Abstract
The poor prognosis associated with patients afflicted with the acquired immunodeficiency syndrome and primary central nervous system lymphoma (AIDS-PCNSL) is due in part to the intrinsic resistance of this Epstein-Barr virus (EBV)-associated tumor to conventional antineoplastic therapy. Fas (CD95) is a transmembrane protein receptor that transmits an intracellular signal leading to rapid programmed cell death following ligation with its natural ligand or anti-Fas antibodies. Fas expression and function were assessed in AIDS-PCNSL biopsy samples and in EBV+ human B-cell tumors that spontaneously developed in severe combined immune deficient (SCID) mice engrafted with human lymphocytes (hu-PBL-SCID mice). All tumors samples showed high-density surface expression of Fas by flow cytometry or immunohistochemical staining. Cells from two AIDS-PCNSL biopsy samples that did not express pan B-cell markers did not express Fas antigen. All tumors examined were susceptible to Fas-mediated apoptosis, as measured by standard assays for endonucleolytic cleavage of DNA. The response to Fas-mediated apoptosis was dependent on log-fold increases in the concentration of immobilized anti-Fas antibody, but could also be induced with a mobilized anti-Fas antibody. No evidence for intrinsic resistance to Fas-mediated apoptosis (ie, secreted or truncated forms of Fas) could be shown. Radiation-induced apoptosis of neoplastic EBV+ B cells was enhanced by activation of Fas, and prolonged exposure to interleukin-2 increased both Fas expression and Fas-induced apoptosis. As the normal brain parenchyma appears to have either low-density or absent expression of Fas, and antineoplastic therapy can be selectively delivered to the CNS with little systemic toxicity, local delivery of Fas-activating molecules could prove to be a useful component in the multimodal treatment of AIDS-PCNSL.
INDIVIDUALS WITH acquired immunodeficiency syndrome (AIDS) are at increased risk of developing cancer during the course of infection with the human immunodeficiency virus (HIV).1 There is strong evidence implicating the Epstein-Barr virus (EBV) in the pathogenesis of some immunoblastic B-cell lymphomas in patients afflicted with severe immune deficiency.2 Primary central nervous system lymphomas in patients with AIDS (AIDS-PCNSL) demonstrate an invariable association with type III latent EBV infection (ie, expression of nine latent genes), and a rapidly fatal clinical course.3-8 The intrinsic resistance of AIDS-PCNSL to standard chemotherapy and radiotherapy suggests that the molecular mechanisms which prevent death in these cells are likely to be multiple and complex.
It has recently become evident that many of the modalities traditionally used in therapy of cancer function through the induction of some but not all apoptotic pathways.9 In attempting to overcome cytotoxic drug resistance, it may become necessary to identify and exploit additional signaling pathways that induce apoptosis. Fas (APO-1/CD95) is a transmembrane protein receptor that is a member of the tumor necrosis factor (TNF) receptor family. Crosslinking of the Fas receptor via the binding of Fas-ligand (Fas-L) or monoclonal antibodies (MoAbs) specific for Fas induces apoptosis.10-12 Fas has been shown to be expressed on some human non-Hodgkin's B-cell lymphomas,13 but has not been examined in AIDS-PCNSL.
Fas expression has been shown to correlate with specific patterns of EBV latent gene expression. Type I latency is characterized by the expression of only one latent gene product (EBNA-1) whereas type III latency involves the expression of nine latent gene products, some of which have been implicated in B-cell transformation.14,15 The expression of Fas on EBV+ B-cell lines derived from patients with Burkitt's lymphoma (BL) was shown to increase with a shift from a type I latent EBV infection to a type III latent EBV infection.14 This shift in latent gene expression also induces BL lines to assume lymphoblastoid features similar to those seen in AIDS-PCNSL.16 17
Intraperitoneal (ip) transfer of high numbers of human peripheral blood lymphocytes (hu-PBL) from healthy donors seropositive for EBV into severe combined immune deficient (SCID) mice spontaneously gives rise to immunoblastic tumors of human B-cell origin within 8 to 12 weeks.18,19 These tumors contain EBV DNA, demonstrate a type III latent EBV infection, and have cellular and molecular features that bear striking resemblance to AIDS-PCNSL.16,17,20 21 Therefore, we investigated whether both of these human EBV+ B-cell tumors express Fas and, subsequently, evaluated the functional response to Fas activation.
MATERIALS AND METHODS
Animals.Four- to 6-week-old c.b.-17 scid/scid (SCID) mice were obtained from the breeding colony of Dr Richard Bankert (Roswell Park Cancer Institute, Buffalo, NY). Mice were housed in a specific pathogen-free environment provided by the institute animal care facility. All micro isolator cages, food supplements, acidified water, and instruments were autoclaved before use and all manipulations were performed in a laminar flow hood. Animals showed no evidence for the leaky phenotype as determined by serum murine immunoglobulin (Ig).22 Engraftment of human lymphocytes, verified by the presence of huIg, was determined by inhibition enzyme-linked immunosorbent assay (ELISA).19 All procedures were approved by the Institute Animal Care and Use Committee.
Fas (CD95) expression on EBV+ LCLs generated from human EBV+ B-cell tumors of hu-PBL-SCID mice. The solid peak represents staining with the FITC-conjugated anti-Fas MoAb, while the dashed line represents staining with an FITC-conjugated nonreactive isotype control MoAb. Eighteen LCLs generated from 18 individual human EBV+ B-cell tumors from hu-PBL-SCID mice demonstrated virtually identical reactivity for Fas expression as that shown here. The COLO 205 cell line lacks Fas expression and was used as a negative control and the Jurkat T-cell line was used as a positive control (not shown).44
Fas (CD95) expression on EBV+ LCLs generated from human EBV+ B-cell tumors of hu-PBL-SCID mice. The solid peak represents staining with the FITC-conjugated anti-Fas MoAb, while the dashed line represents staining with an FITC-conjugated nonreactive isotype control MoAb. Eighteen LCLs generated from 18 individual human EBV+ B-cell tumors from hu-PBL-SCID mice demonstrated virtually identical reactivity for Fas expression as that shown here. The COLO 205 cell line lacks Fas expression and was used as a negative control and the Jurkat T-cell line was used as a positive control (not shown).44
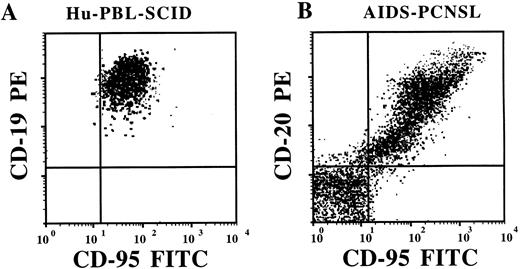
Flow cytometric analysis of Fas (CD95) expression on fresh human EBV+ B-cell tumors from an hu-PBL-SCID mouse (A) and from a patient with AIDS-PCNSL (B). Tumor samples were stained with anti-Fas-FITC MoAb and either with CD19-PE or CD20-PE MoAbs, as indicated. The brain biopsy cells visualized in (B) are ungated and show that cells nonreactive for the pan-B-cell marker CD20 are also nonreactive for Fas.
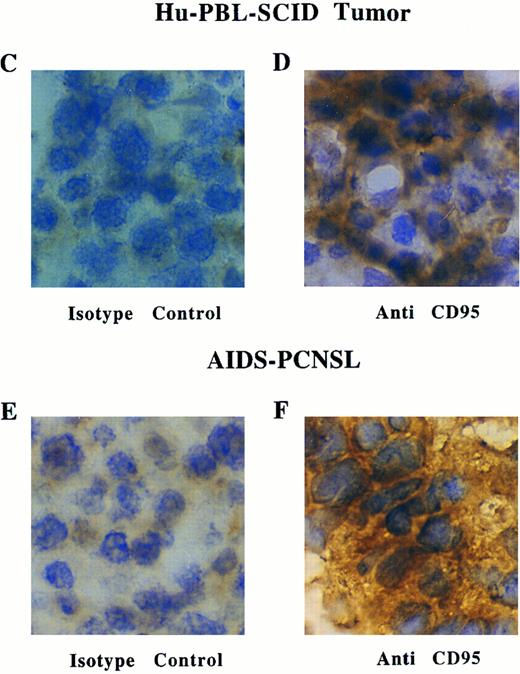
(C through F) Immunoperoxidase staining for Fas expression in a representative frozen section of a human EBV+ B-cell tumor taken from a hu-PBL-SCID mouse (D), and a representative frozen section of a brain biopsy from a patient with AIDS-PCNSL (F). Sections stained with a nonreactive isotype control MoAb are shown in (C) and (E).
Flow cytometric analysis of Fas (CD95) expression on fresh human EBV+ B-cell tumors from an hu-PBL-SCID mouse (A) and from a patient with AIDS-PCNSL (B). Tumor samples were stained with anti-Fas-FITC MoAb and either with CD19-PE or CD20-PE MoAbs, as indicated. The brain biopsy cells visualized in (B) are ungated and show that cells nonreactive for the pan-B-cell marker CD20 are also nonreactive for Fas.
(C through F) Immunoperoxidase staining for Fas expression in a representative frozen section of a human EBV+ B-cell tumor taken from a hu-PBL-SCID mouse (D), and a representative frozen section of a brain biopsy from a patient with AIDS-PCNSL (F). Sections stained with a nonreactive isotype control MoAb are shown in (C) and (E).
Generation of human EBV+ B-cell tumors in hu-PBL-SCID mice.Human leukocytes were obtained from healthy donors with an EBV-VCA titer greater than 1.5, determined by an independent laboratory using an enzyme immunoassay to detect anti-VCA IgG levels (Roche Biomedical Laboratories, West Seneca, NY). Donors were leukopheresed after obtaining informed consent. Peripheral blood mononuclear cells (PBMC) were isolated following Ficoll Hypaque (Sigma Chemical Co, St Louis, MO) density gradient separation. Monocytes were subsequently removed by overnight plastic adherence at 37°C and nonadhered PBL were collected, enumerated, and prepared for injection. Four- to 6-week-old SCID mice were injected ip with 5 × 107 human PBL in 0.5 mL of PBS. Engraftment was documented between weeks 6 and 9. hu-PBL-SCID mice were monitored closely for signs of lymphomagenesis, including physical appearance, elevated huIg levels, and peripheral leukocytosis. When judged to be moribund, mice were anesthetized, killed, and autopsied. All organs involved with tumor were collected for histologic, flow cytometric, and nucleic acid analysis.
Cell lines.EBV+ lymphoblastoid cell lines (LCLs) were generated from human EBV+ B-cell tumors resected from hu-PBL-SCID mice. After removal, tumors were dispersed to single-cell suspension, passed over Ficoll Hypaque gradients, and cultured in RPMI 1640 growth medium supplemented with 10% fetal bovine serum (Sigma). The COLO 205 colorectal carcinoma cell line and the Jurkat T-lymphoblastoid line were obtained from American Type Culture Collection (ATCC; Rockville, MD) and grown under culture conditions recommended by ATCC.
Patients and samples.Fresh samples from eight patients with AIDS-PCNSL were obtained as residual tissue after pathologic examination of stereotactic core brain biopsies. Pathologic examination of all samples showed B-cell lymphoma of the large cell type. Tissues were either dispersed to collect single-cell suspension of tumor cells or embedded in O.C.T. cryopreservation media (Tissue Tek; Miles, Elkhart, IN) for subsequent immunohistochemical evaluation.
Flow cytometric analysis.Human EBV+ B-cell tumors obtained from hu-PBL-SCID mice and patients with AIDS-PCNSL were dispersed to single-cell suspension for analysis of surface antigenic expression of Fas (anti CD95-FITC; Oncor Technologies, Gaithersburg, MD) and pan-B lymphocyte surface markers (anti-CD19-FITC and anti-CD20-FITC; Becton Dickinson Immunology, Mountain View, CA). Directly conjugated non reactive murine isotype control MoAbs were purchased from Beckon Dickinson. For experiments evaluating the effects of cytokines on surface expression of Fas, cell suspensions were first incubated for 4 days in the presence of interleukin-2 (IL-2, 10 ng/mL; Cetus Corp, Emeryville, CA), interferon-γ (IFN-γ, 10 ng/mL; Genentech, San Francisco, CA), or IL-10 (50 ng/mL; Schering Plough Research Institute, Kenilworth, NJ). Samples were then procured, washed, stained with MoAb, and subjected to functional assays as described below or analyzed on a FACScan using LYSIS II software (Becton Dickinson), as previously described.23
Immunohistochemical staining of tumor samples.Fresh tumor tissue was embedded in O.C.T. and frozen in liquid nitrogen. Five micron sections were placed on silicone-coated slides and fixed in 2% paraformaldehyde solution containing 0.1 mol/L lysine, 0.1 mol/L Na2PO4 , 10 mmol/L NaIO4 for 15 minutes at 4°C. Sections for surface antigen staining were fixed for 5 minutes in acetone at −20°C. Saponin (0.1%) was included in all solutions and washes when staining with anti-Fas MoAb or the mouse nonreactive isotype MoAb control. After three washes in Hanks' Balanced Saline Solution (HBSS; GIBCO, Grand Island, NY), all sections were blocked in 100 μL of avidin blocking solution (Vector, Burlingame, CA) for 15 minutes, biotin blocking solution (Vector) for 15 minutes, 0.2 mol/L sodium azide, and 1.0% H2O2 in HBSS for 30 minutes and 500 μg/mL goat IgG (Sigma) for 30 minutes. Primary MoAbs were then added and sections were incubated overnight at 4°C. MoAbs directed at the following human antigens were used: CD19 (Leu12; Becton Dickinson Immunology; San Jose, CA), and M2, M3, and M31 (anti-Fas; Immunex Corp, Seattle, WA). A nonreactive mouse IgG1 MoAb was used as an isotype control to establish background staining (Sigma). Sections were then washed three times with HBSS, blocked with 10% goat serum for 30 minutes, washed three times, and incubated with a secondary biotin-conjugated goat–anti-mouse IgG1 MoAb (Vector) for 30 minutes at room temperature and washed again. One hundred microliters of avidin-HRP conjugate (Vector) was next added to each section for 30 minutes, washed, allowed to dry for 2 minutes, then reacted with 100 μL DAB peroxidase substrate solution (Vector) for 10 minutes. Slides were submerged in ddH2O for 1 minute, counter stained with hematoxylin, and cover slipped with Permount (Sigma).
Fas Expression on AIDS-PCNSL and EBV-LPD
| Tumor Specimen . | Samples* . | Expression . | Method . |
|---|---|---|---|
| AIDS-PCNSL | 2/2 | +++ | FACS |
| AIDS-PCNSL | 6/6 | +++ | IHC |
| hu-PBL-SCID EBV-LPD | 4/4 | +++ | FACS |
| hu-PBL-SCID EBV-LPD | 6/6 | +++ | IHC |
| hu-PBL-SCID LCLs | 18/18 | +++ | FACS |
| Tumor Specimen . | Samples* . | Expression . | Method . |
|---|---|---|---|
| AIDS-PCNSL | 2/2 | +++ | FACS |
| AIDS-PCNSL | 6/6 | +++ | IHC |
| hu-PBL-SCID EBV-LPD | 4/4 | +++ | FACS |
| hu-PBL-SCID EBV-LPD | 6/6 | +++ | IHC |
| hu-PBL-SCID LCLs | 18/18 | +++ | FACS |
Abbreviations: +++, 90%-100% reactive by FACS or IHC; FACS, fluorescence activated cell sorting; IHC, immunohistochemical analysis; hu-PBL-SCID EBV-LPD, SCID mouse engrafted with human peripheral blood lymphocytes with evidence of human EBV+ B-cell tumors; LCLs, lymphoblastoid cell lines derived from hu-PBL-SCID mice with EBV-LPD.
Number of samples testing positive/total number of samples analyzed.
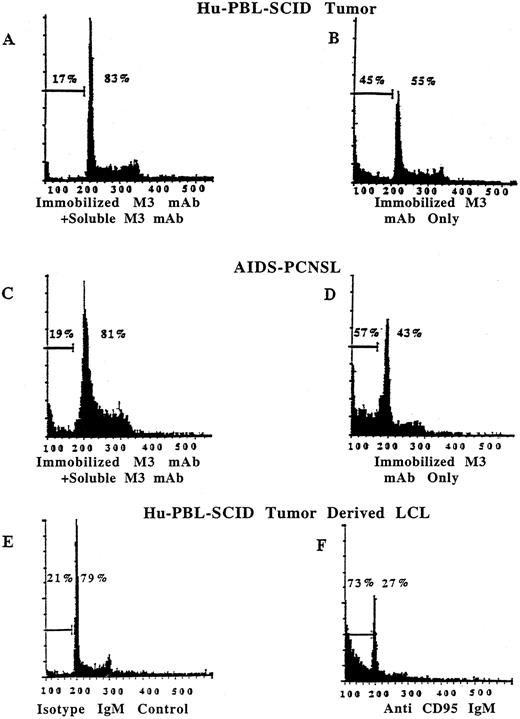
Human EBV+ B-cell tumors from hu-PBL-SCID mice and from patients with AIDS-PCNSL are susceptible to Fas-induced apoptosis. Quantitative assessment of apoptosis was obtained by staining cell nuclei with PI and analyzing 5,000 events by flow cytometry.30 Tumor cells plated in the presence of 10 μg/mL of immobilized anti-Fas M3 MoAb and a fivefold excess of soluble M3 have only 17% of nuclei within the hypodiploid portion of the histogram, indicative of apoptosis (A). Tumor cells plated in the presence of 10 μg/mL of immobilized anti-Fas M3 MoAb alone have 45% of nuclei within the apoptotic portion of the histogram (B). Similar results were obtained with tumor cells from a fresh AIDS-PCNSL biopsy specimen in that only 19% of nuclei were within the hypodiploid portion after exposure to 10 μg/mL of immobilized M3 MoAb with an excess of soluble M3 (C), while 57% were within the apoptotic portion after exposure to 10 μg/mL of immobilized M3 MoAb alone (D). Similar results were obtained when LCLs derived from hu-PBL-SCID B-cell tumors were plated in the presence of 5 μg/mL of the mobilized anti-Fas IgM CH11 MoAb. Twenty-one percent of the cells were in the apoptotic fraction after exposure to an IgM isotype control MoAb (E), while 73% of cells were within the apoptotic fraction after exposure to mobilized anti-Fas IgM CH11 MoAb (F).
Human EBV+ B-cell tumors from hu-PBL-SCID mice and from patients with AIDS-PCNSL are susceptible to Fas-induced apoptosis. Quantitative assessment of apoptosis was obtained by staining cell nuclei with PI and analyzing 5,000 events by flow cytometry.30 Tumor cells plated in the presence of 10 μg/mL of immobilized anti-Fas M3 MoAb and a fivefold excess of soluble M3 have only 17% of nuclei within the hypodiploid portion of the histogram, indicative of apoptosis (A). Tumor cells plated in the presence of 10 μg/mL of immobilized anti-Fas M3 MoAb alone have 45% of nuclei within the apoptotic portion of the histogram (B). Similar results were obtained with tumor cells from a fresh AIDS-PCNSL biopsy specimen in that only 19% of nuclei were within the hypodiploid portion after exposure to 10 μg/mL of immobilized M3 MoAb with an excess of soluble M3 (C), while 57% were within the apoptotic portion after exposure to 10 μg/mL of immobilized M3 MoAb alone (D). Similar results were obtained when LCLs derived from hu-PBL-SCID B-cell tumors were plated in the presence of 5 μg/mL of the mobilized anti-Fas IgM CH11 MoAb. Twenty-one percent of the cells were in the apoptotic fraction after exposure to an IgM isotype control MoAb (E), while 73% of cells were within the apoptotic fraction after exposure to mobilized anti-Fas IgM CH11 MoAb (F).
Viability and DNA fragmentation assays.Tumor cell suspensions were plated into 1-mL wells with culture medium (RPMI 1640 supplemented with 5% fetal bovine serum [FBS] and antibiotics) in the presence or absence of anti-Fas MoAbs. All MoAbs were immobilized on tissue culture–treated, flat-bottom 96-well plates by incubating M3 MoAb (10 μg/mL) at room temperature for 4 hours. The M3 anti-Fas MoAb, when immobilized, induces apoptosis of target cells expressing Fas, while a fivefold excess of soluble M3 MoAb competitively blocks this process. The M31 anti-Fas MoAb recognizes Fas antigen but will not induce an apoptotic signal after immobilization and ligation to Fas.24 Anti-Fas IgG MoAbs were provided by Immunex Corp. The anti-Fas CH11 MoAb (0.1 to 5 μg/mL; Upstate Biotechnology, Lake Placid, NY) is an IgM isotype and induces apoptosis in the absence of immobilization.11 For analysis of viability, cells from three identical wells were enumerated for vital dye exclusion using a hemocytometer. For analysis of programmed cell death, tumor cells were cultured with immobilized M3 anti-Fas MoAb in the absence or presence of a fivefold excess of soluble M3 MoAb, and obtained at various time points to determine extent of apoptosis by either light microscopy of cytospin preparations, flow cytometric analysis of nuclei stained with propidium iodide (PI) (5,000 events analyzed), or gel electrophoresis as previously described.25
Human EBV+ B-cell tumors from hu-PBL-SCID mice and from patients with AIDS-PCNSL are susceptible to Fas-induced cell death. Tumor cells were plated in the presence of 10 μg/mL of immobilized M3 anti-Fas MoAb with or without a fivefold excess of soluble M3 MoAb. Cells were then allowed to incubate for 24 hours and procured. Wells were scored for the number of cells/well that excluded vital dye. Three human EBV+ B-cell tumors of hu-PBL-SCID mice and two human EBV+ B-cell tumors from patients with AIDS-PCNSL were analyzed. Cells were enumerated from two wells under each experimental condition for each tumor. Values represent the mean percent ± SEM of viable cells/well.
Human EBV+ B-cell tumors from hu-PBL-SCID mice and from patients with AIDS-PCNSL are susceptible to Fas-induced cell death. Tumor cells were plated in the presence of 10 μg/mL of immobilized M3 anti-Fas MoAb with or without a fivefold excess of soluble M3 MoAb. Cells were then allowed to incubate for 24 hours and procured. Wells were scored for the number of cells/well that excluded vital dye. Three human EBV+ B-cell tumors of hu-PBL-SCID mice and two human EBV+ B-cell tumors from patients with AIDS-PCNSL were analyzed. Cells were enumerated from two wells under each experimental condition for each tumor. Values represent the mean percent ± SEM of viable cells/well.
Activation of Fas in EBV+ LCLs after exposure to sublethal radiation.For assays evaluating the combined effect of sublethal radiation and Fas activation, EBV+ B cells were exposed to gamma (γ) irradiation at a dose rate of 9.34 Gy/min using a 137Cs Gammacell 40 irradiator (Nordion International, Kanata, Ontario, Canada). The sublethal dose (9.34 Gy) was determined by performing a radiation dose escalation between 9.34 and 46.7 Gy and assessing EBV+ B-cell apoptosis by PI and viability by vital dye exclusion. Sublethally irradiated or nonirradiated tumor cells were then plated with increasing concentrations of immobilized M3 anti-Fas MoAb and incubated for 24 hours, after which they underwent nuclear staining with PI to measure apoptosis by flow cytometry (5,000 events).
Molecular detection of Fas isoforms and mutants, and ELISA for soluble Fas.Assessment for Fas transcripts encoding the full-length receptor, Fas soluble variants and a Fas truncated receptor (FasExo8Del) was performed by reverse transcriptase-polymerase chain reaction (RT-PCR) under conditions previously described.26-28 PCR products were analyzed on polyacrylamide gels. ELISA was used to assess for all known soluble Fas proteins from sera of tumor-bearing hu-PBL-SCID mice and from culture supernatants of tumor-derived LCLs, as previously described.26
RESULTS
Expression of Fas on EBV+ B-cell tumors from Hu-PBL-SCID mice and AIDS-PCNSL.We first looked for the expression of Fas on 18 EBV+ LCLs generated from 18 hu-PBL-SCID mice with markedly advanced human EBV+ B-cell tumors. Flow cytometric analysis showed that all 18 LCLs had high-density surface expression of the Fas antigen. The mean percent of cells staining positive for Fas was 95.7% ± 0.84% and the mean log fluorescence intensity (MFI) was 380 ± 35, with background MFI of 32.5 ± 4.4. Figure 1 shows a representative profile. We next examined Fas expression on fresh human EBV+ B-cell tumor samples which spontaneously develop in hu-PBL-SCID mice. In four tumors examined, 90% ± 4.4% of cells expressed Fas, with an MFI of 515 ± 64 (background MFI 182 ± 34). Figure 2A shows a representative profile. Immunohistochemical analysis consistently showed similar intensity of staining for Fas antigen expression in frozen sections from six additional human EBV+ B-cell tumors generated in hu-PBL-SCID mice using three human donors (Fig 2C and D).
AIDS-PCNSLs have striking phenotypic and genotypic similarity with human EBV+ B-cell tumors that spontaneously develop in hu-PBL-SCID mice.16,17 21 Therefore, we assessed Fas expression in eight patient AIDS-PCNSL biopsy samples. In a representative two-color ungated fluorescence-activated cell sorter (FACS) profile of a fresh AIDS-PCNSL sample, 97.1% of CD20+ B cells express Fas, while uninvolved brain parenchyma does not express the pan-B cell marker CD20, nor the Fas antigen (Fig 2B). An additional fresh AIDS-PCNSL sample had identical staining (not shown). Flow cytometric results of Fas expression on the two AIDS-PCNSL samples were verified by immunohistochemical staining of six additional AIDS-PCNSL biopsy samples. In each tumor examined, staining with three different anti-Fas MoAbs showed strong reactivity compared with nonreactive isotype control MoAbs. Representative samples are shown in Fig 2E and F. A summary of Fas antigen expression in human EBV+ B-cell tumors examined is provided in Table 1.
Human EBV+ B-cell tumors from Hu-PBL-SCID mice and AIDS-PCNSL are susceptible to Fas-induced apoptosis.Fas expression does not necessarily predict susceptibility to Fas-induced apoptosis.29 Therefore, we screened LCLs derived from human EBV+ B-cell tumors of hu-PBL-SCID mice for susceptibility to Fas-mediated cell death. All 18 LCLs tested showed significant cell death as determined by vital dye exclusion when exposed to immobilized anti-Fas M3 MoAb (32.7% ± 2.7% viability), compared with results of the identical assay performed in the presence of an excess of soluble anti-Fas M3 MoAb (90.4% ± 0.94% viability). The viability of fresh tumor samples from hu-PBL-SCID mice and from patients with AIDS-PCNSL was then evaluated by a vital dye exclusion assay after a 24-hour exposure to immobilized anti-Fas M3 MoAb in the absence or presence of excess soluble M3 MoAb. Both tumor types displayed substantial cell death when exposed to immobilized M3 MoAb, as determined by vital dye exclusion, compared with the same tumor samples exposed to immobilized M3 MoAb in the presence of an excess of soluble M3 MoAb (Fig 3).
Because loss of membrane integrity alone is not sufficient to characterize cell death as apoptotic, genomic DNA integrity was evaluated with PI staining of nuclei to quantitate DNA fragmentation.30 The efficacy of Fas-mediated apoptosis was evaluated on LCLs derived from human EBV+ B-cell tumors of hu-PBL-SCID mice, fresh human EBV+ B-cell tumors of hu-PBL-SCID mice, and fresh tumor cell preparations from AIDS-PCNSL biopsy samples. After overnight incubation in the presence of immobilized IgG anti-Fas M3 MoAb without or with an excess of soluble M3 MoAb, or in the presence of the mobilized IgM anti-Fas CH11 MoAb or the nonreactive isotype control MoAb, cells were procured and nuclei were stained with PI. Figure 4 shows representative results obtained by PI staining of fresh tumor samples from hu-PBL-SCID mice, from patients with AIDS-PCNSL, or from LCLs derived from hu-PBL-SCID B-cell tumors. A single peak (200 on the X-axis) is characteristic of normal diploid DNA content of viable cells. Hyperdiploid DNA from replicating cells appears to the right of the diploid peak whereas the hypodiploid DNA to the left of the peak measures the DNA fragmentation that characterizes apoptosis.30 Tumor cells plated in the presence of immobilized anti-Fas M3 MoAb and a fivefold excess of soluble M3 showed 17% of nuclei within the hypodiploid or apoptotic portion of the histogram (Fig 4A), while tumor cells plated in the presence of immobilized anti-Fas M3 MoAb alone have 45% of nuclei within the hypodiploid portion of the histogram (Fig 4B). Similar results were obtained with tumor cells from a fresh AIDS-PCNSL biopsy specimen in that 19% of nuclei were within the hypodiploid portion after exposure to immobilized M3 MoAb with an excess of soluble M3 (Fig 4C), while 57% were within the hypodiploid portion after exposure to immobilized M3 MoAb alone (Fig 4D). Likewise, when LCLs derived from three different hu-PBL-SCID B-cell tumors were plated in the presence the mobilized IgM anti-Fas CH11 MoAb (5 μg/mL), 58% to 73% apoptosis was noted. The mobilized IgM anti-Fas CH11 MoAb was 20-fold more potent than the immobilized IgG anti-FAS MoAb, M3, in inducing apoptosis of EBV+ LCLs in these experiments (not shown). Cells incubated in the presence of the IgM isotype control MoAb showed 19% to 21% apoptosis (Fig 4E and F).
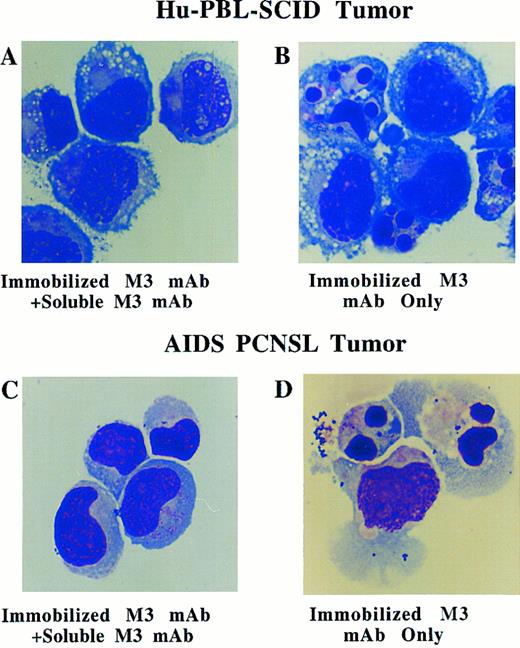
Morphologic evaluation of Fas-mediated apoptosis was assessed with cytospin preparation of fresh human EBV+ B-cell tumors from hu-PBL-SCID mice and from patients with AIDS-PCNSL (Fig 5A through D, see page 1739). Morphologic features of apoptosis such as nuclear degradation and membrane blebbing were consistently observed in the majority of cells after only a 2-hour incubation with immobilized anti-Fas M3 MoAb (Fig 5B and D), while normal lymphoblastoid morphology was observed in most cells incubated with immobilized M3 MoAb and an excess of soluble M3 MoAb (Fig 5A and C). For EBV+ tumors from hu-PBL-SCID mice, PI and morphologic evidence of apoptosis was confirmed by viewing DNA fragmentation on gel electrophoresis (not shown). In total, 3 separate AIDS-PCNSL biopsy samples, 6 separate hu-PBL-SCID biopsy samples, and 18 LCLs derived from 18 hu-PBL-SCID B-cell tumors were assessed for functional response to Fas ligation by vital dye exclusion, agarose gel electophoresis, PI nuclear staining, or morphology. Apoptosis was documented to occur in greater than 50% of cells in all samples assessed by PI or morphologic examination.
Morphologic analysis of fresh human EBV+ B-cell tumors from hu-PBL-SCID mice (A and B) and from a patient with AIDS-PCNSL (C and D) in the presence of 10 μg/mL of immobilized anti-Fas M3 MoAb for 2 hours with or without a fivefold excess of soluble M3 MoAb. Morphologic characteristics of apoptosis (ie, nuclear fragmentation and membrane blebbing) are noted in (B) and (D). Wright Giemsa stain, original magnification × 330.
Morphologic analysis of fresh human EBV+ B-cell tumors from hu-PBL-SCID mice (A and B) and from a patient with AIDS-PCNSL (C and D) in the presence of 10 μg/mL of immobilized anti-Fas M3 MoAb for 2 hours with or without a fivefold excess of soluble M3 MoAb. Morphologic characteristics of apoptosis (ie, nuclear fragmentation and membrane blebbing) are noted in (B) and (D). Wright Giemsa stain, original magnification × 330.
Evaluation for Fas variants soluble Fas.Fas-sensitive cell lines show nearly 100% apoptosis after ligation of Fas-specific MoAbs.31 However, EBV+ LCLs, fresh B-cell tumors from hu-PBL-SCID mice, and AIDS-PCNSL biopsy samples only demonstrated approximately 50% apoptosis following Fas activation, despite high surface density expression of Fas. This improved to 70% to 80% with log-fold increases in the concentration of anti-Fas antibody (not shown). Alternatively spliced variants of the Fas transcript can produce soluble isoforms that interfere with Fas-induced apoptosis,26-28 and mutant forms of Fas can be nonfunctional.28 RNA from one fresh AIDS-PCNSL sample, five hu-PBL-SCID B-cell tumors, and tumor-derived LCLs were therefore analyzed for expression of these variants by RT-PCR. Alternatively spliced variants of the Fas transcript could be faintly detected, along with abundant wild-type transcript, in some of the fresh hu-PBL-SCID B-cell tumors and tumor-derived LCLs by RT-PCR. However, we did not detect soluble Fas protein in the supernatants of the same LCLs or in the sera of hu-PBL-SCID mice with lethal B-cell tumor burden. No expression of the FasExo8Del transcript28 was detected by RT-PCR (data not shown). Thus, we can exclude the possibility that soluble Fas or FasExo8Del are responsible for the partial susceptibility to Fas-mediated apoptosis.
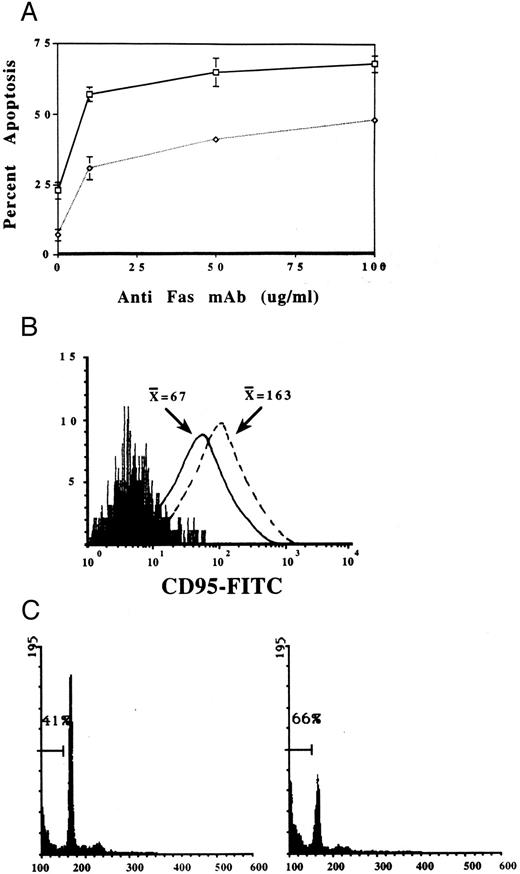
Effect of radiation or exogenous cytokines on Fas-mediated apoptosis.Radiation can induce apoptosis of EBV+ tumor cell lines,32 and whole brain radiation therapy is an integral part of the treatment for AIDS-PCNSL.33 Therefore, we assessed the effects of anti-Fas MoAb treatment on LCLs derived from human EBV+ B-cell tumors of hu-PBL-SCID mice, immediately after treatment of LCLs with a sublethal dose (9.34 Gy) of radiation. The subsequent exposure of irradiated LCLs to immobilized anti-Fas MoAb consistently increased the percentage of cells undergoing apoptosis, compared with cells exposed to either treatment modality alone (Fig 6A). We also tested whether IL-2, IFN-γ, or IL-10 were able to directly alter the expression of Fas on the surface of fresh tumor cells as has previously been reported in other cell types after exposure to IFN-γ.34 Fresh tumor biopsy specimens from hu-PBL-SCID mice with human EBV+ B-cell tumors were plated in the presence of either IL-2 (10 ng/mL), IFN-γ (10 ng/mL), IL-10 (50 ng/mL), or medium alone for 4 days. Figure 6B shows results of a representative assay where the addition of IL-2 to fresh tumor cells in serum-free medium resulted in a marked upregulation of Fas expression (MFI = 163) compared with the same tumor cells incubated in medium (MFI = 67), IFN-γ (MFI = 55, not shown), or IL-10 (MFI = 50, not shown). Further, upregulation of Fas expression after culture in IL-2 potentiated Fas-mediated apoptosis by 50% (Fig 6C).
(A) Sublethal radiation enhances Fas-mediated apoptosis of LCLs generated from human EBV+ B-cell tumors of hu-PBL-SCID mice. Irradiated (□) or nonirradiated (⋄) LCLs were incubated for 24 hours without or with an increasing concentration of immobilized anti-Fas MoAb, and then assessed for induction of apoptosis using nuclear staining with PI.30 Each point represents the mean ± SEM of three different experiments on three different LCLs. (B) Exogenous IL-2 upregulates Fas expression on fresh human EBV+ B-cell tumors from hu-PBL-SCID mice and (C) potentiates Fas-mediated apoptosis. FACS profile of Fas expression on EBV+ tumor cells stained with anti-Fas MoAb after a 96-hour incubation in the absence ( — ) or presence (– – –) of IL-2 (10 ng/mL). Nonreactive isotype control MoAb profile is represented by the solid peak. X̄ = mean fluorescence intensity. Apoptosis was assessed following a 24-hour incubation with 10 μg/ml of immobilized anti-Fas MoAb, and then quantitated by nuclear staining with PI as described in Materials and Methods.
(A) Sublethal radiation enhances Fas-mediated apoptosis of LCLs generated from human EBV+ B-cell tumors of hu-PBL-SCID mice. Irradiated (□) or nonirradiated (⋄) LCLs were incubated for 24 hours without or with an increasing concentration of immobilized anti-Fas MoAb, and then assessed for induction of apoptosis using nuclear staining with PI.30 Each point represents the mean ± SEM of three different experiments on three different LCLs. (B) Exogenous IL-2 upregulates Fas expression on fresh human EBV+ B-cell tumors from hu-PBL-SCID mice and (C) potentiates Fas-mediated apoptosis. FACS profile of Fas expression on EBV+ tumor cells stained with anti-Fas MoAb after a 96-hour incubation in the absence ( — ) or presence (– – –) of IL-2 (10 ng/mL). Nonreactive isotype control MoAb profile is represented by the solid peak. X̄ = mean fluorescence intensity. Apoptosis was assessed following a 24-hour incubation with 10 μg/ml of immobilized anti-Fas MoAb, and then quantitated by nuclear staining with PI as described in Materials and Methods.
DISCUSSION
Patients diagnosed with AIDS-PCNSL have an average life expectancy of 2 to 3 months despite intensive chemotherapy and radiotherapy.33 Alternative treatment strategies aimed at circumventing the intrinsic resistance to conventional therapies and reducing systemic toxicities should therefore be explored. In recent years it has become clear that effective tumor cell kill often involves activation of multiple intracellular pathways that induce programmed cell death or apoptosis.9 In the current report we provide the first evidence that EBV+ human B-cell tumors obtained from both hu-PBL-SCID mice and patients with AIDS-PCNSL have high-density surface expression of Fas, a member of the TNF receptor family of proteins that induces a distinct pathway of programmed cell death after activation.35
We also show that these human EBV+ B-cell tumors from hu-PBL-SCID mice and from patients with AIDS-PCNSL demonstrate susceptibility to Fas-induced apoptosis in the absence of chemotherapeutic or radiotherapeutic agents. Although the apoptotic response to Fas activation was dependent on log-fold increases in the concentration of anti-Fas IgG MoAb, cell kill in these tumors was consistently induced despite their constitutive expression of several endogenous survival factors associated with type III latent EBV infection, including LMP-1, BCL-2, IL-6, and IL-10.25,36 BCL-2 expression has previously been shown to inversely correlate with Fas-induced apoptosis in mouse hepatocytes.37 Therefore, it is likely that the requirement for log-fold increases in anti-Fas MoAb to enhance apoptosis is at least in part reflective of the tumors' abundant expression of LMP-1 which can directly regulate BCL-2 expression.25 36 We found no evidence for a role of Fas variants or mutants in any of the EBV+ tumor samples tested.
We were also able to show that an IgM anti-Fas MoAb that crosslinks Fas without immobilization was 20-fold more potent than the immobilized IgG anti-Fas MoAb. The use of a larger, more potent, mobilized mediator of Fas activation has potentially greater clinical utility. In vivo immobilization may not be required and leakage across the blood brain barrier to the systemic circulation may be minimized, thus limiting toxicity to organs such as liver that express abundant Fas.38
Importantly, we were able to demonstrate enhanced EBV+ B-cell tumor kill in a greater-than-additive fashion when combining sublethal amounts of radiation with activation of the Fas pathway. This also has potential clinical relevance, as patients with AIDS-PCNSL are often treated with whole brain radiation therapy, followed by an additional fraction of radiation localized to the tumor. Incubation in the presence of IL-2 was able to increase surface density expression of Fas on EBV+ B-cell tumors and was also able to enhance Fas-mediated apoptosis. Whether increased surface density expression of Fas increased susceptibility to Fas-mediated apoptosis or whether this resulted from other IL-2–mediated effects remains unclear. IL-2 can also increase expression of Fas ligand on cytotoxic effector cells, which in turn increases cell-mediated cytotoxicity.39,40 Thus, if Fas-activating molecules can be effectively delivered to the site of AIDS-PCNSL, there appears to be promise of combining this therapy with standard radiation therapy and with existing cytokine therapy.41
Results from the current study and previous work40 indicate that the majority of normal human brain astrocytes, neurons, oligodendrocytes, microglial cells, or ependymal cells have either very low density or absent expression of Fas. This suggests that the restriction of high-density Fas expression on EBV+ tumor cells within the CNS could be exploited with localized anti-Fas therapy. Systemic delivery of anti-Fas therapy results in lethal toxicity secondary to extensive hepatic parenchymal damage of Fas+ hepatocytes,38 although more recent work suggests that administration of an IL-1β converting enzyme (ICE) inhibitor can prevent this complication.42 Local delivery of Fas-activating therapy to the CNS, possibly in combination with systemic administration of an ICE inhibitor, may therefore prove to be a feasible adjunct to the more conventional therapies discussed above. Experimental CNS tumor models of human EBV+ B-cell tumors in immunodeficient animals should therefore be pursued to test this therapy alone and in combination with chemotherapy, immunotherapy, and radiotherapy.43 Further, the nearly universal association between type III latent EBV gene expression in human EBV+ B-cell tumors of the hu-PBL-SCID mouse and in AIDS-PCNSL supports the potential utility of this immunodeficient chimeric mouse model of human lymphomagenesis to evaluate the pathogenesis and treatment of AIDS-PCNSL.16,17,20 21
Supported by National Institutes of Health Grants No. CA65670 CA09581, and 9403-80 AIDS, an AIRC grant, the Coleman Leukemia Research Fund, Ministero della Sanitá, Associazione Italiana per la Ricerca sul Cancro, and an American Society of Hematology Medical Student Scholar Fellowship. G.P. is a fellow of Fondazione Italiana per Ricerca sul Cancro.
Address reprint requests to Michael A. Caligiuri, MD, The Arthur G. James Cancer Hospital and Research Institute, Ohio State University, 300 W Tenth Ave, Columbus, OH 43210-1228.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal