Abstract
Anti–Sia-lb (formerly anti-Gd) cold agglutinins (CAs) recognize sialylated carbohydrates on both adult and neonate red blood cells (RBCs). RBC CA activity inhibition experiments reported here indicate that the domain NeuNAcα2-3Gal, as found in sialyllactose, synthetic sialyl(s) Lewis(Le)x and sLea, sialyllactosamine, sialyl-fucosyllactose, and nonfucosylated sLea, constitutes the minimal epitope for these CAs, implicating that these autoantibodies could be able to bind this domain in sLex and sLea and related carbohydrates expressed on nucleated cells and in soluble cancer-related mucins. The following data obtained with the previously characterized monoclonal IgMk anti-Sia-lb CA, GAS, show that this is the case. GAS epitope expression among leukocytes that lack sLea parallels that of sLex determinant as detected by mouse monoclonal antibodies (MoAbs), especially MoAb KM-93. It is also found on epithelial malignant cells bearing both sLex and sLea. GAS epitope on these nucleated cells, (1) like that present on RBC, is abolished by sialidase, unaffected by proteases, and inhibited by sialyllactose; and (2) is overlapping and/or proximal to that recognized by anti-sLex MoAb, CSLEX-1, and KM-93. Moreover, CAGAS binds soluble cancer-associated mucins bearing sLex and sLea determinants. This binding is inhibited by sialyllactose and these mucins inhibit the RBC CA activity of CAGAS. The possible significance of anti–Sia-lb (anti-Gd) CAs as autoantibodies directed to carbohydrate ligands of host adhesion molecules that might be receptors of microbial adhesins of some CA-inducing pathogens is discussed.
COLD AGGLUTININS (CAs) are autoantibodies, mostly of IgMk isotype, recognizing carbohydrates on human red blood cells (RBCs) that cause RBC agglutination at temperatures less than 37°C and, optimally, in the cold (4°C). Low titers (<32) of IgMk CAs, mainly of anti-I specificity, are usually found in the serum of healthy adult individuals. Pathologically increased CA titers occur transiently following the infection by certain pathogens such as Mycoplasma Pneumonia (M.Pn), Epstein-Barr virus (EBV), cytomegalovirus, rubella, and varicella virus; and occur persistently as the result of a monoclonal B-cell expansion.1,2 Several antigenic specificities of CAs are known,1-3 with the anti-I being the most frequent, followed by the anti-i one.1,3 These CAs recognize epitopes in poly-N-aceytllactosamine or type 2 chains (repeating Galβ1-4GlcNAcβ1-3Gal-R units), with either branched (I antigen) or linear sequences (i antigen). Less information is available about the carbohydrate chains recognized by other infrequent CA specificities such as those designated anti–Pr1-3, anti–Sia-lb (formerly anti-Gd), anti–Sia-b (formerly anti-Fl), anti–Sia-l (formerly anti-Vo), and anti-Sa.1-3 In contrast to I/i antigens that are sialidase-resistant, the epitopes recognized by all these infrequent CAs are abolished by sialidase.
CAs are of great interest as a model of human autoimmunity because they establish a link between natural harmless autoantibodies, pathogenic infection-induced autoantibodies, and proliferated B-cell clones secreting such pathogenic monoclonal autoantibodies. There is a correlation between some CA-inducing pathogens and certain CA specificities, the best known being M.Pn. with anti-I, EBV with anti-i, and rubella and varicella with anti-Pr.1,3 This indicates that the infectious agent determines the specificity of CAs they induce. Although initially this was attributed to a possible molecular mimicry between CA-inducing pathogens such as M.Pn and RBC, experimental data do not support this, but indicate that CAs are autoantibodies directed to the carbohydrates that act as receptors of CA-inducing pathogens.4-6 It was suggested that the binding of the pathogen to their host receptors renders immunogenic these self components4,5 and/or that1,2 CAs might be autoantiidiotypic antibodies to antipathogen antibodies recognizing the pathogen's molecule that bind to the host receptor.7 Both hypothesis predict that the pathogen and the CAs they induce should bind similar/proximal structures, which should be mainly found on nucleated cells and tissues, because RBCs are not an infection site for the above-mentioned CA-inducing pathogens. Accordingly, I/i carbohydrates as detected by human monoclonal CAs5,8-13 or mouse monoclonal antibodies (MoAbs)14-16 are known to be abundantly expressed on nucleated cells, including the primary site of M.Pn infection (ie, bronchial epithelium); and, among leukocytes, the i antigen is prominently expressed on B lymphocytes,10-13 a primary site of EBV infection. Data about the expression on nucleated cells of antigens recognized by CAs other than the anti-I/i ones are very scarce.17 18
In that setting, the present study was aimed to precisely identify the carbohydrate sequences recognized by anti–Sia-lb (anti-Gd) CAs and to investigate the presence of such structures on leukocytes and other nucleated cells using the previously reported anti–Sia-lb (anti-Gd) CAGAS.19 Data show that the domain NeuNAcα2-3Gal constitute the minimal epitope of anti–Sia-lb (anti-Gd) CAs and that these autoantibodies bind such a domain in sLex and sLea and related carbohydrates on the surface of nucleated cells and in soluble cancer-related mucins. The possible significance of anti–Sia-lb CAs as autoantibodies to epitopes found in carbohydrates that are ligands of host adhesion molecules20-23 and might act as receptors of some CA-inducing pathogens is discussed.
MATERIALS AND METHODS
Human Igs and cold hemagglutination inhibition with oligosaccharides.Monoclonal IgMk CAGAS19 and other monoclonal IgMk CAs of anti-i (BER) and anti-I (MES) were purified as reported.19 Purification of Igs was monitored by immunofixation and enzyme-linked immunosorbent assay (ELISA) as already described.19 Cold hemagglutination and inhibition of cold hemagglutination with oligosaccharides listed in Table 1 and with mucin preparation (see below) was performed as reported.2 19
Inhibition of RBC Cold Agglutination Capacity of Anti-Sia-lb CAs, GAS, and Kn by Oligosaccharides
| Oligosaccharide . | Inhibition (mmol/L) . | ||
|---|---|---|---|
| Designation . | Structure . | GAS . | Kn . |
| 1.Sialyllactose | NeuNAcα2-3Galβ1-4Glc | 2.5 | 1.25 |
| 2.Sialyllactosamine | NeuNAcα2-3Galβ1-4GlcNAc | 1.25 | ND |
| 3.Sialylfucosyllactose | NeuNAcα2-3Galβ1-4[Fucα1-3]Glc | 1.25 | 1.25 |
| 4.sLex | NeuNAcα2-3Galβ1-4[Fucα1-3]GlcNAc | 1.25 | ND |
| 5.Nonfucosylated sLea | NeuNAcα2-3Galβ1-3GlcNAcβ1-3Galβ1-4Glc | 1.25 | 0.63 |
| 6.sLea | NeuNAcα2-3Galβ1-3[Fucα1-4]GlcNAc | 1.25 | ND |
| 7. | Galβ1-3GalNAcβ1-4[NeuNAcα2-3]Galβ1-4Glc | 0.63 | 1.25 |
| 8.Lex | Galβ1-4[Fucα1-3]GlcNAc | NI | NI |
| 9.Lea | Galβ1-3[Fucα1-4]GlcNAc | NI | NI |
| Oligosaccharide . | Inhibition (mmol/L) . | ||
|---|---|---|---|
| Designation . | Structure . | GAS . | Kn . |
| 1.Sialyllactose | NeuNAcα2-3Galβ1-4Glc | 2.5 | 1.25 |
| 2.Sialyllactosamine | NeuNAcα2-3Galβ1-4GlcNAc | 1.25 | ND |
| 3.Sialylfucosyllactose | NeuNAcα2-3Galβ1-4[Fucα1-3]Glc | 1.25 | 1.25 |
| 4.sLex | NeuNAcα2-3Galβ1-4[Fucα1-3]GlcNAc | 1.25 | ND |
| 5.Nonfucosylated sLea | NeuNAcα2-3Galβ1-3GlcNAcβ1-3Galβ1-4Glc | 1.25 | 0.63 |
| 6.sLea | NeuNAcα2-3Galβ1-3[Fucα1-4]GlcNAc | 1.25 | ND |
| 7. | Galβ1-3GalNAcβ1-4[NeuNAcα2-3]Galβ1-4Glc | 0.63 | 1.25 |
| 8.Lex | Galβ1-4[Fucα1-3]GlcNAc | NI | NI |
| 9.Lea | Galβ1-3[Fucα1-4]GlcNAc | NI | NI |
Values correspond to the concentration (in millimoles per liter) inhibiting RBC cold agglutination. Oligosaccharides were obtained from Sigma (no. 1), from BioCarb Chemicals (Lund, Sweden) (nos. 2, 3, 5, and 7), and from Oxford Glycosystems Ltd (Oxford, UK) (nos. 4, 6, 8, and 9).
Abbreviations: NI, not inhibitory at 10 mmol/L; ND, not done.
Murine MoAbs.Purified mouse MoAb KM-9324,25 that recognize sLex and mouse MoAb 2D320,26 that recognize sLea and nonfucosylated sLea (sLec) were from Seikagaku Corp (Tokio, Japan). Hybridomas producing mouse MoAbs 19.9 (anti-sLea)27,28 and CSLEX-1(anti-sLex)29 were obtained from the American Tissue Culture Collection (Rockville, MD). Rat MoAb HECA-452 that recognizes a common domain shared by both sLex and sLea20,21,30 was donated by Dr L.J. Picker (Dallas, TX). Hybridomas producing MoAbs CD8 (109-2D4), CD14 (Cris-6), CD45 (138-3), CD4 (Edu-2), CD3 (Cris-7), CD20 (B-C1), CD19 (A3.B1), EDU-1 (anti-HLA class II antigens), and CD55 (143-30) have been obtained in our laboratory and clustered in the International Workshops On Human Leukocyte Differentiation Antigens (De la Calle-Martı́n et al,31 Inglés et al,32 and Engel et al33 and the references therein). These MoAbs were used as unconjugated or biotin-labelled purifed Ig. Other commercial fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated MoAbs to human leukocyte differentiation antigens and the corresponding negative controls from Becton Dickinson España (Madrid, Spain) were also used.
Cells and cell cultures.Peripheral blood mononuclear cells (PBMCs) of healthy individuals or patients were obtained from heparinized venous blood by standard Ficoll centrifugation method.31-33 To isolate polymorphonuclear leukocytes (PMN), the pellets of centrifuged Ficoll gradients, were collected and resuspended in phosphate-buffered saline (PBS; 4× volumes of the original venous blood used to obtain the pellet), and then the RBCs were eliminated by sedimentation in 2% Dextran 500 (Pharmacia Biotech S.A., Barcelona, Spain) and residual RBCs were further eliminated by hypotonic lysis. To obtain purified T lymphocytes, PBMCs were subjected to complement-mediated lysis with the above indicated MoAbs CD14, Edu-1 (anti-HLA class II), CD20, and CD19, and then natural killer (NK) cells and residual Mo and dead cells were eliminated by centrifugation over a discontinuous (40%-45%-50%-55%-60%-65%) gradient of Percoll (Pharmacia),31,32 in which only cells banding less than 55% Percoll were collected. B cells from tonsils (of children subjected to amygdalectomy) were obtained by complement-mediated lysis with the above-indicated MoAbs CD3, CD4, CD8, and CD14 and then subfractionated by centrifugation over a discontinuous Percoll gradient.32 PBMCs were cultured with 1% (vol/vol) of phytohemagglutinin (PHA; Munex Diagnostics, Dartford, UK) and viable activated cells were used for cell surface staining with antibodies.31 Hybridomas, leukocyte cell lines (indicated in the Results), and the colon carcinoma cell line COLO205 were cultured as described.19 Human umbilical venous endothelial cells (HUVECs) were obtained and cultured as reported.34
Immunofluorescence studies by flow cytometry.Washed (3×) viable (≥96%) cells (0.5 × 106 in 0.05 mL) were incubated with a saturating dose of biotinylated (Bio) human Igs for 45 minutes and, after washing (2×), incubated for 30 minutes with streptavidin (SA) conjugated to either PE (SA-PE; Becton Dickinson) or FITC (SA-FITC; Tago Inc, Burlingame, CA) and then washed once and immediately analyzed by flow cytometry in a FACScan (Becton Dickinson). Significant differences in histograms were assessed on the basis of Kolmogorof-Smirnov statistics (LYSISII software; Becton Dickinson).19,31 33 The entire assay steps (incubations and washes) were performed in the cold (ice bath or 4°C). Cold PBS containing 3% fetal calf serum and 0.05 % of sodium azide was used to dilute cells and antibodies and for washes. The same procedure was used for staining with unconjugated mouse MoAbs, which were detected by means of FITC-conjugated antimouse Igs goat antibodies from Sigma (Sigma-Aldrich Quı́mica S.A., Alcobendas, Madrid, Spain). Unless otherwise stated, human and murine primary antibodies and the corresponding unreactive isotype-matched Igs were used at 5 μg/mL. Dual-staining with biotinylated human Igs (detected by means of SA-FITC) and commercial PE-conjugated MoAbs was performed in the same manner. In some experiments, cells (5 × 106 cells/mL in saline solution, pH 5.9) were treated without or with 0.05 or 0.1 U/mL of sialidase from Clostridium perfingens (type VIII; Sigma) or with 30 μg/mL of pronase (Sigma) for 45 minutes at 37°C and then washed (3×) before being incubated with antibodies. For inhibition binding experiments with carbohydrates, a subsaturating dose (1.5 μg/mL) of Bio-GAS (0.05 mL) was incubated with the carbohydrate solution (0.05 mL) for 30 minutes, and then cells (0.5 × 106 in 0.05 mL) were added to the mixture and the assay was continued as described above. For cross-inhibition experiments between CAGAS and murine antibodies, cells (0.5 × 106 in 0.05 mL) were preincubated for 30 minutes in the cold with the unconjugated antibody (0.05 mL) and then a subsaturating dose (1.5 μg/mL) of biotinylated antibody (0.05 mL) was added to the mixture and the assay was continued as described above. The unconjugated antibody concentration was 25 to 50 times higher than that of biotinylated antibody. In the cross-blocking experiments between CAGAS and mouse MoAb KM-93 (IgM isotype), this MoAb was used as purified Ig and its binding was detected by means of FITC-conjugated goat antibodies to mouse IgM heavy chains (Sigma).
ELISA.A capture ELISA with plate-bound CAGAS was established to analyze the capacity of CAGAS to bind mucins bearing sLex and/or sLea determinants defined by the mouse or rat MoAbs indicated above. Briefly, Nunc Maxisorb ELISA 96-well plates (provided by Labclinics, Barcelona, Spain) were coated (0.1 mL/well) overnight at 4°C with human Igs (10 μg/mL in PBS). PBS containing 2% bovine serum albumin (Merck Farma y Quı́mica S.A., Barcelona, Spain) and 0.05% Tween 20 (Merck) was used for blocking (2 hours at room temperature) as well as for dilution of samples and showing antibodies. Washes (6×) were performed with PBS-0.05% Tween. Incubation times were overnight for samples (0.1 mL/well) to bind to plate-bound human Igs and 2 hours for the binding of primary mouse or rat MoAbs (0.1 mL/well) to CAGAS-captured material, as well as for the binding of secondary peroxidase-conjugated rabbit antibodies (0.1 mL/well) specific for mouse Igs (ref. P 0260; from Dako, ATOM, Barcelona, Spain) or rat IgM (ICN Biomedicals/Immunochemicals, Costa Mesa, CA). All assay steps (washes, incubations, and dilutions of samples and antibodies) were performed in the cold (4°C). The remaining procedures in the ELISA were performed as already reported.19 Results correspond to background-subtracted optical density (OD) mean values of 2 to 4 replicates with OD values ≤2% of the mean. To perform inhibition binding experiments with sialyllactose and sialic acid (Sigma), lactose, and sucrose (Pharmacy Service, Hospital Clı́nic, Barcelona, Spain), CAGAS-coated wells were incubated for 8 hours in the cold with 0.05 mL of either PBS or carbohydrate solutions in PBS, and then the sample (0.05 mL) was added without washing the inhibitor and the ELISA was continued as described above.
Human sera.The sera from 12 patients with cancer exhibiting high serum levels of 19.9 antigen as measured by the commercial kit Centocor CA19.9 RIA (Centocor Diagnostics, Malvern, PA) and from 14 healthy individuals with undetectable or normal CA19.9 serum levels were tested in the above-mentioned ELISA.
Cancer-related mucin characterization and preparation.Percloric acid (PCA) from Merck was added (0.6 N, final concentration) to culture supernatants of cell lines35,36; after centrifugation to eliminate the precipitated material, the supernatant was extensively dialyzed against PBS and then concentrated (Urifil-10; Millipore España, Madrid, Spain) to achieve the original culture medium volume. In other experiments, culture medium supernatants were concentrated (5×) and then passed through a 700-mL gel bed-column of Sephacryl S-300 (Pharmacia). The elution volume fraction containing CAGAS-captured components was concentrated until the original volume used in the column and then treated with 0.6 N PCA as described above and the PBS-dialyzed supernatant concentrated 4× (ie, 20× concentrated with respect the original culture supernatant used in the entire procedure). These mucin-enriched preparations were used to perform RBC hemagglutination inhibition studies and for dot-blotting onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), which were used to perform the periodic acid shiff (PAS) reaction.37
RESULTS
Inhibition of RBC cold agglutinating capacity of anti–Sia-lb (anti-Gd) CACAS and CAKn by oligosaccharides.Anti–Sia-lb (anti-Gd) CAGAS19 and the reference anti–Sia-lb (anti-Gd) CAKn (Roelcke1,3 and references therein), both of IgMk isotype, detect sialidase-sensitive and protease-resistant epitopes on both newborn and adult RBCs, and their RBC CA activity is inhibited by sialyllactose (NeuNAcα2-3Galβ1-4Glc), but not by sialic acid or lactose alone.1,3,19 To characterize the precise carbohydrate structure detected by these CAs, RBC CA activity inhibition studies were performed using sialyllactose and eight additional oligosaccharides indicated in Table 1. Except for Lex and Lea, which lacked inhibitory ability, all the oligosaccharides, including 3′sialyl-lactosamine, Sialyl-fucosyl-lactose, synthetic sLex, synthetic sLea, nonfucosylated sLea (sLec), and oligosaccharide no. 7 were even more effective (0.63 to 1.25 mmol/L) than sialyllactose (2.5 mmol/L) to inhibit the CA activity. Because the domain NeuNAcα2-3Gal is the only one common to the inhibitory oligosaccharides, these data indicate that such domain contains the minimal epitope structure. It is noteworthy that this notion was already suggested by earlier studies (reviewed in Roelcke1,3 ). RBCs lack sLea and sLex as detected by mouse MoAbs CSLEX-1 and 19.9, respectively.27,29 Therefore, the fact that these carbohydrate inhibit the RBC CA activity of anti–Sia-lb CAs suggests that these autoantibodies could be able to bind sLex and sLea on nucleated cells. To test this, we next investigated by flow cytometry the cell-surface binding of biotinylated (Bio) CAGAS (Bio-GAS) to nucleated cell types such as leukocytes, HUVECs, and the colon carcinoma cell line COLO205, in which the expression of sLea and/or sLex has been studied.15,16,20,29,30 38-47
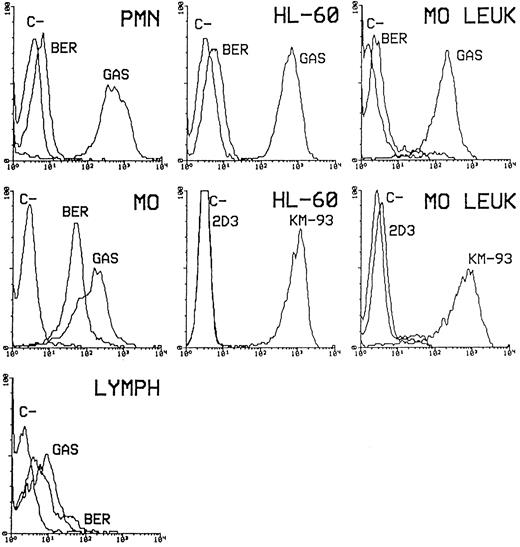
Flow cytometry analysis of GAS epitope expression on the surface of nucleated cells.GAS epitope was strongly expressed on PMN, Mo, and HL-60 cells, as well as on monocytic leukemia cells that were negative for CD14, CD34, CD61, CD5, CD7, CD3, CD2, CD19, and CD22 and positive for CD45, CD33, HLA class II antigens, CD15, CD13, CD11b, and CD11c (Fig 1). As expected,20-23,30-33,43,44 anti-sLex mouse MoAbs CXLEX-1 (not shown) and KM-93 (Fig 1) also strongly bound these myeloid cell types, with a binding profile nearly identical to that of CAGAS. A considerable proportion (36% to 60%, depending on the donor) of normal blood lymphocytes, from which Mo were gated out on the basis of forward and side scatter parameters, also expressed GAS epitope moderately (Fig 1); similar results were found with MoAb KM-93,38 whereas CSLEX-1 MoAb15,16,20,29 39-43 bound only a few (≤9 %) of these cells (not shown). Data with normal leukocytes in Fig 1 are from 1 representative experiment of 30 performed with cells from different donors.
Binding of biotinylated (Bio) CAGAS (GAS) to normal leukocytes, HL-60 cell line, and monocytic leukemia cells (MO. LEUK), which was analyzed in parallel with the binding of unconjugated anti-sLex (KM-93 and CSLEX-1) and anti-sLea (2D3 and 19.9) mouse MoAbs and biotinylated anti-i IgMk CABER (BER). The binding of CSLEX-1 to myeloid cells (PMN, MO, HL-60, and MO.LEUK) was nearly identical to that shown for Bio-GAS and KM-93. Whereas the binding of MoAb KM-93 to lymphocytes (LYMPH) was similar to that shown for BIo-GAS, CSLEX-1 MoAb bound only a few (≤9%) of these cells. Data with normal leukocytes are from 1 representaive experiment of 30 performed with cells from different healthy donors. For each donor, PMN and PBMC were obtained, and Mo and lymphocytes present in PBMCs were gated on the basis of forward and side scatter parameters. MO contained ≤0.1% of CD3+ cells and CD19+ cells, and lymphocytes contained ≤1% of CD14+ cells. Bound antibodies were detected by means of SA-PE and FITC-conjugated goat antibodies to mouse Igs. Negative controls (C−) for CAs and for mouse Igs were, respectively, biotinylated (Bio) polyclonal human IgM (MPoly) and unreactive isotype-matched mouse MoAbs. X-axis, Log fluorescence intensity; Y-axis, relative number of cells.
Binding of biotinylated (Bio) CAGAS (GAS) to normal leukocytes, HL-60 cell line, and monocytic leukemia cells (MO. LEUK), which was analyzed in parallel with the binding of unconjugated anti-sLex (KM-93 and CSLEX-1) and anti-sLea (2D3 and 19.9) mouse MoAbs and biotinylated anti-i IgMk CABER (BER). The binding of CSLEX-1 to myeloid cells (PMN, MO, HL-60, and MO.LEUK) was nearly identical to that shown for Bio-GAS and KM-93. Whereas the binding of MoAb KM-93 to lymphocytes (LYMPH) was similar to that shown for BIo-GAS, CSLEX-1 MoAb bound only a few (≤9%) of these cells. Data with normal leukocytes are from 1 representaive experiment of 30 performed with cells from different healthy donors. For each donor, PMN and PBMC were obtained, and Mo and lymphocytes present in PBMCs were gated on the basis of forward and side scatter parameters. MO contained ≤0.1% of CD3+ cells and CD19+ cells, and lymphocytes contained ≤1% of CD14+ cells. Bound antibodies were detected by means of SA-PE and FITC-conjugated goat antibodies to mouse Igs. Negative controls (C−) for CAs and for mouse Igs were, respectively, biotinylated (Bio) polyclonal human IgM (MPoly) and unreactive isotype-matched mouse MoAbs. X-axis, Log fluorescence intensity; Y-axis, relative number of cells.
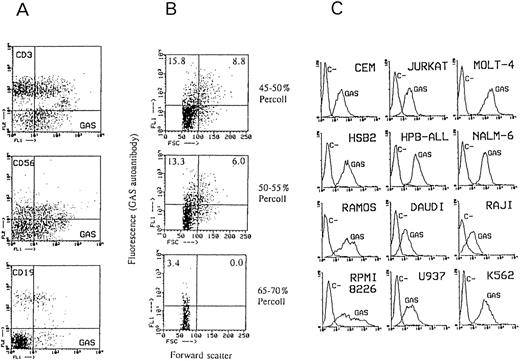
Two-color immunofluorescence with gated lymphocytes from 3 different donors (Fig 2A) showed that GAS epitope was moderately expressed on 15% to 35% (depending on the donor) of T lymphocytes (CD3+) and on 40% to 50% of NK cells (CD56+) and was poorly expressed on 15% to 30% of B cells (CD19+). The binding of CAGAS to 15% to 35% of blood T lymphocytes was confirmed by using PBMC depleted of Mo, NK cells, and B lymphocytes, and it was similar to that found with the MoAb KM-9338 (not shown). The expression of sLex on NK cells has been documented using most anti-sLex murine MoAbs.16,20,39 42
Binding of Bio-GAS (A) to normal blood lymphocyte subsets, (B) to purified tonsil B cells of different size obtained at different Percoll gradients, and (C) to leukocyte cell lines. In (A), two-color immunofluorescence with Bio-GAS/SA-FITC and PE-conjugated CD3, CD56, and CD19 MoAbs was performed; lymphocytes were gated from PBMCs on the basis of forward and side scatter parameters and they contained no detectable CD14+ cells. In (B), the forward scatter dot marker was established arbitrarily. These purified tonsil B cells mostly expressed (≥90%) CD19 and (70% to 85%) i antigen as detected by CABER,10,11 13 whereas they contained ≤4% of CD3+ cells and no detectable CD56+ cells and CD14+ cells. In (C), the immunofluorescence technique was as described in Fig 1.
Binding of Bio-GAS (A) to normal blood lymphocyte subsets, (B) to purified tonsil B cells of different size obtained at different Percoll gradients, and (C) to leukocyte cell lines. In (A), two-color immunofluorescence with Bio-GAS/SA-FITC and PE-conjugated CD3, CD56, and CD19 MoAbs was performed; lymphocytes were gated from PBMCs on the basis of forward and side scatter parameters and they contained no detectable CD14+ cells. In (B), the forward scatter dot marker was established arbitrarily. These purified tonsil B cells mostly expressed (≥90%) CD19 and (70% to 85%) i antigen as detected by CABER,10,11 13 whereas they contained ≤4% of CD3+ cells and no detectable CD56+ cells and CD14+ cells. In (C), the immunofluorescence technique was as described in Fig 1.
The expression of sLex on T and B lymphocytes is upregulated after their activation, and some anti-sLex MoAbs, such as SNH-3 and FH6 MoAbs, do not bind these cells until they are activated, although CSLEX-1 MoAb fails to bind these cells even when they are activated.16,20,38-41 The expression of GAS epitope increased on activated T lymphocytes, because in two independent experiments with cells from different donors, 50% to 60% of CD3+ cells of 3-day PHA-blasts were GAS+, whereas in the same unstimulated PBMCs only 18% to 23% of CD3+ cells were GAS+. As expected,38 the binding of anti-sLex KM-93 MoAb to PHA-blasts also increased and was similar to that of CAGAS (not shown). As shown in Fig 2, GAS epitope expression was also increased on in vivo activated B cells, because the number of GAS+ cells among purified tonsil B cells increased as their density decreased.32 GAS epitope was highly or clearly expressed on lymphoid T-cell (JURKAT, MOLT-4, CEM, HSB2, and HPB-ALL) and B-cell lines (RAMOS, NALM-6, DAUDI, and the myeloma cell line RPMI8226), the monocytic cell line U937, and the erythroleukemia cell line K562, whereas it was poorly expressed on the B-cell line RAJI. As expected,16,20,29 CSLEX-1 epitope was undetectable in all these cell lines, including K562, except RPMI8226, in which it bound 47% of cells. The binding of MoAb KM-93 with these cells could not be assessed, but most of them have been found to express sLex as detected by mouse MoAbs different from CSLEX-1 such as SHN3 or even FH6 or the MoAb 2F3.16,38-41 As expected,20 anti-sLea MoAbs 19.9 (not shown) and 2D3 (Fig 1) did not bind myeloid cells (Fig 1) or lymphoid cells (not shown).
The similarity in the cell distribution of CAGAS and KM-93 epitopes among leukocytes also occurred in cultured HUVEC, because we found that MoAb KM-93, but not MoAb CSLEX-1,44 moderately bound most cultured HUVECs and that the same thing occurred with CAGAS (data not shown).
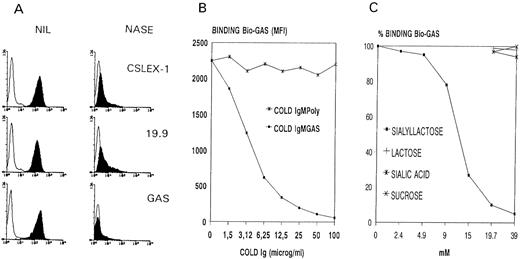
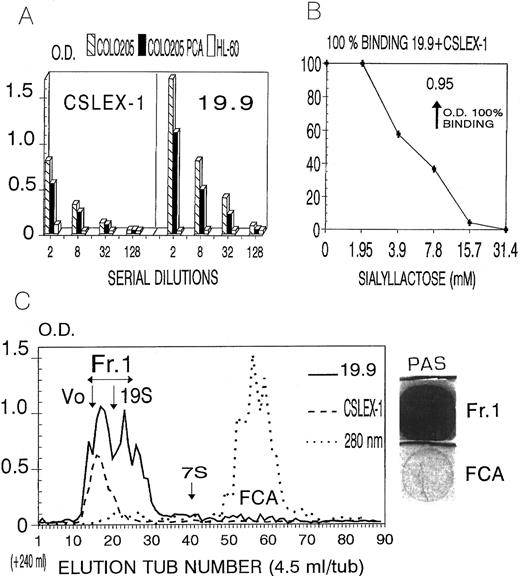
Lastly, as shown in Fig 3A, CAGAS also strongly bound colon carcinoma cell line, COLO205, with a binding profile nearly identical to that of anti-sLex (CSLEX-1) and sLea (19.9) mouse MoAbs.45-47
(A) Binding of Bio-GAS and mouse MoAbs CSLEX-1 (anti-sLex) and 19.9 (anti-sLea) to mock-treated (NIL) and sialidase-treated (NASE) colon carcinoma cell line COLO205. (B and C) Inhibition studies of the binding of Bio-GAS to HL-60 cells by unconjugated CAGAS (Cold IgMGAS), unconjugated IgMPoly (Cold IgMPoly), sialyllactose, lactose, sialic acid, and sucrose. The immunofluorescence technique was as described in Fig 1. Solid and open curves in (A) correspond, respectively, to antibodies and negative controls. In (B), MFI indicates mean fluorescence intensity as obtained by flow cytometry. The MFI value of the 100% CAGAS binding in (C) corresponded to 2,450.
(A) Binding of Bio-GAS and mouse MoAbs CSLEX-1 (anti-sLex) and 19.9 (anti-sLea) to mock-treated (NIL) and sialidase-treated (NASE) colon carcinoma cell line COLO205. (B and C) Inhibition studies of the binding of Bio-GAS to HL-60 cells by unconjugated CAGAS (Cold IgMGAS), unconjugated IgMPoly (Cold IgMPoly), sialyllactose, lactose, sialic acid, and sucrose. The immunofluorescence technique was as described in Fig 1. Solid and open curves in (A) correspond, respectively, to antibodies and negative controls. In (B), MFI indicates mean fluorescence intensity as obtained by flow cytometry. The MFI value of the 100% CAGAS binding in (C) corresponded to 2,450.
The binding of CAGAS to nucleated cells is not Fc receptor-dependent but due to the recognition of sialo-dependent epitopes as those found on human RBCs.This conclusion is based on the following data. The negative control for flow cytometry experiments was biotinylated polyclonal IgM (Mpoly) used at the same concentration as Bio-GAS. Although not shown in all figures for simplicity, the binding of anti-i CABER (IgMk like CAGAS) to nucleated cells was examined in parallel and showed a different cell distribution pattern, as can be observed in Fig 1. Moreover, unconjugated Mpoly failed to inhibit the binding of Bio-GAS to HL-60 cell line, whereas unconjugated CAGAS caused a concentration-dependent inhibition (Fig 3B); the same pattern occurred using PMN and Mo (not shown). In addition, sialyllactose, but not by sialic acid or lactose or sucrose, inhibited the binding of CAGAS to HL-60 cell line (Fig 3C), Mo, PMN, and COLO205 cell line (not shown). Furthermore, sialidase treatment of COLO205 cells abolished GAS epitope as well as CSLEX-1 and 19.9 epitopes (Fig 3A). Sialidase treatment of Mo, PMN, and HL-60 cells also abolished GAS and CSLEX-1 epitopes and increased the expression of CD15, but not that of i antigen as detected by CABER (data not shown). On the other hand, it is noteworthy that treatment of PMN and HL-60 cells with pronase or trypsin did not reduce the binding of CAGAS (not shown); and the same thing occurred with the binding of anti-sLa MoAbs CSLEX-1 and KM-93 (not shown), an expected finding given data from others.20,22,39,42 43
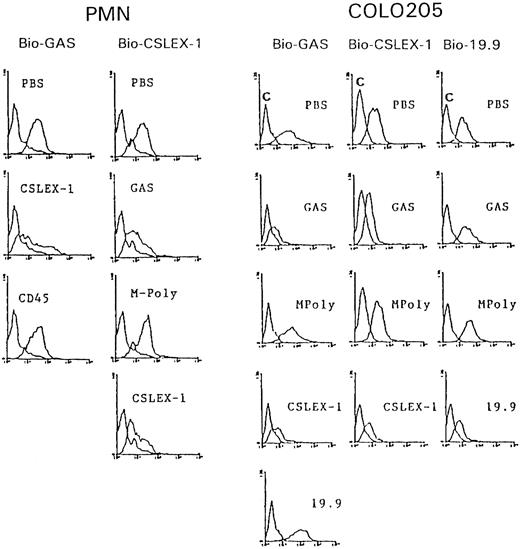
Competitive cross-inhibition experiments between CAGAS and anti-sLex (CSLEX-1, KM-93) and anti-sLea (19.9) murine MoAbs.Because the domain NeuNAcα2-3Gal constitutes the minimal epitope of CAGAS and both this domain and fucose play a critical for the epitope recognition of mouse MoAbs directed to sLex and sLea, we investigated whether CAGAS and these mouse MoAbs exhibit a certain degree of competition in their binding to their respective epitopes. We found that CSLEX-1 and CAGAS antibodies clearly inhibited each other in their respective binding to PMN (Fig 4A) as well as to COLO205 (Fig 4B) and HL-60 cells (not shown). Moreover, in three independent series of cross-blocking experiments between CAGAS and the anti-sLex murine MoAb, KM-93, using HL-60 cells, it was found that the KM-93 MoAb inhibited significantly the binding of CAGAS, whereas CAGAS did not affect the KM-93 binding (not shown). Because CSLEX-1, KM-93, and GAS are of IgM isotype, these differential inhibition effects of CSLEX-1 and KM-93 are not attributable to a isotype size-dependent effect but are consistent with the fact that KM-93 and CSLEX-1 mouse MoAbs recognize different epitopes in sLex carbohydrate.24 It should be noted that mouse MoAbs CD8 (not shown) and CD45 (Fig 4A) of IgM isotype such as CSLEX-1 and KM-93 did not affect the binding of CAGAS and that IgMpoly also failed to affect the binding of CSLEX-1 (Fig 4). Overall, these data indicate that GAS epitope overlaps and/or is very proximal to CSLEX-1 and KM-93 epitopes. In contrast, the binding of 19.9 murine MoAb (anti-sLea; IgG1 isotype) and CAGAS to COLO205 cells lacked any degree of cross-competition (Fig 4B). Cross-inhibition experiments between IgM isotype MoAb 2D3 MoAb (anti-sLea plus anti-nonfucosylated sLea) and CAGAS could not be performed.
Cross-blocking experiments between CAGAS and mouse MoAb CSLEX-1 (anti-sLex) for their binding to PMN leukocytes (PMN) and between CAGAS and mouse MoAbs CSLEX-1 and 19.9 (anti-sLa) for their binding to COLO205 cells (COLO205). Histograms show the binding of a subsaturating dose of biotinylated (Bio) antibody (indicated at the top of histograms) in the presence of PBS or an excess of unconjugated antibody (indicated within each histogram). For control purposes, mouse MoAb CD45 (138-3) of IgM isotype-like CSLEX-1 was also included as inhibitor. The binding of antibodies was detected by means of SA-PE. Negative control fluorescence curves are the most proximal to the Y-axis and were Bio-MPoly for Bio-GAS and biotinylated isotype-matched unreactive mouse for Bio-CSLEX-1 and Bio-19.9.
Cross-blocking experiments between CAGAS and mouse MoAb CSLEX-1 (anti-sLex) for their binding to PMN leukocytes (PMN) and between CAGAS and mouse MoAbs CSLEX-1 and 19.9 (anti-sLa) for their binding to COLO205 cells (COLO205). Histograms show the binding of a subsaturating dose of biotinylated (Bio) antibody (indicated at the top of histograms) in the presence of PBS or an excess of unconjugated antibody (indicated within each histogram). For control purposes, mouse MoAb CD45 (138-3) of IgM isotype-like CSLEX-1 was also included as inhibitor. The binding of antibodies was detected by means of SA-PE. Negative control fluorescence curves are the most proximal to the Y-axis and were Bio-MPoly for Bio-GAS and biotinylated isotype-matched unreactive mouse for Bio-CSLEX-1 and Bio-19.9.
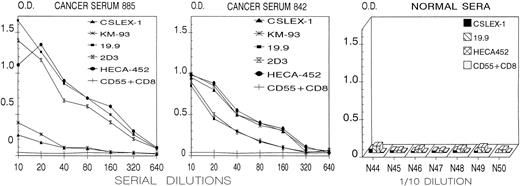
CAGAS binds soluble cancer-associated mucins bearing sLex and sLea determinants.We next investigated whether CAGAS binds cancer-related mucins bearing sLex and/or sLea determinants. High levels of these mucins are found in the serum of many patients with cancer and in the culture supernatant of malignant epithelial cell lines such as COLO205 cells.28,29,35,36,45-47 We found that plate-bound CAGAS captured, in a dose-dependent manner, components reactive with the murine MoAbs recognizing sLea (19.9 and 2D3) and sLex (CSLEX-1 and KM-93) in the sera of 12 patients with cancer, but not in the sera of 14 healthy individuals (Fig 5). In most cancer sera, the components bearing sLea predominated over those bearing sLex (cancer serum 885 in Fig 5), whereas in few cancer sera similar levels of both components were found (cancer serum 842 in Fig 5). Note that CAGAS-captured components also reacted with the rat MoAb HECA-452, an expected finding given that this MoAb detects an epitope common to sLex and sLea.20 21
ELISA plate-bound CAGAS captured cancer-associated serum components reactive with anti-sLex (CSLEX-1 and KM-93) and anti-sLea (19.9 and 2D3) murine MoAbs as well as with rat MoAb HECA-452 that recognize a common epitope in both sLex and sLea.20,21 30 The results with serial dilutions of 2 (885 and 842) of 12 cancer sera and with 7 (N44-N50) of 14 normal sera (1/10 dilution) are shown. Plate-bound CAGAS did not capture serum components reactive with MoAb CD55 (143-30, of IgG1 isotype such as 19.9) plus MoAb CD8 (109-2D4, of IgM isotype such as CSLEX-1, KM-93, and 2D3). Normal rat serum (1/100 dilution) used in place of HECA-452 showed values not different from those shown for CD55 + CD8 murine MoAbs. The results with plate-bound MPoly or plate-bound anti-i CABER (not shown) were not different from those shown for plate-bound CAGAS with CD55 + CD8 MoAbs. Y-axis and X-axis show, respectively, background-subtracted OD and reciprocal values of dilutions.
ELISA plate-bound CAGAS captured cancer-associated serum components reactive with anti-sLex (CSLEX-1 and KM-93) and anti-sLea (19.9 and 2D3) murine MoAbs as well as with rat MoAb HECA-452 that recognize a common epitope in both sLex and sLea.20,21 30 The results with serial dilutions of 2 (885 and 842) of 12 cancer sera and with 7 (N44-N50) of 14 normal sera (1/10 dilution) are shown. Plate-bound CAGAS did not capture serum components reactive with MoAb CD55 (143-30, of IgG1 isotype such as 19.9) plus MoAb CD8 (109-2D4, of IgM isotype such as CSLEX-1, KM-93, and 2D3). Normal rat serum (1/100 dilution) used in place of HECA-452 showed values not different from those shown for CD55 + CD8 murine MoAbs. The results with plate-bound MPoly or plate-bound anti-i CABER (not shown) were not different from those shown for plate-bound CAGAS with CD55 + CD8 MoAbs. Y-axis and X-axis show, respectively, background-subtracted OD and reciprocal values of dilutions.
In the supernatant of COLO205 cell line (COLO Sup), but not in the supernatants of HL-60 (Fig 6A) JURKAT, MOLT-4, RAJI, or CEM cell lines (not shown), plate-bound CAGAS also captured, in a dose-dependent manner, components bearing sLex and sLea determinants, with the latter predominating over the former (Fig 6A). As it occurred in cancer sera, these components also reacted with rat MoAb HECA-452 (not shown). A considerable proportion of these components remained soluble in 0.6 N PCA, a property indicative of their mucin nature35,36 (Fig 6A). Such components were eluted in the void volume (Vo) peak (molecular weight higher than 1.5 × 106; assessed by Dextran Blue 2000) and in the elution peak of 19S components (970,000 Daltons, human IgM) of a sephacryl S-300 column and were strongly stained in the PAS reaction, a finding corroborating their mucin nature37 (Fig 6C).
(A) ELISA plate-bound CAGAS captured components bearing sLex and sLea determinants in the supernatant of COLO205 cells, but not in the culture supernatant of HL-60; and a considerable proportion of these components remained soluble in 0.6 N percloric acid (COLO-PCA). The results shown with MoAbs KM-93 and 2D3 were similar to those shown for MoAbs CSLEX-1 and 19.9, respectively. (B) Inhibition by sialyllactose of the binding of plate-bound CAGAS to sLex- and sLea-bearing components present in COLO205 supernatant (1/8 dilution). (C) Sephacryl S-300 column chromatography of CAGAS-captured components bearing sLex and sLea present in COLO205 culture supernatant. The void volume (Vo) and the elution peaks of human IgM (19S) and IgG (7S) are indicated. The large peak at OD 280 nm corresponds to the fetal calf serum albumin (FCA) present in the culture medium. The staining of elution fraction Fr1 in the PAS reaction was analyzed using FCA as negative control.
(A) ELISA plate-bound CAGAS captured components bearing sLex and sLea determinants in the supernatant of COLO205 cells, but not in the culture supernatant of HL-60; and a considerable proportion of these components remained soluble in 0.6 N percloric acid (COLO-PCA). The results shown with MoAbs KM-93 and 2D3 were similar to those shown for MoAbs CSLEX-1 and 19.9, respectively. (B) Inhibition by sialyllactose of the binding of plate-bound CAGAS to sLex- and sLea-bearing components present in COLO205 supernatant (1/8 dilution). (C) Sephacryl S-300 column chromatography of CAGAS-captured components bearing sLex and sLea present in COLO205 culture supernatant. The void volume (Vo) and the elution peaks of human IgM (19S) and IgG (7S) are indicated. The large peak at OD 280 nm corresponds to the fetal calf serum albumin (FCA) present in the culture medium. The staining of elution fraction Fr1 in the PAS reaction was analyzed using FCA as negative control.
Sialyllactose inhibits the binding of CAGAS to Sialyl Lex- and sialyl Lea-bearing mucins and these mucins inhibit the RBC CA activity of this CAGAS.Data shown in Fig 6B indicate that plate-bound CAGAS binds sLex- and sLea-bearing mucins by recognizing the NeuNAcα2-3Gal domain, because sialyllactose inhibited this binding in a dose-dependent manner (Fig 6B); lactose and sialic acid alone did not inhibited this binding (not shown). As sialyllactose inhibits the CA activity of CAGAS as well as its binding to nucleated cells and mucins, such mucins constitute a soluble form of CAGAS epitope-bearing antigen, and therefore they should inhibit the RBC CA capacity of CAGAS. To test this, we performed RBC cold agglutination inhibition experiments with the Sephacryl S300-isolated and PCA-soluble mucin preparation (20× concentrated with respect the native COLO205 culture supernatant from which it was obtained) and with a similar PCA-soluble preparation from HL-60 cells, and we found that the former, but not the latter, inhibited the RBC CA activity of CAGAS while it failed to inhibit the RBC CA activity of CAMES (anti-I) and CABER (anti-i) that were tested in parallel (data not shown).
DISCUSSION
This study shows that the domain NeuNAcα2-3Gal, as found in sLea and sLex and related carbohydrates, constitutes the minimal recognition epitope of anti–Sia-lb (anti-Gd) CAs and that these autoantibodies bind such domain in sLea, sLex, and related carbohydrates expressed on both the surface of nucleated cells and in soluble cancer-related mucins.
Sialylated I and i antigens are present on RBC4,48 and therefore anti–Sia-lb CAs (anti-Gd) can bind the domain NeuNAcα2-3Gal in sialylated I on adult RBCs and in sialylated i on neonate RBCs. On normal myeloid cells, sialylated forms of I/i antigen appear to be absent or very low.48 Accordingly, we found that the desialylation of myeloid cells did not or poorly increase the epitopes recognized by anti-i CABER (see the flow cytometry analysis in the Results) and I antigen as detected by a CA of anti-I CAMES (unpublished data), whereas GAS epitope and sLex disappeared and Lex expression increased. Therefore, on leukocytes, sLex might be the main carbohydrate in which GAS epitope is found, although on myeloid cells, it could be also found in the glycolipid CDw65, which is strongly expressed on both Mo and PMN.49 The carbohydrate of CDw65 glycolipid (Neu-NAc;ga2 - 3Gal;gb1 - 4ClcNAc;ga1 - 3Gal;gb1 - 4 ;ob Fuc;ga1 - 3 ;cb GlcNAc ) -is nearly identical to sLex and it was found to act as a Selectin ligand.50 Moreover, there is evidence that the epitopes of CDw65, sLex, and Lex MoAbs are in close proximity on granulocytes.49 On COLO205 cell line and soluble cancer-related mucins, GAS epitope can be found in both sLex and sLea as well as in nonfucosylated sLea (sLec), because this carbohydrate can be expressed on colon carcinoma cell lines and in serum-cancer mucins20; whether COLO205 cell line express CDw65, as it occurs with some other colocarcinoma cell lines,49 remains unknown to our knowledge.
GAS epitope on both RBCs and nucleated cells is resistant to proteases, indicating that it is found in glycolipids or protease-resistant glycoprotein regions. There is evidence indicating that, on RBCs, anti–Sia-lb (anti-Gd) bind only glycolipids1,3 and the same is likely to occur on leukocytes, because CAGAS failed to detect protein bands by high-sensitivity Western blot and immunoprecipitation analysis with lysates of HL-60 cells and PMN (unpublished data). Although studies on sLex-bearing molecules as ligands of selectins have mainly focused those of glycoprotein nature, sLex-bearing glycolipids are also functionally active as ligands of selectins, a phenomenon that can be of physiologic importance given that the proportion of sLex-bearing glycolipids on myeloid cells is far predominant over sLex-bearing glycoproteins.22 Therefore, it could be argued that GAS epitope is present in both cell-surface glycolipids and glycoproteins, and that the latter, due to their low proportion, appear to be undetectable. Alternatively, and more likely, GAS epitope could be only recognized in cell-surface glycolipids but not in cell-surface glycoproteins as it occurs with conventional murine MoAbs recognizing either sLex (CSLEX-1, FH-6, SNH-3, and KM-93) or sLea (19.9 and 2D3). Although all of these MoAbs bind protease-resistant epitopes on the surface of cells, all of them strongly bind soluble cancer-derived mucins as they occur with CAGAS. It is possible that the large size and high glycosilation of mucins might provide a high epitopic density or availability resembling that found in glycolipids on the surface of cells. In fact, carbohydrates such as sLex or sLea are found in a great variety of glycolipids and glycoproteins and their antigenicity can be largely influenced by modifications of the core carbohydrate where the determinant is found as well as by the particular molecule species carrying the carbohydrate. This antigenic heterogeneity is clearly reflected by the different tissue distribution of epitopes detected by conventional anti-sLex MoAbs, CSLEX-1, KM-93, FH6, and SHN-3, that detect the epitope only in glycolipids on the surface of cells and in soluble mucins (see flow cytometry analysis of the Results).15,16,20,24,25,29,35,38-44 Moreover, there are other nonconventional anti-slex MoAbs (AM3, 2F3, and 2H5) that recognize the epitope mainly on cancer mucins (MoAb AM3)51 or in both glycolipids and glycoproteins expressed on some particular cell types such as memory CD4 T cells (MoAb 2F3)41 or in high endothelial venules (HEV; MoAb 2F5).52 Moreover, the HECA-452 epitope, which corresponds to a common domain shared by both sLex and sLea, is expressed in cell-surface glycolipids and glycoproteins in both myeloid cells, HEV, and activated T cells migrating to the skin.21,30 Remarkably, the HECA-452 epitope corresponds in reality to the domain recognized by selectins.21 As shown in the present study, MoAb HECA-452 also binds soluble cancer-associated mucins bearing sLex and sLea.
It is worth noting that some members of the sialoadhesin family of adhesion molecules (sialoadhesin and CD33) recognize on their natural ligands the same sugar domain as CAGAS (NeuNAcα2-3Gal; Freeman et al23 and the references therein). Therefore, the cellular distribution of GAS epitope is likely to delineate that of the natural ligands of such sialoadhesin members. In accord with this notion, recombinant sialoadhesin has been shown to bind mainly cells of the granulocytic lineage in which sLex and CDw65 are abundantly expressed.53
Blood group-related carbohydrate antigens constitute onco-developmental molecules, which are probably involved in systemic cell-cell or cell-soluble molecule interactions,9-18,24,25,28,30,34-36,38-47 and their expression on RBCs might simply reflect a residual epiphenomenic trait. This is illustrated by the fact that sLea and sLex have been studied as onco-developmental markers27-29,35,36 many years before they were identified as the main ligands of selectins (Berg et al21 and the references therein). The present data with CAGAS clearly reinforce this notion. Moreover, the systemic relevance of blood group-related sugars is further stressed by their role as target molecules for the microbial carbohydrate-binding proteins (also called adhesins) that mediate the attachment of pathogens to host cells and tissues,4-6,54-56 as indicated by the fact that (1) M.Pn binds to the NeuNAcα2-3Gal β 1-4GlcNAc sequence,56 mainly when it is found in long type II chains such as sialylated I/i antigen type4-6; (2) Helicobacter pylori binds to Leb57; (3) a great variety of microbial adhesins bind the Galα1-4Gal domain of blood-group P antigen (Zopf and Roth55 and the references therein), which is defined by CD77 mouse MoAbs58; (4) the influenza A virus binds granulocytes by recognizing sialo groups in sLex59; and (5) the pertussis toxin of B pertussis shares similarities with mammalian selectins and binds sialo groups in the selectin ligands, sLex, and sLea (Heereze et al60 and the references therein). On the other hand, many immunologically important cell-surface molecules, including CD21, CD4, CD36, CD55, CD14, β2 -integrin CD11β/CD18, CD54 or ICAM-1 and chemokine receptors act as the receptors of a number of pathogens (Schlossman et al,61 Hoepelman and Toumanen,62 Isberg and Tran Van Nhieu,63 and Moore64 and the references therein). Moreover, a wide spectrum of microbial adhesins bind extracellular matrix ligands of nonleukocyte integrins,65 and a number of such integrins also act as the receptors of several pathogens (Schlossman et al,61 Hoepelman and Toumanen,62 and Isberg and Tran Van Nhieu63 and the references therein). In addition, CD77, which, as indicated above, constitutes the receptor of a great variety of pathogens, is able to act as the natural ligand of CD19, a functionally important molecule of B lymphocyes.58 In most of these cases, it remains still unknown whether the microbial adhesin binds an aminoacid portion or saccharides decorating these proteins.
These data suggest that one strategy of pathogens to subvert the host defence mechanisms might reside in their capacity to simulate host adhesion receptors and bind their ligands or bind the host adhesion receptors themselves or other immunologically important host molecules. Such a scenario is also relevant for the capacity of pathogens to elicit autoimmune responses to host components including those of carbohydrate nature that are usually poorly immunogenic. A prokaryotic adhesin that binds a host carbohydrate can act as a carrier and/or an adjuvant or a T-cell superantigen that could activate CD4+ helper T cells, which, in turn, would promote the activation of B cells bearing surface receptors autoreactive for the complexed host carbohydrate, and therefore autoantibodies to the self carbohydrate, without cross-reactivity for the pathogen, could appear. Certainly, infection-induced CAs constitute an excellent model for this possible mechanism of infection-induced autoimmunity as there is evidence that M.Pn-induced CAs are directed to the host carbohydrates that act as the receptors of M.pn.4-6 56
A great variety of pathogens can induce CAs, but M.Pn appears to be the most effective.1,2 Although M.Pn-induced CAs are thought to be mainly of anti-I specificity, anti–Sia-b (anti-Fl) CA is usually associated with the anti-I one, in the sera of patients with recent M.Pn infection2; at a lower frequency, the same is true for anti–Sia-lb (anti-Gd) CA.2 Anti–Sia-b CAs recognize sialic acid α2-3–linked to branched sequences of I antigen type1-3,6 and can act as a receptor for M.Pn.6 These data strongly suggest that CAs of anti–Sia-b (anti-Fl) and anti–Sia-lb (anti-Gd) specificities induced by M.Pn could recognize different epitopes in the sialylated groups directly involved in the binding of M.Pn, whereas M.Pn-induced anti-I CAs could be directed to the backbone of type II chain sequence, where such sialylated groups are found.
It is clear that the NeuNAcα2-3Galβ1-4GlcNAc sequence recognized by M.Pn adhesins is found not only in sialylated I/i antigens but also in sLex and CDw65. Influenza A virus can also bind sialic α2-3-linked in these carbohydrates, and pertussis toxin binds sLex (see above). Moreover, all these carbohydrates (sLex, sialylated I/i, and CDw65) are expressed at the natural infection site of these pathogens, ie, bronchial epithelium and lung macrophages.5,14,25 Nevertheless, B pertussis and influenza A virus infections, in contrast to M.Pn infection, have not been reported to induce CAs. Therefore, other factors of a pathogen, apart from the capacity of its adhesins to bind a given host carbohydrate, play a critical role for the induction of autoantibodies to this carbohydrate. Such factors might depend on adhesin characteristics and/or adhesin-related accessory proteins or other pathogen's components serving as superantigens and/or adjuvants as known to be present in the case M.Pn.56
ACKNOWLEDGMENT
The invaluable technical assistance of Mònica Cristina Rión, a recipient of a “FP II CIRIT” grant (“Generalitat de Catalunya”, Spain), is highly appreciated. We are indebted to Dr L.J. Picker (University of Texas, Dallas, TX) for his generosity in providing HECA450 rat MoAb and to Dr G. Dighiero (Institut Pasteur, Paris, France) and Dr J. Vives Puiggròs (Servei d'Immunologia, Hospital Clı́nic, Barcelona, Spain) for helpful suggestions in reviewing the manuscript.
Supported by Grants No. FIS93/0178, FIS96/0883, FIS96/0274, and FIS96/2145 from Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo (Spain) and by Marató TV3 95/3009.
Address reprint requests to Teresa Gallart, MD, Servei d'Immunologia, Hospital Clı́nic Universitari, Villarroel, 170, 08036 Barcelona, Spain.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal