Abstract
Leukemic B cells in chronic lymphocytic leukemia (B-CLL) typically exhibit low or undetectable surface Ig. Because the B29 (CD79b and Igβ) and mb-1 (CD79a and Igα) gene products are required for surface Ig display in the B-cell receptor complex (BCR), we analyzed the expression of these genes in B-CLL cells. The majority (83%) of the randomly selected B-CLL patient samples analyzed exhibited low or undetectable surface BCR measured by μ heavy chain and B29 expression. Levels of mb-1 mRNA in these B-CLL samples with low surface BCR were similar to those in normal B cells. Among those with decreased surface expression, B29 mRNA was not detected in half of these B-CLL samples. The remaining B-CLL samples with diminished surface BCR contained normal levels of B29 mRNA. Further analysis of cDNA clones from the majority of these latter samples contained point mutations, insertions, or deletions that were largely located in the B29 transmembrane and cytoplasmic domains. These results indicate the occurrence of somatic mutations predicted to affect B29 expression and/or function in the majority of B-CLL and suggest that these aberrations underlie the diminished surface BCR display and loss of BCR signaling characteristic of this leukemia.
B-CHRONIC lymphocytic leukemia (B-CLL) is the most common form of leukemia in the United States. It is characterized by the prolonged accumulation of monoclonal B lymphocytes that often express CD5.1,2 The molecular origins of this leukemia are still unresolved. B-CLL karyotypes are often very complex with multiple cytogenetic abnormalities.3,4 Recurrent nonrandom alterations of chromosomes 12, 13, 14, 17, and 18 have been described, yet no specific chromosomal abnormalities or translocations have been shown to lead to CLL. In addition to the expression of CD5, B-CLL cells characteristically exhibit several other hallmark properties, including elevated BCL-2 expression and decreased levels of surface Ig in comparison to normal B cells.5-7 Intracellular Ig content is reportedly either normal or elevated in B-CLL cells, suggesting possible defects in Ig transport and surface display.8 9 It is postulated that these features may contribute to the prolonged survival and resistance to apoptosis characteristic of B-CLL.
Surface Ig on B cells is complexed with heterodimers of two critical accessory proteins called B29 (Igβ and CD79b) and mb-1 (Igα and CD79a) in the B-cell antigen receptor (BCR). B29 is critical for the intracellular assembly and subsequent surface expression of the BCR in human and murine lymphocytes. Its presence is absolutely essential for the in vitro reconstitution of a functional BCR in both lymphoid and nonlymphoid cell lines.10-12 In addition to blocking the surface display of Ig, targeted knockouts of either B29 or mb-1 genes severely disrupt pre-B–cell and B-cell development.13-15 This establishes a very early role for the B29-mb-1 heterodimer complex in pre-B–cell development consistent with the finding that both genes are expressed in early precursor cells in which Ig μ heavy chain genes have not been fully rearranged. Signaling through the BCR is mediated by the so-called ITAM motifs in the cytoplasmic tails of the heterodimeric B29-mb-1 chains that are phosphorylated on critical tyrosine residues.12,16 B29-mb-1 signaling triggers Src and Syk family kinases leading to B-cell activation.17 18
The critical role of the B29/mb-1 heterodimer in BCR surface display and signal transduction strongly suggests that perturbation or alteration of either of these genes could prevent surface Ig expression and cause diminished Ig signaling characteristic of B-CLL cells. We report here that the majority (83%) of B-CLL examined exhibited low surface B29 that was directly correlated with surface Ig expression. Approximately half of these B-CLL samples with low or undetectable surface B29 had either undetectable or greatly reduced B29 mRNA levels. The remaining half contained moderate to apparently normal B29 mRNA levels. However, in this latter group, cDNA clones of B29 mRNA were found to contain single or multiple mutations that are likely to prevent intracellular assembly of B29 into BCR complexes and/or affect signaling by B29 in surface displayed BCR complexes. These findings indicate that aberrant B29 expression or autosomal B29 mutations occur in the majority of B-CLL and suggest that these may account for the loss of surface BCR and defective BCR signaling that are hallmarks of B-CLL.
MATERIALS AND METHODS
Patient population and isolation of B-CLL cells.Blood samples were obtained with informed consent from 18 patients randomly selected from a group of 60 patients in the UCLA system who were previously diagnosed with CLL using standard clinical criteria. A minority had bone marrow aspirates or lymph node biopsies performed for diagnosis. None were receiving cytotoxic chemotherapy at the time samples were obtained, but several had previously received some form of therapy. Disease status or degree of leukocytosis was not used in selecting patients for this study. Peripheral blood lymphocytes (PBLs) were separated by centrifugation on a Hypaque density gradient, washed with phosphate-buffered saline, and stored at −70°C. Aliquots also were resuspended in RPMI tissue culture media and 10% dimethyl sulfoxide (DMSO) before freezing at −80°C.
Immunofluorescence analysis of cell surface molecules.Ficoll Hypaque-isolated mononuclear CLL cells were assessed by two-color flow cytometry. B29 expression was measured by indirect immunofluorescence using CB3-1, a mouse γ1 monoclonal antibody (MoAb) to human B29,19 as the primary antibody followed by phycoerythrin (PE)-labeled goat antibodies to mouse Ig (Becton Dickinson, San Jose, CA). Dose titrations were performed to ensure that the amount of B29 MoAb used provided optimal detection (data not shown). Staining was performed in the presence of 50% human AB serum to block potential Fc binding. After B29 staining, fluorescein isothiocyanate (FITC)-conjugated MoAbs directed against μ, δ, κ, and γ, CD19 or CD20 (Becton Dickinson) was used to determine surface expression of these molecules. The flow cytometric data were acquired on a FACScan (Becton Dickinson) and analyzed using the LYSIS II Software. Mean fluorescence for various markers was compared by the Mann-Whitney U test for nonparametric measurements.
RNA isolation and Northern blotting.Frozen pellets containing approximately 1 × 108 cells were pulverized under liquid N2 , resuspended in 4 mol/L guanidinium isothiocyanate (Sigma, St Louis, MO), and then incubated at 4°C with 200 mmol/L KOAc, pH 4.0, and acid phenol (Ambion, Austin, TX). After centrifugation at 15,000g for 30 minutes, the aqueous layer was collected and total cellular RNA was precipitated with an equal volume of isopropyl alcohol at −20°C. Ten micrograms of RNA was denatured with 7 mol/L glyoxal at 50°C for 1 hour, electropheresed on a 1% agarose gel in 10 mmol/L sodium phosphate buffer, and then transferred to a charged nylon membrane (MSI, Westboro, MA) by overnight capillary transfer in 20× SSC. Membranes were baked under vacuum at 80°C for 60 minutes. A 32P-labeled human B29 cDNA probe was prepared by random priming and was hybridized with the membranes at 65°C overnight, washed at high stringency, and then exposed to film at −70°C.
Simultaneous RNAse protection assays.Total cellular RNA was extracted from CLL cells using guanidinium-acid phenol-chloroform method as described above. A 700-bp Apa I fragment of pBS27-1B/Kpn I containing the first exon of the B29 gene was subcloned into the (−) vector (Promega, Madison, WI) and used as the template for in vitro transcription. A 900-bp Nco I B29 cDNA containing most of the coding region and a 115-bp mb-1 cDNA fragment were similarly subcloned. Using 1 μg of linearized plasmid, α32P-CTP (650 Ci/mmol; ICN, Costa Mesa, CA), and SP6 RNA polymerase (20 U; Promega), RNA probes with high specific activity were synthesized. After DNAse treatment to remove the template, the probe was purified over a G-50 Sephadex spin column and 8 × 104 cpm of the probe was ethanol precipitated with 20 μg of tRNA and then hybridized at 48°C overnight. Unannealed probe was digested with a 1:100 dilution of RNAse A/T1 (Ambion) and the protected fragment was separated on a 6% denaturing polyacrylamide gel. Gels were fixed and dried before autoradiography.
Reverse transcription-polymerase chain reaction (RT-PCR) and mRNA cloning.One microgram of total cellular RNA was reverse transcribed at 70°C for 15 minutes using the rTth enzyme (Perkin Elmer Cetus, Foster City, CA), 10 mmol/L deoxynucleotides, and the antisense primers 5′TGTCCTCATAGGTGGCTGTCTGG3′ (corresponding to nucleotides 723-701) in the cDNA sequence20 or 5′CCATCCCATGTGTG GGGACGGATC3′ (corresponding to nucleotides 1067-1044). First-strand cDNA was immediately amplified using the same DNA polymerase and the sense primers 5′GGAATCCCAAAGGTAGTG3′ (201-218) or 5′AGAAGTGCAACAACACCTCGGAGGTCTACC3′ (464-494) under the following conditions: 94°C for 2 minutes, 56°C for 1 minute, and 72°C for 1 minute for 25 cycles. Quality of the PCR reaction was confirmed by 2% Metaphor (FMC BioProducts, Rockland, ME) gel electrophoresis followed by ethidium bromide staining. Amplified PCR products were subcloned into a TA cloning vector (Invitrogen, San Diego, CA) and transformed into DH5α. Sequence analysis of purified denatured plasmids was performed by the dideoxy method of Sanger using Sequenase (US Biochemical, Cleveland, OH) and was confirmed by sequencing the complementary strand.
RESULTS
Fluorescence-activated cell sorting (FACS) analyses of Ig and B29 on B-CLL cells.The expression of Ig μ heavy chains and B29 on the surface of B-CLL cells was measured using dual-color flow cytometry. Figure 1 shows the results from such analyses for three representative CLL patient samples and control normal B lymphocytes. The three B-CLL panels are representative of the categories of B-CLL cells designated normal (++), low (+), and negative (−) based on their relative expression of these two BCR components (summarized in Table 1). The B-cell population in a normal individual shows two populations: the majority showing bright B29 and μ heavy chain staining and a far smaller population exhibiting dim B29 and μ heavy chain staining (Fig 1A).
Immunocytometric analyses of surface B29 and Ig μ heavy chain expression on normal and CLL patient B-cell samples. Surface B29 and μ chain expression was determined by dual staining on peripheral blood mononuclear B cells from a normal individual ([A], designated ++) and from 3 representative CLL patients distinguished on their expression of surface B29 and μ proteins. B-CLL cells from patient 44 ([B], designated ++) displayed apparently normal surface B29 and μ levels. B-CLL cells from patient 19 ([C], scored +) exhibited low levels of both proteins. B-CLL cells from patient 21 ([D], designated −) were scored negative for surface B29 and μ expression.
Immunocytometric analyses of surface B29 and Ig μ heavy chain expression on normal and CLL patient B-cell samples. Surface B29 and μ chain expression was determined by dual staining on peripheral blood mononuclear B cells from a normal individual ([A], designated ++) and from 3 representative CLL patients distinguished on their expression of surface B29 and μ proteins. B-CLL cells from patient 44 ([B], designated ++) displayed apparently normal surface B29 and μ levels. B-CLL cells from patient 19 ([C], scored +) exhibited low levels of both proteins. B-CLL cells from patient 21 ([D], designated −) were scored negative for surface B29 and μ expression.
Summary of Immunocytometric Analyses of Cell Surface Proteins on B-CLL and Normal B Cells
| CLL . | μ . | δ . | B29 . | Light Chain . |
|---|---|---|---|---|
| 2 | + | + | + | κ |
| 4 | + | + | + | κ |
| 12 | ++ | − | ++ | κ |
| 14 | + | + | + | κ |
| 18 | + | + | + | κ |
| 19 | + | − | + | λ |
| 21 | − | − | − | − |
| 25 | + | − | + | λ |
| 32 | − | − | − | − |
| 36 | + | + | + | κ |
| 37 | ++ | − | ++ | λ |
| 38 | + | + | + | κ |
| 39 | − | − | − | − |
| 40 | − | − | + | − |
| 41 | − | − | − | − |
| 42 | − | − | − | − |
| 43 | − | − | − | − |
| 44 | ++ | − | ++ | κ |
| Normal | ++/+ | ND | ++/+ | κ/λ |
| CLL . | μ . | δ . | B29 . | Light Chain . |
|---|---|---|---|---|
| 2 | + | + | + | κ |
| 4 | + | + | + | κ |
| 12 | ++ | − | ++ | κ |
| 14 | + | + | + | κ |
| 18 | + | + | + | κ |
| 19 | + | − | + | λ |
| 21 | − | − | − | − |
| 25 | + | − | + | λ |
| 32 | − | − | − | − |
| 36 | + | + | + | κ |
| 37 | ++ | − | ++ | λ |
| 38 | + | + | + | κ |
| 39 | − | − | − | − |
| 40 | − | − | + | − |
| 41 | − | − | − | − |
| 42 | − | − | − | − |
| 43 | − | − | − | − |
| 44 | ++ | − | ++ | κ |
| Normal | ++/+ | ND | ++/+ | κ/λ |
FACS results on surface B29 and Ig μ heavy chains are scored relative to the bright staining (++) population of normal B cells. All B-CLL samples were CD19/20+. All were CD5+ except CLL 25, 38, and 39.
Abbreviation, ND, not determined.
B29 and μ chain expression on three B-CLL cell samples (ie, CLL 12, 37, and 44, designated ++ in Table 1) were comparable in intensity for both markers to the bright B-cell population in normal B-cell controls (Fig 1A and B). Intense bright staining was also seen with Ramos cells, an established B-cell line derived from a patient with Burkitt's lymphoma (data not shown). Eight B-CLL samples represented by CLL 19 (Fig 1C) exhibited diminished staining for B29 and μ chains compared with the bright staining seen in normal B cells or the three B-CLL patient cell samples with intense staining previously mentioned. These 8 B-CLL samples with low staining of surface B29 and μ chains are designated positive (ie, +) in Table 1. The remaining 7 B-CLL samples represented by CLL 21 (Fig 1D; designated − in Table 1) had barely detectable B29 and μ chain staining, with few if any cells staining even with the diminished intensity of the low B-CLL category. Based on these FACS results, 15 of the 18 B-CLL samples (ie, 83%) scored low or negative for surface B29 and μ chain expression. This high frequency of diminished or absent Ig expression is in accord with larger published surveys6 21 wherein 85% of B-CLL cell samples exhibited low to undetectable surface Ig expression. In all samples, the surface expression of B29 and μ chains were concordant and proportional, consistent with the requirement of both chains for surface BCR display.
All of the B-CLL samples examined expressed the B-cell–specific antigens, CD19 and CD20, on the vast majority of their cells. Clonality of the B-CLL cells was evaluated by light chain use determined in FACS (Table 1). The majority (15 of 18 [83%]) of the B-CLL cell samples were positive for CD5, in accordance with larger surveys of B-CLL cell surface molecule expression.6 21 There was no correlation between CD5 and surface Ig or B29 expression. In some patients there was a small population of companion CD3+, CD19−, CD20− cells that were not included in the analyses of mean channel fluorescence or in the analyses of B29 and μ chain expression in the normal B-cell samples.
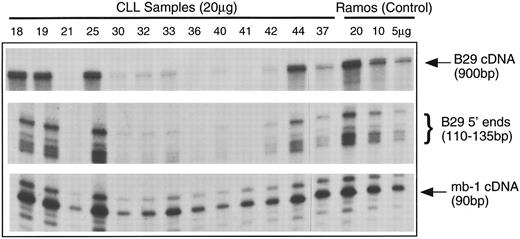
B29 and mb-1 mRNA in B-CLL cells.B29 mRNA was not detectable in a number of B-CLL samples analyzed by Northern blot analysis (data not shown). Accordingly, we used the highly sensitive RNAse protection assay (RPA) technique to examine B29 and mb-1 mRNA. Total cellular RNA extracted from B-CLL cells was hybridized simultaneously with three antisense ribonucleotide probes and then digested with RNAses and analyzed in gels. The B29 cDNA probe encompassed most of the coding region. A second B29 genomic probe, which included part of the first exon and the immediate 5′ flanking region, was designed to examine differences in the usage of the mapped transcription initiation sites in B-CLL cell samples compared with normal B cells. An additional riboprobe corresponding to a small internal fragment of the mb-1 cDNA allowed comparison of steady-state mb-1 mRNA levels. Ramos B-cell RNA was used as a positive control. A β actin riboprobe was included in each reaction to control for equivalent loading (results not shown).
Figure 2 is a representative panel of B-CLL mRNA samples analyzed by RPA. The levels of B29 mRNA were quite variable among these B-CLL samples. The three B-CLL samples that displayed normal B29 surface staining (Table 1) had B29 mRNA levels equivalent to the Ramos B-cell control. Of the 15 B-CLL cell samples that had poor surface expression of B29 protein, 8 samples (ie, 53%) had either drastically reduced levels or undetectable B29 mRNA. The remaining 7 samples (ie, 47%) had diminished to apparently normal B29 mRNA levels.
Analyses of B29 and mb-1 mRNA expression in B-CLL cell samples using simultaneous RPA. Antisense riboprobes were hybridized with total cellular RNA samples from B-CLL cells samples (20 μg/lane) and from control Ramos B cells (5, 10, and 20 μg/lane), RNAse-digested, and analyzed in gels. RNAse protected probe fragments corresponding to the majority of the B29 mRNA coding sequence (900 bp), the multiple 5′ B29 mRNA termini (110 to 135 bp), and an internal mb-1 coding region segment (90 bp) are displayed for 14 representative B-CLL cell samples and the Ramos controls.
Analyses of B29 and mb-1 mRNA expression in B-CLL cell samples using simultaneous RPA. Antisense riboprobes were hybridized with total cellular RNA samples from B-CLL cells samples (20 μg/lane) and from control Ramos B cells (5, 10, and 20 μg/lane), RNAse-digested, and analyzed in gels. RNAse protected probe fragments corresponding to the majority of the B29 mRNA coding sequence (900 bp), the multiple 5′ B29 mRNA termini (110 to 135 bp), and an internal mb-1 coding region segment (90 bp) are displayed for 14 representative B-CLL cell samples and the Ramos controls.
The 5′ B29 probe showed that the B-CLL cells exhibited the same pattern of multiple transcription start sites seen in control B cells.22 In addition, the relative intensities of the 5′ B29 mRNA RPA fragments exactly duplicated the findings for the coding region of B29 mRNA. In contrast, the levels of mb-1 mRNA were similar in all B-CLL samples and comparable to the mb-1 mRNA levels in the Ramos B-cell control. Other studies that have examined mb-1 expression in B-cell neoplasms reported that mb-1 protein is expressed at apparently normal levels in the all B-CLL cases evaluated.23 24 These RPA results findings indicate that mb-1 mRNA is present at normal levels in the majority of B-CLL cell samples, whereas B29 mRNA is absent or greatly diminished in nearly half of B-CLL cell samples. This establishes that genetic aberrations affecting B29 mRNA levels underlie the poor surface B29 protein expression in a substantial fraction of B-CLL cell samples.
Mutations in B29 mRNA from B-CLL cells.Seven B-CLL samples with low or absent surface B29 protein exhibited B29 mRNA levels similar to the Ramos B-cell control. B29 mRNA from 6 of these B-CLL samples was amplified in RT-PCR reactions and the products were analyzed by sequencing to detect mutations possibly affecting B29 expression or function. Two different pairs of PCR primers were used to prepare two partially overlapping RT-PCR DNA fragments of 603 and 522 bp, respectively, largely covering B29 mRNA from the extracellular Ig-like domain through the 3′ untranslated region (Fig 3A). The RT-PCR DNA products were cloned and multiple isolates were sequenced in both directions. All mutations reported were identified in two or more independently isolated B29 mRNA clones. Mutations reported here were detected in more than 1 CLL patient sample using different primer pairs, making it unlikely that the alterations in these B29 mRNA clones represent PCR errors.
Summary of mutations and alterations identified in cDNA clones generated from B-CLL cell B29 mRNA species by RT-PCR. RT-PCR reactions were performed with 2 μg of cellular RNA from 6 selected B-CLL cell samples using two overlapping pairs of B29-specific primers (A). RT-PCR products were cloned and fully sequenced. A summary of the alterations detected in these B29 mRNA sequences and of their predicted consequences for B29 protein translation and function is shown (B). Two kinds of B29 mRNA clones (designated A and B) were detected in all B-CLL cell samples and are presumed to correspond to the products of B29 alleles. Numbering of the cDNA and amino acid sequences begins with the first base of the ATG methionine codon initiating translation. IG Domain denotes the B29 Ig-like domain. TM and CYTO denote the B29 transmembrane and cytoplasmic segments, respectively.
Summary of mutations and alterations identified in cDNA clones generated from B-CLL cell B29 mRNA species by RT-PCR. RT-PCR reactions were performed with 2 μg of cellular RNA from 6 selected B-CLL cell samples using two overlapping pairs of B29-specific primers (A). RT-PCR products were cloned and fully sequenced. A summary of the alterations detected in these B29 mRNA sequences and of their predicted consequences for B29 protein translation and function is shown (B). Two kinds of B29 mRNA clones (designated A and B) were detected in all B-CLL cell samples and are presumed to correspond to the products of B29 alleles. Numbering of the cDNA and amino acid sequences begins with the first base of the ATG methionine codon initiating translation. IG Domain denotes the B29 Ig-like domain. TM and CYTO denote the B29 transmembrane and cytoplasmic segments, respectively.
Two classes of B29 mRNA clones were identified in the RT-PCR–generated cDNA clones from all 6 B-CLL samples analyzed, presumably corresponding to the transcripts from both B29 alleles (summarized in Fig 3B). B-CLL sample 37 contained two B29 mRNA species that are both predicted to code for the wild-type B29 protein, consistent with the finding of normal surface B29 expression on these cells. One of the B29 mRNA species exactly matched the known B29 coding sequence, whereas the other differed by a single base change (ie, TGT → TGC) in the codon for Cys122 involved in the Ig-like domain loop. This same silent base change was also detected in sequenced B29 mRNA clones from 2 other B-CLL samples (ie, CLL 19 and 44). Accordingly, we presume that this silent base change represents a previously undetected normal B29 allele.19,20,25 26 B29 sequences are being determined from a panel of normal individuals to confirm this prediction.
Two of the sequenced B-CLL samples (CLL 2 and 25) each contained a B29 mRNA species bearing a single missense mutation along with a normal B29 mRNA species with the previously known B29 sequence. In CLL 2, the CAG → CAA substitution is predicted to produce the nonconservative amino acid change, Q87R, in the extracellular Ig-like domain of B29. This mutation closely follows the conserved Trp76 and Trp78 residues that are known to facilitate interchain interactions between Ig-like domains.27 In CLL 25, the ATC → ACC substitution is expected to generate the nonconservative amino acid change, I201T, in the cytoplasmic tail just after the YEGLD sequence of the B29 ITAM motif. It is not clear if this mutation would affect B29 function.
As mentioned above, CLL 19 contained a B29 mRNA species with the silent TGC base change (Cys122) expected to code for the wild-type B29 protein. The other B29 mRNA species from CLL 19 contained two in-frame deletions presumably resulting from multiple deletional events. The first removed the codons encoding amino acids 91-167 (ie, most of the B29 Ig domain with 7 residues of the TM region), whereas the second eliminated amino acids 184-185 (ie, both Asp residues at the beginning of the B29 cytoplasmic tail). These two negatively charged Asp may function in anchoring the TM region in the plasma membrane. In addition, Asp185 is highly conserved in ITAM motifs.16 28 The codons for 184-185 directly follow the splice junction preceding exon 5, suggesting that this deletion may be due to alterations affecting B29 mRNA splicing. Beginning with Ser186, the remaining portion of the cytoplasmic tail is expressed in-frame. These two internal deletions in this B29 mRNA species are expected to prevent the resultant B29 protein from forming heterodimers with mb-1, thereby presumably reducing the surface BCR displayed on these B-CLL cells.
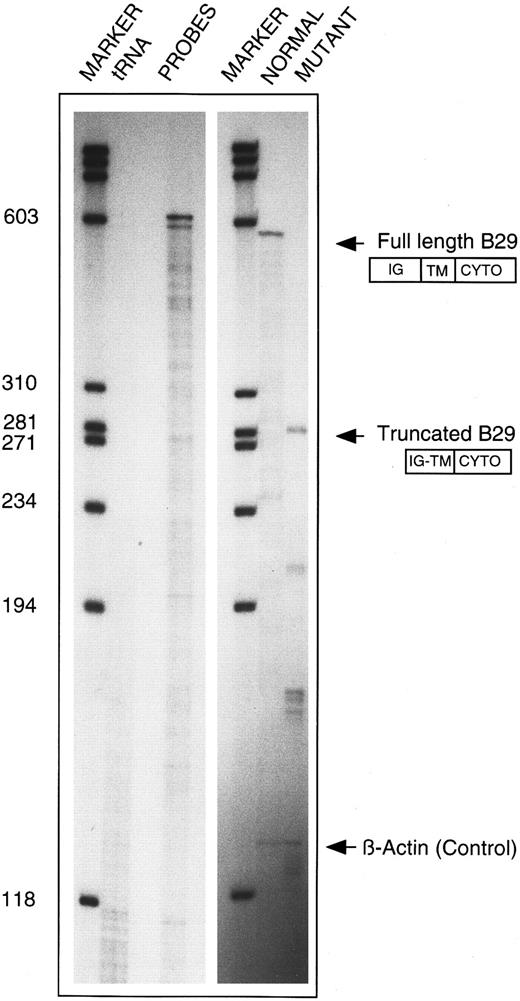
An additional RPA was performed to substantiate the RT-PCR finding on the internally deleted B29 mRNA species from CLL 19. A 590-bp BsiAKHI/Kpn I B29 cDNA fragment that contains exons 3 to 6 was subcloned into a plasmid vector and used to prepare an antisense riboprobe. The truncated RT-PCR product from CLL 19 was similarly subcloned. Figure 4 shows RNAse protection of mRNA species from the patient that correspond to both B29 alleles. The wild-type riboprobe consisting of exons 3 to 6 detected a 585-bp fragment, as expected. When the truncated sequence was used as the probe, a novel 285-bp fragment corresponding to the truncated B29 mRNA was protected. Additional smaller fragments of 210, 171, and 48 bp in the same lane correspond to the expected sizes for protected products from exons 3 through 6 of the full-length B29 mRNA.
Confirmation of internally deleted variant B29 mRNA in B-CLL 19 cells. Total cellular RNA samples from patient CLL 19 (10 μg each) were independently reacted with an antisense riboprobe for either wild-type B29 mRNA (ie, 630-nt long) or for the internally deleted variant B29 mRNA cloned from this patient (ie, 588-nt long) and analyzed in RPA. Lanes (left to right) 1 and 4, molecular weight markers; lane 2, control of both riboprobes digested with tRNA; lane 3, undigested wild-type (630 nt) and variant (588 nt) B29 riboprobes; lane 5, protected RPA product obtained with wild-type (630 nt) B29 riboprobe; lane 6, protected RPA products obtained with internally deleted CLL 19 variant (588 nt) B29 riboprobe. β-Actin riboprobe digestion products are shown in lanes 5 and 6 to confirm equivalent RNA inputs.
Confirmation of internally deleted variant B29 mRNA in B-CLL 19 cells. Total cellular RNA samples from patient CLL 19 (10 μg each) were independently reacted with an antisense riboprobe for either wild-type B29 mRNA (ie, 630-nt long) or for the internally deleted variant B29 mRNA cloned from this patient (ie, 588-nt long) and analyzed in RPA. Lanes (left to right) 1 and 4, molecular weight markers; lane 2, control of both riboprobes digested with tRNA; lane 3, undigested wild-type (630 nt) and variant (588 nt) B29 riboprobes; lane 5, protected RPA product obtained with wild-type (630 nt) B29 riboprobe; lane 6, protected RPA products obtained with internally deleted CLL 19 variant (588 nt) B29 riboprobe. β-Actin riboprobe digestion products are shown in lanes 5 and 6 to confirm equivalent RNA inputs.
B-CLL samples (CLL 18 and 44) each contained two variant B29 mRNA species, implying the occurrence of mutations affecting both B29 alleles in these cells. One B29 mRNA species from CLL 18 contained two independent G insertions predicted to produce frameshifts leading to chain termination in the TM domain. The second B29 mRNA species in CLL 18 had two independent point mutations predicted to generate amino acid changes (ie, F145L and K158R) and a third silent base change in the codon for L179. Both the B29 mRNA species from CLL 44 are predicted to encode truncated proteins. The presumed mRNA product of one CLL 44 B29 allele is predicted to produce a severely truncated B29 protein fragment terminated shortly after a single G insertion shifting the reading frame at G58 in the Ig-like domain. The B29 mRNA from the other CLL 44 B29 allele contained two mutations: a single base change (CTG → CGG) predicted to result in the L157 R mutation directly preceding the TM domain and the deletion of a single A in the codon for the critical ITAM residue (Y207) predicted to produce a frameshift and chain termination after only two codons. The protein products from this latter B29 allele presumably are capable of heterodimer formation with mb-1 and assembly into surface-displayed BCR because the B-CLL cells from this patient exhibited normal surface B29 (and μ chains) in FACS. However, the truncation of the ITAM motif at the Y207 kinase phosphorylation site and the loss of the remainder of the cytoplasmic tail are predicted to abrogate signaling by this variant B29 protein.
DISCUSSION
These findings strongly indicate that aberrations affecting B29 gene expression and/or function frequently underlie the diminished surface BCR expression in B-CLL. Greater than 80% of the B-CLL cell samples analyzed here had low or negative surface B29 protein that directly correlated with the level of surface Ig expression. Applying a combination of molecular approaches, we detected reduced levels of B29 mRNA or mutations in B29 genes in most of the B-CLL cell samples examined. The combined results of RPA studies of B29 mRNA in B-CLL cells and immunocytometry on surface B29 have identified three subsets of B-CLL cells with regard to the level of B29 expression in B-CLL: those with normal B29 surface protein expression and normal mRNA levels (3/18 [17%]); those with low or absent surface protein and low or no B29 mRNA (8/18 [44%]); and a third group with low B29 surface expression, but normal levels of B29 mRNA (7/18 [39%]). This latter group with the discordant result of low B29 protein expression, but apparently normal B29 mRNA levels, was the focus of the detailed mutational analyses reported here.
Mutations were detected by sequencing in either one or both B29 mRNA species from 5 of these 6 B-CLL samples. In three samples, mutations were detected in 1 of the 2 B29 mRNA species and in the remaining two samples alterations were found in both B29 mRNA species. We presume that these findings respectively reflect mutations affecting either one or both B29 alleles in these cells, respectively. More than one mutation was detected in the B29 mRNA species from three of the B-CLL samples, suggesting that the B29 alleles encoding these mRNA species have sustained multiple intraclonal mutational events. Most of the abnormalities detected were either frameshifts (from deletions or insertions) or missense mutations. A similar constellation of mutations have been described in other genetic diseases including the WAS gene in Wiskott-Aldrich syndrome, BTK in X-linked agammaglobulinemia, and BRCA1 in breast cancer,29-31 where these alterations generally result in qualitative defects in protein processing, transport or function. In this regard, the pattern of B29 mutations is especially intriguing with respect to their possible effects on B29 protein function in B-CLL. The mutations are concentrated in protein domains required for B29 function. All but two mutations are located within exons 4 and 5 that encode the B29 TM and CYTO domains. These two segments comprise the most highly conserved sequences between murine and human B29 proteins with greater than 95% amino acid identity.20 26 This extraordinarily high degree of amino acid sequence conservation points to the critical role of these regions in mediating B29 interactions with other BCR members and in signal transduction. Accordingly, it is likely that the mutations in these regions could account for the diminished surface B29 expression seen in their respective B-CLL cell samples. These mutations are also likely to affect B29 signaling, and the functional consequences of these mutations on the intracellular assembly of B29, mb-1 and Ig into BCR and on signaling by surface BCR are currently being analyzed.
One B29 mRNA species from CLL 19 was found to contain a large internal deletion removing 76 amino acids from the Ig-like and TM domains, along with a second smaller deletion removing two amino acids from the CYTO domain. RPA analysis independently confirmed the structure of this internally truncated B29 mRNA species. Two other studies have reported findings predicting deleted B29 mRNA species.32 33 In one study, a minor truncated version of B29 protein (called Ig-γ) was found to lack 30 to 36 amino acids from the C-terminus and not to be phosphorylated, consistent with the postulated loss of the CYTO domain containing the ITAM motif. This minor truncated protein was detected in subsets of normal B cells. A variant mRNA species encoding the Ig-γ product was not identified. A second study identified an internally truncated B29 cDNA clone lacking the entire extracellular Ig-like domain presumably through removal of exon 3 through alternative splicing. A minor mRNA species corresponding to this internally truncated mRNA was detected in a panel of established B-cell lines. The RT-PCR primers used to generate our B29 mRNA clones were designed to allow detection of these two alternately spliced B29 mRNA species. However, we found no evidence for either of these truncated mRNA species in B-CLL cells.
Recent studies using transgenic mouse models have expanded our understanding of the critical role of B29 in early lymphocyte development. The cytoplasmic domain of B29 appears sufficient for the antigen-independent pro-B– to pre-B–cell transition.14 Mutations of a mouse heavy chain transgene that hinder its association with endogenous B29 result in a block in the transition beyond the pre-B–cell stage. Inactivation of the B29 gene by homologous recombination resulted in a specific block in the early pre-B–cell stage of bone marrow cells in homozygous mice.15 Germline mutations of B29 would be expected to result in a B-lineage phenotype that is much less mature than that seen in B-CLL. The phenotype of CLL B cells is more consistent with a transforming event at the B-cell stage that results in somatic B29 gene mutations.
What then is the origin of these somatic mutations? We previously mapped the human B29 gene to chromosome locus, 17q23. This locus is known to undergo recurrent nonrandom translocations in B-CLL cells, albeit at a very low frequency (ie, ≤5%). Genomic DNA Southern blots probed with human B29 cDNA showed no detectable alterations in 12 B-CLL samples (results not shown). Thus, translocations affecting B29 genes cannot account for the aberrant B29 expression seen in the majority of the B-CLL cases examined here. The 17q region reportedly exhibits a high frequency of mutations and deletions in other malignancies. Loss of heterozygosity (LOH) is also prevalent over the region of human chromosome 17q that includes the B29 locus. In one study of 146 tumors, 70% of ovarian tumors exhibited loss of allelic heterozygosity detected by a 17q23-qter probe.34 Studies of breast cancer LOH identified the BRCA1 gene that is located at 17q21.30,35 High mutation rates of the tk locus at 17q23-25 in a lymphoblastoid cell line have been attributed to LOH.36 The high potential for mutation and LOH within this region may be important determinants contributing to the prevalence of B29 mutations in B-CLL. That these mutations or other B29 alterations are manifested in B-CLL is presumably due to the absolute tissue specificity of the B29 gene.
Strikingly different results of B29 expression were reported recently in chronic B-cell diseases using the SN8 MoAb.37 SN8 was produced by immunizing mice with an antigen preparation from human prolymphocytic leukemia cells.38 It identifies an extracellular epitope of the human B29/mb-1 heterodimer. Because only 5% of CLL cases in their large series had detectable surface expression of B29, they concluded that SN8 may be a useful marker for non-CLL B-cell disorders. The CB3-1 MoAb used in our studies also recognizes an extracellular epitope. Its clear specificity for human B29 that can be detected throughout B-lymphocyte differentiation would perhaps make it a more selective antibody for further studies of B29 expression.19
Surface expression of Ig defines B-cell maturation and represents a fundamental event in B-cell progression toward proliferation or cell death. Greater than 80% of the CLL samples examined here and in other studies have decreased surface Ig expression.1,6 CLL B cells typically exist in a noncycling G0 state that may confer a survival advantage for these cells. We hypothesize that mutations or other perturbations affecting B29 gene expression or function produce B-CLL cells with diminished surface BCR that are unresponsive to antigen binding and therefore unable to initiate either growth-stimulatory or apoptotic signal cascades. In support of this prediction, a recent study showed that BCR-signaled apoptosis of B cells required both B29 and mb-1.39 The key issue to be resolved is whether these B29 gene mutations are a direct cause of B-CLL or a secondary event that follows another primary transforming event. Finally, we predict that B29 gene mutations that result in reduced surface Ig expression may have prognostic significance because normal surface Ig is reportedly correlated with poor survival in CLL.6 40
Supported by Grants No. CA12800 and GM40185 from the National Institutes of Health (R.W. and A.S.), by a Minority Medical Faculty Development Award from the Robert Wood Johnson Foundation (A.A.T.), and by a Stein-Oppenheimer Award from the UCLA School of Medicine (A.A.T. and R.W.). M.D.C. is a Howard Hughes Medical Institute Investigator.
Address reprint requests to Alexis A. Thompson, MD, Department of Pediatrics, UCLA School of Medicine, 10833 LeConte Ave, Los Angeles, CA 90095.

![Fig. 1. Immunocytometric analyses of surface B29 and Ig μ heavy chain expression on normal and CLL patient B-cell samples. Surface B29 and μ chain expression was determined by dual staining on peripheral blood mononuclear B cells from a normal individual ([A], designated ++) and from 3 representative CLL patients distinguished on their expression of surface B29 and μ proteins. B-CLL cells from patient 44 ([B], designated ++) displayed apparently normal surface B29 and μ levels. B-CLL cells from patient 19 ([C], scored +) exhibited low levels of both proteins. B-CLL cells from patient 21 ([D], designated −) were scored negative for surface B29 and μ expression.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1387/3/m_bl_0039f1.jpeg?Expires=1763520512&Signature=srLqAzT3aJAUR0EDWojxKgUSfn2gZtqinw5q6rxtEmYYJQu1zOjg5sTNETxmVWnfl-RQbUH1S6zasCGPC43ueBPjd-MoZ1wBoO0LN07pvcv9MDR2pUsE8cmAwzsD2eK-TGQIaKud3-tBA7~g93dGt0iKJAY38fMPE6uFudfUOKhNRTxeR3BXECqU4~G0Wjb6PkzPgmEO~9UTP5akaoImiBfy78MAx43XWLaK6eAG1l7D7Gu7NoqCeE9NhVs~R73GhF3oSkReqio4SRqCD4LXLGA0nHEGDjZGqXKSlGaRdm7luISD4e2UPCR~Q9SJCoVWAVqOVCMczzRW41M9RkhFQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal