human juvenile chronic myelogenous leukemia (CML) have been recently characterized by their ability to establish the tumor in primary and secondary SCID mouse recipients.27
Altogether, these data indicated that human leukemias can be closely approximated following tumor cell injection into SCID mice. The resulting xenochimeric models are amenable to investigations pertaining to the biology of the leukemic blasts, as well as to the development of strategies to control tumor cell expansion.28-31
Yet in the presently described experimental systems, factors involved in human tumor cell expansion and dissemination besides administered growth factors are provided by tissues of the mouse host. As already stressed by Uckun,23 SCID mouse microenvironments are not likely to be representative of microenvironments in human organs. It is conceivable that these xenogeneic cellular environments modify some characteristics of the engrafted malignant cells. Indeed, T-ALL–derived cell lines underwent dramatic phenotypic changes when transplanted into SCID mice.32 Lücking-Famira et al30 also observed, albeit more rarely, CD3 upregulation on T-ALL blasts engrafted in SCID mice. On the other hand, transferring the intact cellular support of human malignant hematopoiesis into a laboratory animal could help to experimentally evaluate the debated existence of a “leukemic stroma.”33 We therefore considered transplanting human leukemic cells inside their natural microenvironment into tolerant mice. To that end, combining the observations that intact human fetal bones can sustain long-term hematopoiesis on engraftment into immunodeficient mouse recipients and that these animals are also appropriate hosts for human leukemic cells, we implanted BM biopsies from AML and ALL pediatric patients into SCID mice.
MATERIALS AND METHODS
Human Tissues
BM samples were obtained, with informed consent of the parents, from 11 and three pediatric patients admitted to the Robert Debré Children's Hospital for ALL and AML, respectively, between May 1994 and June 1995 (Table 1). Bone trephine biopsies were made at the same time BM was harvested under general anesthesia, for diagnosis. Normal marrow cells were from aspirations performed on donors for transplantation.
ALL and AML Marrow Biopsy Donor Patients: Clinical Observations
| Patient No. . | Sex/Age (yr) . | Diagnosis . | Risk Factor Level . | Evolution . |
|---|---|---|---|---|
| R19 | M/9 | Pre-pre-B-ALL | High | Relapse after 6 mo |
| R20 | M/3 | Pre-pre-B-ALL (first relapse) | Standard | Relapse after 3 yr; alloBMT |
| R28 | M/5.5 | Pre-pre-B-ALL | Standard | CCR |
| R29 | F/5 | Pre-pre-B-ALL | Standard | CCR |
| R31 | M/12 | AML (first relapse) | High | Death 16 mo after first relapse |
| R35 (relapsed R19) | M/9 | Pre-pre-B-ALL | High | Death 8 mo after first relapse |
| R36 | M/4 | Pre-pre-B-ALL | Standard | CCR |
| R38 | F/15 | AML | High | AlloBMT; CCR |
| R39 | F/8 | Pre-pre-B-ALL (first relapse) | Standard | AlloBMT; CCR |
| R41 | M/3.5 | Pre-pre-B-ALL | Standard | CCR |
| R43 | M/2 | Pre-pre-B-ALL | Standard | CCR |
| R44 | M/2 | Pre-pre-B-ALL | High | CCR |
| R46 | F/3.5 | AML-2 | High | CCR |
| R48 | F/14 | ALL | Standard | CCR |
| R49 | M/6 | Pre-pre-B-ALL | Standard | CCR |
| Patient No. . | Sex/Age (yr) . | Diagnosis . | Risk Factor Level . | Evolution . |
|---|---|---|---|---|
| R19 | M/9 | Pre-pre-B-ALL | High | Relapse after 6 mo |
| R20 | M/3 | Pre-pre-B-ALL (first relapse) | Standard | Relapse after 3 yr; alloBMT |
| R28 | M/5.5 | Pre-pre-B-ALL | Standard | CCR |
| R29 | F/5 | Pre-pre-B-ALL | Standard | CCR |
| R31 | M/12 | AML (first relapse) | High | Death 16 mo after first relapse |
| R35 (relapsed R19) | M/9 | Pre-pre-B-ALL | High | Death 8 mo after first relapse |
| R36 | M/4 | Pre-pre-B-ALL | Standard | CCR |
| R38 | F/15 | AML | High | AlloBMT; CCR |
| R39 | F/8 | Pre-pre-B-ALL (first relapse) | Standard | AlloBMT; CCR |
| R41 | M/3.5 | Pre-pre-B-ALL | Standard | CCR |
| R43 | M/2 | Pre-pre-B-ALL | Standard | CCR |
| R44 | M/2 | Pre-pre-B-ALL | High | CCR |
| R46 | F/3.5 | AML-2 | High | CCR |
| R48 | F/14 | ALL | Standard | CCR |
| R49 | M/6 | Pre-pre-B-ALL | Standard | CCR |
Abbreviations: CCR, continuous complete remission; BMT, BM transplantation.
SCID-hu Mouse Construction and Follow-Up Evaluation
C.B.17 scid/scid (SCID) mice were bred aseptically in our own facility. Six- to 8-week-old mice were conditioned 24 hours before transplantation by total-body irradiation with a single dose of 200 cGy γ-rays delivered by a 137Cs source. Larger BM biopsies were divided into two fragments, 5 to 10 mm in length, which were implanted subcutaneously on each side of the back in anesthetized mice. One of the grafts was removed for analysis 5 to 6 weeks posttransplant from the anesthetized host from which blood was also taken by retro-orbital puncture. Mice were killed 3 to 7 weeks later for harvesting the second implant. Smaller bone biopsies were implanted undivided into mice under identical conditions and harvested for analysis from the dead host 8 to 19 weeks later. On a total of 11 engrafted mice, the lungs (six of 11), heart (one of 11), liver (nine of 11), long bones (nine of 11), spleen (nine of 11), thymus (three of 11), pancreas (two of 11), mesentery (one of 11), kidneys (six of 11), and peripheral blood (PB) (11 of 11) were also harvested for histologic and cytologic examination, even in the absence of macroscopic evidence of neoplastic dissemination.
Cytologic and Histologic Analyses
Single cell suspensions were prepared mechanically from the murine BM, spleen, and liver. Human bone grafts were opened along the longer axis, and the enclosed marrow was dissociated by treatment with 0.05% collagenase (Boehringer, Mannheim, Germany) in Ca,Mg-free phosphate-buffered saline (PBS) for 30 minutes at 37°C. Mononuclear cells were isolated from mouse marrow and blood by centrifugation over Ficoll (Lymphoprep; Pharmacia, Uppsala, Sweden) and then washed in PBS. Mouse PB and BM and human BM cells were centrifuged onto gelatin-coated glass slides and then stained with May-Grünwald/Giemsa. Blasts per 100 total cells were counted under the microscope. In parallel, mouse long bones, spleen, liver, lung, heart, kidney, thymus, pancreas, and mesentery and human bone grafts were fixed with 10% Formalin. The mouse bones and human bone grafts were also decalcified for 2 to 4 hours with nitric acid 6.5% and then extensively water-rinsed. Samples were embedded in paraffin; 3-μm thickness microtome sections were stained with hematoxylin and eosin (HE), and also with Giemsa and Gordon-Sweet dyes in the case of human graft tissues.
Flow Cytometry
Single mononuclear cell suspensions were prepared as already described from mouse BM, spleen, liver, and PB and human graft marrow and then adjusted to 105 cells per tube, which were double-stained with the following monoclonal antibodies: CD19, CD15, and CD33 (Dako, Glostrup, Denmark) directly conjugated with phycoerythrin (PE), and CD45 (Dako) revealed with an anti-mouse Ig reagent conjugated with fluorescein isothiocyanate (FITC). PE- and FITC-conjugated IgG isotype controls (Dako) were used in all experiments. Stained cells were exposed to propidium iodide (PI) for dead-cell exclusion and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using Lysis II software (Becton Dickinson).
Analysis of Gene Rearrangements
DNA preparation.Patients' BM samples were collected on EDTA before induction therapy, and mononuclear cells were separated over Ficoll. Graft marrow cells were dispersed mechanically, and then washed in 0.9% NaCl. Remaining erythrocytes were lysed in 1% Triton X-100 (Sigma, St Louis, MO), 5 mmol/L MgCl2 , 0.32 mmol/L sucrose, and 10 mmol/L Tris HCl, pH 7.5, and pelleted cells were stored at −80°C. Thawed samples were centrifuged at 10,000 rpm, and the cells were lysed in 0.45% Tween-20 (Difco, Detroit, MI), 0.45% Nonidet P-40 (Sigma), 2.5 mmol/L MgCl2 , and 10 mmol/L Tris HCl, pH 7.5, and incubated for 2 hours at 60°C in the presence of 1 mg/mL proteinase K. Cells were adjusted to 107/mL. After 10 minutes at 95°C, lysates were stored at −20°C.
Polymerase chain reaction.The reaction mixture (25 μL) consisted of 10 mmol/L Tris HCl, pH 8.3, 50 mmol/L KCl, 2.5 mmol/L MgCl2 , 0.5 U Taq polymerase (Appligene, Strasbourg, France), 0.2 mmol/L dNTP, 0.4 mmol/L spermidine, 20 pmol of each primer (Genset, Paris, France), and 3 μL cell lysate. Polymerase chain reaction (PCR) was performed with a fluorescein-labeled 5′ upstream primer. The following primers were used34,35: γ chain: Vγ1L, 5′-TACCTACACCAGGAGGGGAAGGCCCCA-3′; J1J2L (fluorescent primer), 5′-TCCTGCTTTCCCTCTATTACCTTGGAAATGTTTG-3′; Vγ9L, 5′-AGTTCCTGGTGTCCATTTCATATGACGGC-3′; δ chain: Vδ2 (fluorescent primer), 5′-TGGCCCTGGTTTCAAAGACAATTTCCA-3′; Dδ3-AS, 5′-TGCTTGCTGTGTTTGTCTCCTGAGGCA-3′; and IgH chain: VH, 5′-ACACGGC(C/T)(G/C)TGTATTACTGT-3′; JH (fluorescent primer), 5′-GTGACCAGGGT(A/C/G/T)CCTTGGCCCCAG-3′. The “hot-start PCR” procedure recommended for the detection of rare sequences was used. The PCR was started by mixing template DNA and Taq polymerase at 80°C, followed by 30 to 35 2-minute cycles at the appropriate hybridization temperature in an UNO-Thermoblock (Biometra, Göttingen, Germany) (Table 2). Each PCR series included two negative controls, one without DNA and the other with DNA from polyclonal cells. Amplification was monitored by electrophoresis in 2% agarose gels followed by ethidium bromide staining.
PCR Conditions for Different Sets of Primers
| Primer . | Hybridization Temperature (°C) . | MgCl2 Concentration (mmol/L) . | Mean Size of the Products (bp) . |
|---|---|---|---|
| Vγ1L-J1J2Lc | 63 | 2.5 | 295 |
| Vγ9L-J1J2Lc | 63 | 2.5 | 270 |
| Vδ2c-Dδ3-AS | 61 | 2.5 | 250 |
| VH-JHc | 58 | 2.5 | 96 |
| Primer . | Hybridization Temperature (°C) . | MgCl2 Concentration (mmol/L) . | Mean Size of the Products (bp) . |
|---|---|---|---|
| Vγ1L-J1J2Lc | 63 | 2.5 | 295 |
| Vγ9L-J1J2Lc | 63 | 2.5 | 270 |
| Vδ2c-Dδ3-AS | 61 | 2.5 | 250 |
| VH-JHc | 58 | 2.5 | 96 |
Analysis of PCR products.PCR products were analyzed using a fluorescent automated laser DNA sequencer (A.L.F., Pharmacia LKB Biotechnology). Electrophoresis was performed on a 6% long ranger gel (Bioprobe, Paris, France) containing 8 mol/L urea in a 0.6× Tris-borate-EDTA buffer. PCR products were loaded onto the sequencer after denaturation at 100°C for 5 minutes and then submitted to electrophoresis. Gene rearrangements in a polyclonal lymphoid population are heterogeneous in length and give rise to a gaussian distribution of PCR products. Conversely, a discrete peak is obtained when a rearrangement is present in a clonal population.
RESULTS
Twelve pre-pre-B-ALL and three AML BM biopsies (Table 1) were implanted subcutaneously into SCID mice conditioned by 200 cGy total-body irradiation and were maintained therein for 5 to 19 weeks. When large enough, the biopsies were divided into two pieces that were separately engrafted in the same host, so that two grafts from the same patient could be analyzed at different time points.
Absence of human tumor cell spread in SCID mice engrafted with human ALL and AML BM biopsies.None of the engrafted mice ever developed clinical symptoms suggesting pathogenic human tumor cell dissemination in the tissues of the host. No human cells could even be detected by flow cytometry in cell suspensions prepared from the PB, BM, liver, and spleen of recipient mice and double-stained with CD45 and CD19 for ALL grafts and CD15 and CD33 for AML implants (not shown). Mouse host tissues were also analyzed histologically after HE staining of paraffin sections. No evidence of human blast cell infiltration in mouse BM, liver, spleen, lung, heart, kidney, thymus, pancreas, and mesentery was seen (not shown). In one case only (R49), human blasts were encountered in the mouse tissues closely surrounding the human bone graft, 9 weeks after implantation (see Table 4); human leukemic cells were principally located in muscle and around nerves (see Fig 2C).
Marrow Analysis in Leukemic Bone Biopsies Engrafted in SCID Mice for Two Different Periods
| . | Human BM Biopsy . | |||
|---|---|---|---|---|
| . | R43 . | R44 . | R48 . | R49 . |
| First graft harvest | ||||
| Engraftment period | 6 wk | 5 wk | 6 wk | 6 wk |
| Cytology | 98% CD19+ lymphoblasts | 88% CD19+ lymphoblasts | 27% CD19+ lymphoblasts | 92% CD19+ lymphoblasts |
| Histology | Diffuse blastic infiltration; myelofibrosis; areas of necrosis | Diffuse blastic infiltration; myelofibrosis; areas of necrosis | Myelofibrosis; small foci of blastic infiltration | Diffuse blastic infiltration; myelofibrosis |
| Second graft harvest | ||||
| Engraftment period | 12 wk | 12 wk | 9 wk | 9 wk |
| Cytology | 85% CD19+ lymphoblasts | 90% CD19+ lymphoblasts | Rare blasts | 52% CD19+ lymphoblasts |
| Histology | Diffuse blastic infiltration; myelofibrosis | Diffuse blastic infiltration; myelofibrosis | Small foci of blastic infiltration; myelofibrosis | Blast dissemination in surrounding murine tissues (muscle, nerve); diffuse blastic infiltration; myelofibrosis |
| . | Human BM Biopsy . | |||
|---|---|---|---|---|
| . | R43 . | R44 . | R48 . | R49 . |
| First graft harvest | ||||
| Engraftment period | 6 wk | 5 wk | 6 wk | 6 wk |
| Cytology | 98% CD19+ lymphoblasts | 88% CD19+ lymphoblasts | 27% CD19+ lymphoblasts | 92% CD19+ lymphoblasts |
| Histology | Diffuse blastic infiltration; myelofibrosis; areas of necrosis | Diffuse blastic infiltration; myelofibrosis; areas of necrosis | Myelofibrosis; small foci of blastic infiltration | Diffuse blastic infiltration; myelofibrosis |
| Second graft harvest | ||||
| Engraftment period | 12 wk | 12 wk | 9 wk | 9 wk |
| Cytology | 85% CD19+ lymphoblasts | 90% CD19+ lymphoblasts | Rare blasts | 52% CD19+ lymphoblasts |
| Histology | Diffuse blastic infiltration; myelofibrosis | Diffuse blastic infiltration; myelofibrosis | Small foci of blastic infiltration; myelofibrosis | Blast dissemination in surrounding murine tissues (muscle, nerve); diffuse blastic infiltration; myelofibrosis |
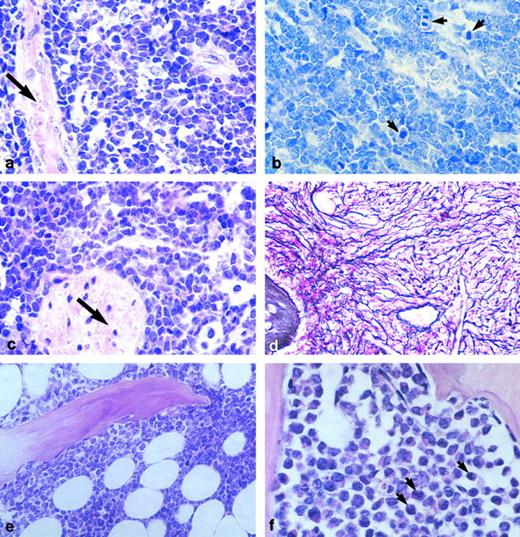
Sections of human malignant marrow tissue engrafted in SCID mice. (a) Sample R49, a pre-pre-B-ALL, 60 days posttransplantation; blasts diffusely infiltrate the tissue. Arrow, bone trabecula (HE staining, original magnification × 100). (b) Many blast cells (in the same graft) are mitotic (arrows) (Giemsa staining, original magnification × 100). (c) In this same case, but in none of the others studied, blast cells also disseminated in the mouse tissues immediately adjacent to the graft, notably around nerves (arrow) (HE staining, original magnification × 100). (d) Same sample as in a, b, and c, showing myelofibrosis (Gordon-Sweet dye, original magnification × 33). (e) R41, another pre-pre-B-ALL, was one of 6 samples in which blastic infiltration was only partial and contained adipocytes; here, at 11 weeks posttransplantation (HE staining, original magnification × 33). (f ) R46, an AML-2 in which evidence of myeloid differentiation is observed, 11 weeks after engraftment (arrows) (HE staining, original magnification × 100).
Sections of human malignant marrow tissue engrafted in SCID mice. (a) Sample R49, a pre-pre-B-ALL, 60 days posttransplantation; blasts diffusely infiltrate the tissue. Arrow, bone trabecula (HE staining, original magnification × 100). (b) Many blast cells (in the same graft) are mitotic (arrows) (Giemsa staining, original magnification × 100). (c) In this same case, but in none of the others studied, blast cells also disseminated in the mouse tissues immediately adjacent to the graft, notably around nerves (arrow) (HE staining, original magnification × 100). (d) Same sample as in a, b, and c, showing myelofibrosis (Gordon-Sweet dye, original magnification × 33). (e) R41, another pre-pre-B-ALL, was one of 6 samples in which blastic infiltration was only partial and contained adipocytes; here, at 11 weeks posttransplantation (HE staining, original magnification × 33). (f ) R46, an AML-2 in which evidence of myeloid differentiation is observed, 11 weeks after engraftment (arrows) (HE staining, original magnification × 100).
Persistent malignant hematopoiesis in engrafted BM biopsies.Eleven human BM biopsies implanted subcutaneously into SCID mice were harvested 8 to 19 weeks later for analysis (Table 3). Four other bone biopsies were large enough to be split into two pieces that were engrafted separately in the same mouse and then analyzed at two different time points (Table 4), from 5 to 12 weeks. The presence of human tumor cells in the grafts was assessed either by FACS analysis after CD45/CD19 double-staining, by microscopic examination of decalcified, paraffin-embedded tissue sections or of cytocentrifuged preparations, or by two or three of these methods simultaneously as permitted by the size of the graft.
Marrow Analysis in Leukemic Bone Biopsies Engrafted in SCID Mice
| . | Human BM Biopsy . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | R19 . | R20 . | R28 . | R29 . | R31 . | R35 . | R36 . | R38 . | R39 . | R41 . | R46 . |
| Engraftment period (wk) | 19 | 15 | 8 | 8 | 17 | 10 | 13 | 15 | 9 | 11 | 14 |
| Cytology | 100% CD19+ lymphoblasts | 68% CD19+ lymphoblasts | 19% CD19+ lymphoblasts | 58% CD19+ | |||||||
| lymphoblasts | 20% myeloblasts | 100% lymphoblasts | Dead cells | 15% myeloblasts | Rare blasts | 91% CD19+ lymphoblasts | 95% myeloblasts | ||||
| Histology | ND | ND | ND | ND | Small foci of blasts showing signs of myeloid differentiation; myelofibrosis | Diffuse blastic infiltration; myelofibrosis | No blasts; myelofibrosis | Rare blasts; moderate myelofibrosis | Rare blasts; moderate myelfibrosis | Partial blastic infiltration (2/3) with adipocytes; myelofibrosis | Diffuse blastic infiltration with signs of myeloid differentiation; myelofibrosis |
| . | Human BM Biopsy . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | R19 . | R20 . | R28 . | R29 . | R31 . | R35 . | R36 . | R38 . | R39 . | R41 . | R46 . |
| Engraftment period (wk) | 19 | 15 | 8 | 8 | 17 | 10 | 13 | 15 | 9 | 11 | 14 |
| Cytology | 100% CD19+ lymphoblasts | 68% CD19+ lymphoblasts | 19% CD19+ lymphoblasts | 58% CD19+ | |||||||
| lymphoblasts | 20% myeloblasts | 100% lymphoblasts | Dead cells | 15% myeloblasts | Rare blasts | 91% CD19+ lymphoblasts | 95% myeloblasts | ||||
| Histology | ND | ND | ND | ND | Small foci of blasts showing signs of myeloid differentiation; myelofibrosis | Diffuse blastic infiltration; myelofibrosis | No blasts; myelofibrosis | Rare blasts; moderate myelofibrosis | Rare blasts; moderate myelfibrosis | Partial blastic infiltration (2/3) with adipocytes; myelofibrosis | Diffuse blastic infiltration with signs of myeloid differentiation; myelofibrosis |
BM implants in SCID mice were analyzed by flow cytometry or on tissue sections and cell smears. The 3 approaches were used simultaneously when permitted by the size of the graft.
At harvesting, all grafts appeared enclosed in a well-vascularized fibrous capsule. At variance with human embryonic and early fetal bone rudiments engrafted in the same conditions (B. Péault, S. Chen, unpublished observations, March 1993), no evidence of osteogenesis, resulting in graft enlargement, was ever seen. On sectioning the harvested bone implant under the dissecting microscope, the marrow tissue appeared similar to that present in the fresh biopsy.
Human leukemic blasts were detected by FACS and/or cell-smear analysis in the marrow cavity of 14 of 15 analyzed grafts 5 to 19 weeks posttransplant, in a proportion approximating or exceeding 90% of the nucleated cells in seven (Tables 3 and 4 and Fig 1). Samples R43, R44, R48, and R49 could be reanalyzed at a second time point 5 to 6 weeks and 9 to 12 weeks later, respectively (Table 4). Sample R48 contained only about 30% malignant cells at 6 weeks and even fewer at 9 weeks, although small blastic foci were detected on sections. In contrast, the three other grafts that were replete with leukemic cells at 5 to 6 weeks posttransplantation exhibited a similar content of malignant cells 3 to 6 weeks later (Table 4).
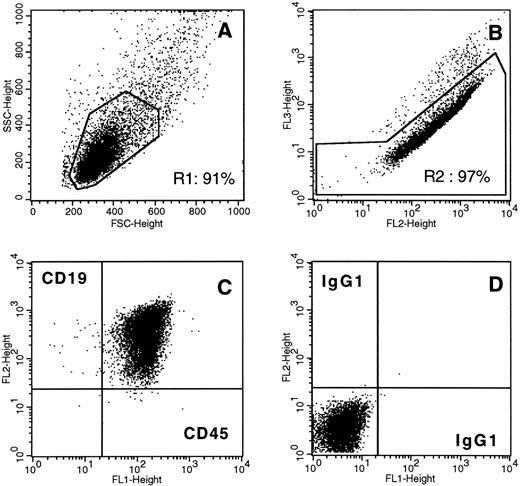
Human tumor cells in a pre-pre-B-ALL (first relapse) BM biopsy implanted subcutaneously for 19 weeks in the SCID mouse. BM cells from engrafted sample R19 were dispersed, stained, and analyzed by flow cytometry. The implant marrow cell population is homogeneous in terms of physical parameters (A, forward-scatter v side-scatter analysis) and virtually exclusively composed of viable (B, PE (fl2) v PI (fl3) signals) leukemic CD45+CD19+ cells (C, gates R1 + R2). (D), control isotype antibody staining.
Human tumor cells in a pre-pre-B-ALL (first relapse) BM biopsy implanted subcutaneously for 19 weeks in the SCID mouse. BM cells from engrafted sample R19 were dispersed, stained, and analyzed by flow cytometry. The implant marrow cell population is homogeneous in terms of physical parameters (A, forward-scatter v side-scatter analysis) and virtually exclusively composed of viable (B, PE (fl2) v PI (fl3) signals) leukemic CD45+CD19+ cells (C, gates R1 + R2). (D), control isotype antibody staining.
In parallel, a total of 15 human marrow grafts were analyzed histologically; as for the cytology analysis, four biopsies could be studied at two different time points posttransplantation. In all but one case (R36), human blast cells were present. Blastic infiltration was diffuse in eight of 15 samples (Fig 2a), and only partial with maintenance of adipocytes in one case (R41; Fig 2e). In the five other samples, more rare, randomly distributed blasts were detected in the engrafted marrow (not shown). R36, the only graft in which blasts could not be detected, was small and exhibited systemic myelofibrosis of the medullary cavities, as was also the case with all samples studied. Blast cells were typically of small to medium size, with a small ring of eosinophilic cytoplasm and a nucleus containing a thin meshwork of chromatin (not shown). Mitoses were numerous (Fig 2b). In two cases, R31 and R46, blasts exhibited a different shape suggesting myeloid differentiation (Fig 2f ). Necrosis was seen on sections from two grafts, R43 and R44, implanted for 6 and 5 weeks, respectively. However, when the same samples were reanalyzed after 12 total weeks of residence in the mouse host, no sign of necrosis was detected anymore and marrow spaces appeared diffusely infiltrated by blast cells (Table 4, and not shown). Bone trabeculae were always present in the grafts; however, they sometimes appeared atrophic. Whether the bone regression was due to tumor spread or was a consequence of mouse macrophage infiltration has not been determined. Finally, as already stressed, in one case only blast cells were found outside the graft, infiltrating the closely neighboring mouse tissues (Fig 2c).
In summary, on removal from mouse hosts, 16 of 19 leukemic bone grafts contained significant numbers (15% to 100%) of malignant cells; only rare blasts were detected in two other implants, and the last one was totally devoid of viable hematopoietic cells. When permitted by the size of the graft, histologic analysis confirmed the presence of blasts detected previously on cell suspensions. Malignant human hematopoiesis thus seems to be stably maintained in most cases in SCID mouse–engrafted marrow biopsies.
Stability of gene rearrangements in human leukemic blasts maintained long-term in SCID mice.In four studied pre-pre-B-ALL samples, γδTCR or IgH gene rearrangements were amplified by PCR on blasts obtained from BM biopsies freshly excised from the patient, and then from the same tissues maintained for various periods in SCID mice. The length of the rearranged sequence was compared in the distinct samples using a laser fluorescence DNA sequencer. The VH-JH rearrangement that was detected originally in samples R41 and R49 was found to be identical after engraftment for 11 and 6 weeks, respectively, in SCID mouse hosts (Fig 3). Similarly, the δ chain gene rearrangement originally found in blasts from biopsy R43 was retrieved after a 6-week residency in the mouse. R44 blasts initially presented a γ gene rearrangement; the rearranged γ sequence could be detected after 5 weeks of engraftment (Fig 3B). Samples R43, R44, and R49 were reanalyzed after being maintained for 6, 7, and 3 additional weeks, respectively, in the hosts. In these three cases, gene rearrangements identical to those previously detected were identified (not shown).
Analysis of tumor-specific gene rearrangements in pre-pre-B-ALL human BM biopsies engrafted in SCID mice. During lymphoid development, exons encoding TcR and Ig are assembled by a site-specific V(D)J recombination. The joining of coding ends is imprecise, and base loss or addition occurs. As a consequence, PCR amplification of TcR or IgH loci of polyclonal lymphocytes yields products of heterogenous size, reflecting the diversity of the N-region. This can be evidenced by size separation using polyacrylamide gel electrophoresis, which shows a gaussian distribution of PCR products according to size. Conversely, electrophoresis of amplification products obtained from a monoclonal population of lymphoid cells with a given rearranged locus results in a single peak. This latter observation is consistent with the presence of a unique N-region in all lymphocytes, and is thus characteristic of a malignant clone. The size of the rearrangement is highly clone-specific. To look for the presence of blastic cells in BM biopsies, mononucleated cell DNA from tissues before and after engraftment was amplified using fluorescein-labeled oligonucleotides and submitted to high-resolution polyacrylamide electrophoresis on a fluorescent automated laser DNA sequencer. PCR products were detected by fluorescence and visualized as peaks separated according to size. (A) Blast cells from sample R41 engrafted for 11 weeks contain a clonal IgH rearrangement on both alleles (b), identical to that detected in the biopsy before implantation (a). Similarly, R49 blast cells exhibit the same single-allele IgH rearrangement before (e) and after (d) a 6-week implantation in a SCID mouse. As a control, polyclonal IgH rearrangements present in a normal BM cell population were used (c). (B) The same Vγ9-J1J2 single-allele rearrangement is detected in R44 blasts analyzed at first diagnosis (b) and after transplantation of the BM biopsy in a SCID mouse for 5 weeks (a). Y-axis, intensity of fluorescence; X-axis, time (min).
Analysis of tumor-specific gene rearrangements in pre-pre-B-ALL human BM biopsies engrafted in SCID mice. During lymphoid development, exons encoding TcR and Ig are assembled by a site-specific V(D)J recombination. The joining of coding ends is imprecise, and base loss or addition occurs. As a consequence, PCR amplification of TcR or IgH loci of polyclonal lymphocytes yields products of heterogenous size, reflecting the diversity of the N-region. This can be evidenced by size separation using polyacrylamide gel electrophoresis, which shows a gaussian distribution of PCR products according to size. Conversely, electrophoresis of amplification products obtained from a monoclonal population of lymphoid cells with a given rearranged locus results in a single peak. This latter observation is consistent with the presence of a unique N-region in all lymphocytes, and is thus characteristic of a malignant clone. The size of the rearrangement is highly clone-specific. To look for the presence of blastic cells in BM biopsies, mononucleated cell DNA from tissues before and after engraftment was amplified using fluorescein-labeled oligonucleotides and submitted to high-resolution polyacrylamide electrophoresis on a fluorescent automated laser DNA sequencer. PCR products were detected by fluorescence and visualized as peaks separated according to size. (A) Blast cells from sample R41 engrafted for 11 weeks contain a clonal IgH rearrangement on both alleles (b), identical to that detected in the biopsy before implantation (a). Similarly, R49 blast cells exhibit the same single-allele IgH rearrangement before (e) and after (d) a 6-week implantation in a SCID mouse. As a control, polyclonal IgH rearrangements present in a normal BM cell population were used (c). (B) The same Vγ9-J1J2 single-allele rearrangement is detected in R44 blasts analyzed at first diagnosis (b) and after transplantation of the BM biopsy in a SCID mouse for 5 weeks (a). Y-axis, intensity of fluorescence; X-axis, time (min).
DISCUSSION
This study explored the possibility of transplanting human lymphoid and myeloid leukemias in the form of intact BM biopsies into SCID mice. Although implantation in SCID mice of human leukemic cells injected IV or IP had already been well documented, the aim in the present approach was an extended maintenance in the mouse host of the human tumor inside its natural stromal environment. The rationale for this project is the previous demonstration that intact fetal human thymus and BM stromas can reside long-term in the SCID mouse while supporting active human hematopoiesis. A total of 15 distinct transplanted BM biopsies were analyzed by histology, cytology, and flow cytometry. Owing to the small size of some of the original trephine samples, not all three analyses could be performed on each graft. Nonetheless, 14 of 15 grafts contained human malignant blasts when observed 5 to 19 weeks after transplantation. FACS and microscopic analyses of marrow cell suspensions showed the presence of high numbers of malignant human cells, accounting for about 90% of the nucleated marrow cells (85% to 100%) in seven cases. The percentage of blasts retrieved from the grafts was on the same order of magnitude as in the original fresh marrow biopsies. Also, three of four tissues that could be analyzed twice sequentially postengraftment presented an essentially similar cellular composition at these successive time points. Histologic analysis of engrafted marrow biopsies, including those that could not be analyzed by flow cytometry, revealed an architecture similar to that of the fresh tissue, with large areas exclusively consisting of blastic cells. Moreover, rearrangements originally detected in γδTCR and IgH genes in the tumor cells were identified in their long-term engrafted descent. Altogether, these observations demonstrate that human malignant hematopoiesis becomes generally stably established in the SCID mouse following transplantation of acute leukemia solid BM tissue. However, a striking observation was that mice engrafted with leukemic bone fragments never became sick. In contrast, lymphoid leukemias injected IV have been reported to be severely aggressive in SCID mice. In a study reported by Cesano and Santoli,22 11 of 11 mice injected IV with six distinct pre-B-ALL samples became clinically ill; two died spontaneously, and the others were killed. Extensive tumor spread was observed at autopsy in all animals, causing variously extensive hepatosplenomegaly, lymph node and thymus enlargement, and infiltration of BM and PB. In the same study, animals injected IP had fewer or no clinical symptoms, but all of them still exhibited internal tumors at autopsy. In our experiments, SCID mice remained healthy and, in addition, gross anatomic and histologic observation of their internal organs did not reveal any tumor cell infiltration. It may be that low numbers of leukemic cells released from the marrow grafts were efficiently neutralized by the host's natural killer (NK) cells and macrophages, whereas these defense cells are overwhelmed when large amounts (107) of tumor cells are administered IV or IP. Similarly, anti–NK cell treatment of SCID mice has been observed to considerably enhance the metastatic potential of injected human lung cancer cells.36 In agreement with our results, a recent report by Sandhu et al37 has documented, by immunohistochemical staining, the maintenance of tumor cells in adult human AML bone pieces engrafted in SCID mice for 8 weeks. However, in that study, recipient mice were pretreated by a combination of total-body irradiation and anti–asialo GM1 (anti–NK cell) antibody injection. In that setting, massive infiltration of CD68+ human leukemic cells was observed in the mouse spleen, liver, and lungs that resulted in severe, and in three of six mice, eventually fatal sickness. Therefore, our data suggest that limiting host SCID mouse conditioning to low-dose whole-body irradiation permits efficient engraftment and long-term persistence of leukemic BM pieces while keeping NK cell activity high enough to prevent tumor cell spread to the tissues of the host. Indeed, marrow implants containing virtually pure populations of viable leukemic blasts were recovered from perfectly healthy mice 5 months after transplantation (Figs 1 and 2).
Altering the size of the NK cell/macrophage compartment in the SCID mouse host thus seems to allow control of human cell spread arising from the malignant graft. There is evidence that this is also the case with normal, nonmalignant hematopoiesis in the SCID-hu bone model. Although fetal and, to a lesser extent, adult bone can retain some blood-forming activity when transplanted into unconditioned SCID mice,7,37 pretreatment of host mice with irradiation and anti–asialo GM1 antibody significantly improved both local hematopoiesis in the engrafted adult bone and dissemination of human blood cells in the mouse.37 It will be interesting to see whether the simple low-dose x-ray conditioning regimen used here to sustain leukemic development would also favor long-term normal hematopoiesis in adult human bone fragments engrafted in SCID mice.
As already mentioned, ALL aggressivity in the patient is usually predictive of its invasive potential when injected into SCID mice. Conversely, slowly evolving leukemias such as CML hardly engraft, if at all, when inoculated into SCID mice,21,38 unless CML blasts are injected directly into the marrow cavity of a preestablished SCID-hu fetal bone graft.39 So faithfully do human ALL blasts maintain their behavior in SCID mice that the model was assumed to be of predictive value for the outcome in patients with newly diagnosed pre-B-ALL,40,41 as well as for the relapse risk in T-ALL and B-ALL.25 42 In preliminary attempts to transplant CML marrow biopsies in SCID mice, we also failed to establish malignant hematopoiesis (F-X Mahon, I. Khazaal, B. Péault, unpublished results, January 1994). However, no clear correlation could be made between acute leukemia development in the setting described herein and tumor progression in the donor patient. Our best-engrafting samples were indeed R19, a high-risk pre-pre-B-ALL, and R35, its relapsed form 6 months later, both of which contained 100% blasts 19 and 10 weeks posttransplantation in SCID mice. Yet some standard-risk ALL like R41, R43, and R49 also became remarkably established in the mice. At this point, one should therefore be most cautious regarding the potential prognostic value of leukemic marrow maintenance in SCID mice.
Nonetheless, these novel SCID-hu chimeras could be used as models in which to address questions requiring the sustained, stable maintenance of human malignant hematopoiesis inside its intact, three-dimensional primary cellular environment. These include the characterization of the leukemia-associated stroma and its interactions with tumor cells, which in turn could be experimentally altered in the same model by antibody-, drug-, or gene-mediated treatments.
ACKNOWLEDGMENT
We are grateful to Dr Pierre Charbord for useful comments on the manuscript, to Françoise Viala for preparing the color plates, and to Michèle Scaglia for expert secretarial assistance.
Supported in part by SyStemix Inc, Palo Alto, CA.
F.L. and I.K. contributed equally to this study.
Address reprint requests to Bruno Péault, PhD, Institut d'Embryologie Cellulaire et Moléculaire, CNRS UPR 9064, 49bis, Avenue de la Belle Gabrielle, 94736 Nogent-sur-Marne cedex, France.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal