Abstract
Blood dendritic cells (DC) are susceptible to both macrophage (M) and T-cell line (T) tropic human immunodeficiency virus type 1. The CC chemokines RANTES, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, eotaxin, and, to a lesser extent, monocyte chemoattractant protein-1 (MCP-1) and MCP-4 blocked entry of M-tropic virus into blood DC. The CXC chemokine, SDF-1, a fusin (CXCR4 chemokine receptor) ligand, and an antifusin antibody inhibited DC entry by T-tropic virus. Purified blood DC contained CCR1, CCR2, CCR3, and CCR5 as well as the CXCR4 chemokine receptor RNA transcripts and high levels of fusin on the cell surface. The coexpression of multiple chemokine receptors offers a molecular mechanism to explain the permissiveness of DC for both M- and T-tropic viruses.
DENDRITIC CELLS (DC) represent a heterogeneous group of motile cells, including skin Langerhans cells (LC), mucosal and blood DC, interdigitating cells in T-cell areas of lymph nodes, and interstitial (tissue) DC.1,2 When these cells migrate to lymph nodes in vivo or when they are treated with cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF ) in vitro, they differentiate into cells that resemble mature blood DC more closely.2 DC/LC are strongly positive for HLA-DR class II molecules and present antigen efficiently to T cells.3 Upon encountering foreign antigens, DC/LC home to lymph nodes and activate resting T cells; trafficking and homing are believed to be mediated by chemotactic factors.4
Most infections with the human immunodeficiency virus-1 (HIV-1) worldwide involve sexual contact. Although HIV-1 infection of DC or LC in vitro is controversial,5,6 DC are among the first target cells after mucosal infection of rhesus monkeys with the simian immunodeficiency virus (SIV),7 which is closely related to HIV-1. Various groups have shown that primary DC are susceptible to HIV-1 infection,8-11 and virus has been detected in DC derived from blood, skin, and other tissues of infected individuals.12,13 Adenoid mucosa of infected asymptomatic individuals contained multinucleated DC/T-cell conjugates that expressed high levels of Gag protein, indicating active viral replication.14 These data suggest that DC/LC residing in blood, skin, or mucosal surfaces play an important role in primate lentiviral infection.
HIV-1 also infects other cell types, such as T cells, macrophages, and microglia. Virus isolates have been designated as macrophage tropic (M-tropic), T-cell line tropic (T-tropic), or dual tropic, based on their ability to replicate efficiently in primary macrophages, immortalized T-cell lines, or both.15 Although DC support replication of both M- or T-tropic viruses,9,11 strain-dependent differences in the relative replication levels have been noted.7
Soon after the inhibitory effects of chemokines on HIV-1 replication were described,16 several different chemokine receptors were identified as coreceptors for T- and M-tropic strains.17-22 The CXCRs are receptors for CXC (or α-) chemokines and the CCRs for CC (or β-) chemokines. Among the α-chemokine receptors, CXCR417 (also called HUMSTSR, LESTR, or fusin), and among the β-chemokines receptors, CCR119, CCR2b22, CCR321,22, and CCR5,18-22 have been implicated as HIV-1 coreceptors.
Recently, CXCR4 has been identified as a receptor for the stromal cell-derived factor, SDF-1, and as a coreceptor for T-tropic viruses.23,24 CCR5 and, to some extent, CCR1 and CCR3 are coreceptors for some M-tropic viruses,18-22 whereas CCR2b could be used as a coreceptor by the dualtropic strain 89.6.22 Individuals homozygous for a 32-bp deletion in the CCR5 gene were reported to be resistant to HIV-1 infection.25-27 However, HIV-1 infection of an individual with the 32-base deletions in the CCR5 gene has been described recently.28 In heterozygous individuals, this CCR5 deletion was associated with slower HIV-1 disease progression when compared with individuals carrying two normal alleles.27
The mechanism of viral entry into DC is ill defined. Recently, Granelli-Piperno et al29 showed mRNA expression of CCR5 and CXCR4 in DC cultures derived from mononuclear cells by in vitro differentiation with GM-CSF and interleukin-4 (IL-4) for 7 days. In their study, entry of HIV-Ba-L (M-tropic) into DC was blocked by RANTES and less completely by macrophage inflammatory protein-1α (MIP-1α). Whereas SDF-1 partially blocked entry of IIIB, it had no effect on the entry of Ba-L into these DC.29 However, chemokine receptor expression levels in DC isolated directly from blood remain to be established. We report here the expression of multiple chemokine receptor RNA transcripts in blood DC, as we detected RNA for CCR1, CCR2, CCR3, CCR4, CCR5 as well as CXCR4. We also describe the potential of β- and α-chemokines to block entry into these cells by M-tropic HIV-1 strains (Yu-2 and ADA) and the T-tropic isolate NL4-3, respectively.
MATERIALS AND METHODS
Virus stocks.Virus stocks were prepared by infecting either Jurkat cells (with strain NL4-3) or human peripheral blood mononuclear cells (PBMCs; with HIVYu-2 and HIVADA , obtained from the NIAID AIDS Repository, Rockville, MD). NL4-3 was kindly provided by Dr J. Zack (University of California, Los Angeles, CA).
Isolation of blood DC.Leukopacks were obtained from individual, anonymous, normal blood donors (Dana-Farber Cancer Institute Blood Bank, Boston, MA). DC were isolated as described30,31; the procedure was modified to include additional purification steps. Briefly, PBMCs were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density separation from blood donor buffy coats or leukopacks.9 T cells were positively enriched by rosetting with neuraminidase-treated sheep red blood cells (SRBCs) and were depleted of accessory leukocytes and B cells by nylon wool column separation (Polysciences Inc, Warrington, PA). DC were isolated from SRBC-rosette negative cells that were depleted of contaminating monocytes by plastic adhesion and multiple Fc panning on IgG-coated dishes. Low-density cells of this population (enriched for DC) were isolated by metrizamide (Sigma Chemicals Co, St Louis, MO) gradients (14.5%) at 500g for 12 minutes at room temperature. To increase the purity of DC, Fc panning on IgG-coated plates was performed on cells obtained from the second metrizamide gradient; DC were purified further by negative selection using Dynalbeads coated with anti-CD2, anti-CD3, anti-CD8, anti-CD14, and anti-CD19 monoclonal antibodies (MoAbs). The yield of purified DC from individual leukopacks varied from 8 × 105 to 1.5 × 106. DC viability was greater than 95% as confirmed by trypan blue exclusion before culture. Before virus exposure, purified DC were cultured for 12 hours in conditioned medium32 (prepared from human PBMCs stimulated with 10 μg/mL of phytohemagglutinin (PHA) for 72 h; supernatant was then clarified with a 0.22-μm filter, diluted in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and antibiotics, and stored at −20°C. To culture blood DC, conditioned medium was used at a final dilution of 1:10. DC purity was greater than 95% as judged by fluorescence-activated cell sorter (FACS; Fig 1A) or immunofluorescent staining. After HIV-1 pulsing, p24+, HLA-DR+ cells could be detected but neither CD3+ nor CD14+ cells were seen (data not shown). Because of the variation and the relative limitation in the number of DC obtained from each donor, individual experiments could not be duplicated exactly; rather, the inhibitory effects of each chemokine were assayed at least two or more times under similar conditions.
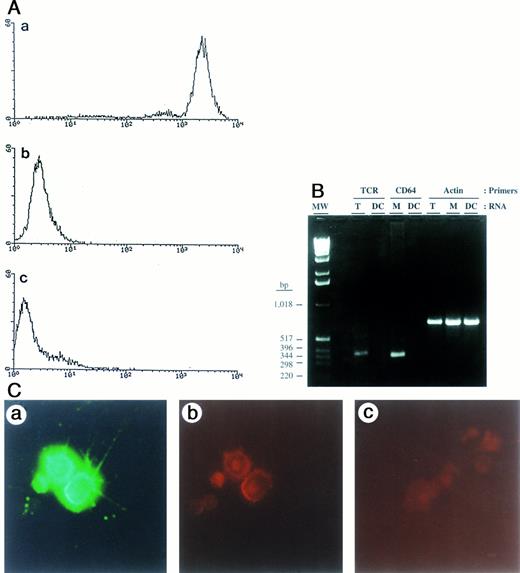
Analysis of surface markers by FACS, expression of mRNA transcripts by RT-PCR, and staining for HIV-1 p24 antigen in purified blood DC. (A) Blood DC stained with (a) anti–HLA-DR, (b) anti-CD14, and (c) anti-CD3. (B) TcR and FcγRI (CD64) mRNA expression in DC, T cells, and monocytes. RT-PCR products were analyzed on a 2% agarose gel: TcR (386 bp), CD64 (345 bp), and actin (665 bp) are indicated. Molecular weight markers are on the far left, with their size in basepairs indicated on the left. (C) Immunofluorescence staining of HIV-1–infected blood DC with antibodies against HLA-DR and p24 antigen. Cells were double-stained with FITC-conjugated anti–HLA-DR MoAb (a), an anti-p24 mouse IgG1 MoAb followed by phycoerythrin (PE)-conjugated rat antimouse antibody (b), and an irrelevant, isotype-specific IgG1 control MoAb followed by PE-conjugated rat antimouse antibody (c). Original magnification × 20.
Analysis of surface markers by FACS, expression of mRNA transcripts by RT-PCR, and staining for HIV-1 p24 antigen in purified blood DC. (A) Blood DC stained with (a) anti–HLA-DR, (b) anti-CD14, and (c) anti-CD3. (B) TcR and FcγRI (CD64) mRNA expression in DC, T cells, and monocytes. RT-PCR products were analyzed on a 2% agarose gel: TcR (386 bp), CD64 (345 bp), and actin (665 bp) are indicated. Molecular weight markers are on the far left, with their size in basepairs indicated on the left. (C) Immunofluorescence staining of HIV-1–infected blood DC with antibodies against HLA-DR and p24 antigen. Cells were double-stained with FITC-conjugated anti–HLA-DR MoAb (a), an anti-p24 mouse IgG1 MoAb followed by phycoerythrin (PE)-conjugated rat antimouse antibody (b), and an irrelevant, isotype-specific IgG1 control MoAb followed by PE-conjugated rat antimouse antibody (c). Original magnification × 20.
DC-T-cell cocultivation.To amplify the p24 signal, virus-pulsed DC were cocultured with mitogen-induced autologous primary T cells. T cells were stimulated with 4 μg/mL of PHA, washed, and resuspended in medium containing IL-2. A total of 3 × 105 T cells was added to chemokine-treated, virus-exposed DC. Culture supernatants were collected every 2 to 4 days and replenished with fresh IL-2–containing medium and chemokines as indicated. Collected supernatants were stored at −70°C for p24 antigen detection by enzyme-linked immunosorbent assay (DuPont, Billerica, MA). Recombinant human chemokines were obtained from PeproTech (Rocky Hill, NJ).
Trypsin treatment of virus-pulsed cells.To remove virus adhering to the cell surface, virus-exposed DC were washed extensively and trypsinized for 10 minutes as described.9 The trypsin was inactivated and removed by washing cells with RPMI-1640 medium containing 10% FBS.
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total RNA prepared from DC using RNA-STAT-60 (Tel-Test “B”, Friendswood, CA) was treated with RNase-free DNase (Promega, Madison, WI) and was used in RT-PCR assays. First-strand cDNA was synthesized from 4 μg of total RNA primed with random hexamers using a cDNA Cycle Kit (Invitrogen, San Diego CA). PCR was performed using the first-strand cDNA and the following primers: actin, TAGCGGGGTCACCCACACTGTGCC and TAGAAGCATTTGCGGTGGACGATG; CCR1, GCAGCCTTCACTTTCCTCACG and ACTCATGGGTGAACAGGAAGTCT; CCR2, TGGCTGTGTTTGCTTCTGTC and TCTCACTGCCCTATGCCTCT; CCR3, TTTGGTGTCATCACCAGCAT and TCATGCAGCAGTGGGAGTAGG; CCR4, GTGGGCTTTTACAGTGGCAT and TAAGATGAGCTGGGGGTGTC; CCR5, GGTGGAACAAGATGGATTAT and CATGTGCACAACTCTGACTG; and CXCR4, CCACCGCATCTGGAGAAC and ACATCTGTGTTAGCTGGAGTGA. Furthermore, purified DC were tested for the presence of contaminating T cells and monocytes by RT-PCR using primers for the T-cell receptor (TcR) β-chain; as a marker for monocytes, we used the high-affinity Fc receptor for IgG (FcγRI or CD64). The primer sequences were as follows: TcR, CCGAGGTCGCTGTGTTTGAGCCAT and GCTCTACCCCAGGCCTCGGC; and CD64 (FcγRI), TGTTCCAAGAGGAAACCGTAACC and TTAAAGGCTTTGCCATTTCGATAGT. PCR reactions were performed in parallel with equivalent amounts of DNase-treated RNA before and after reverse transcription.
FACS analysis of DC and LC for purity and fusin expression.Purified DC were washed twice with washing buffer, and 2 × 105 cells were resuspended in phosphate-buffered saline-containing 2% FBS. The appropriate amount of MoAb (anti–HLA-DR; Becton Dickinson [BD], San Jose, CA), anti-CD3 (Leu 4; BD), anti-CD14 (Leu 18; BD), antifusin MoAb (12G5),33 and an irrelevant isotype control, mouse MoAb IgG2a, was added to the respective wells; the cells were incubated for 30 minutes on ice, washed three times with a wash buffer, and incubated with fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 antimouse IgG for 30 minutes on ice. After incubation, the cells were washed three times, fixed with 1% formaldehyde, and analyzed by FACS analysis.
RESULTS
Purity of blood DC.The purity of DC isolated by the modified procedure described (in the Materials and Methods) was verified by FACS analysis (Fig 1A) and by immunofluorescent staining of cytospin preparations; the latter did not show any contaminating T cells or monocytes/macrophages (data not shown). Additionally, the presence of such contaminating cells was tested by RT-PCR using primers for TcR and FcγRI (CD64). No transcripts for either the T-cell receptor or for the monocyte marker could be detected in the purified DC (Fig 1B). Purified blood DC could support HIV-1 replication as shown by double immunofluorescent staining for HLA-DR and p24 Gag (Fig 1C).
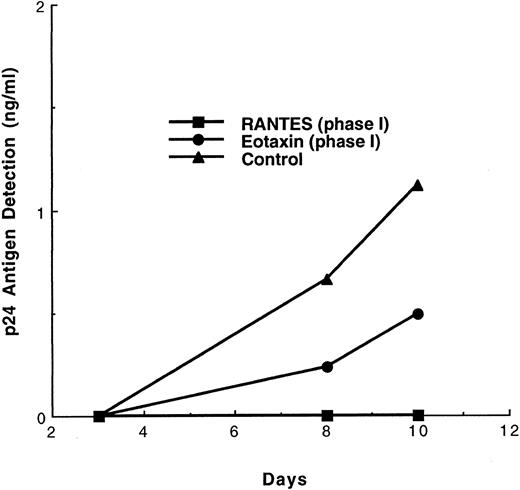
Infection of DC and virus transmission to T cells in the presence of β-chemokines.To examine the effect of chemokines, purified DC were pulsed with M-tropic or T-tropic strains of HIV-1. In earlier experiments, we9 and others34 have shown that isolated, purified blood DC support HIV-1 replication at a low level. Early and late cDNA gag transcripts were detected by PCR,9,34 and low levels of p24 antigen were released into the culture supernatants.9 To amplify the p24 signals, a two-phase system was used. In phase I, purified DC cultured in conditioned medium were treated with chemokines and pulsed with virus. Then, surface-bound virus was removed by extensive washing; in some experiments, an additional trypsin-treatment step was performed (see the Materials and Methods). Then, virus-infected DC were cocultivated with PHA-stimulated autologous T-cell blasts in the subsequent phase II. The cultures were maintained in the presence of IL-2 with or without chemokines. The results indicated that RANTES, even when present only during phase I, was a potent inhibitor of p24 production, whereas eotaxin inhibited p24 production by approximately 60% (Fig 2). However, when eotaxin was present throughout phases I + II, p24 production was reduced further (data not shown). In contrast, addition of eotaxin only during the cocultivation of virus-pulsed (Yu-2) DC with T cells (phase II only) showed some inhibition, which was much lower than the inhibition seen when eotaxin was present either during phase I or phases I + II (data not shown). Clearly, the receptor for eotaxin, CCR3, is involved in virus entry into DC and, possibly, in virus dissemination during the cocultivation phase. Alternatively, eotaxin indirectly alters the expression of other coreceptors required for effective virus spread in the DC/T-cell system. Clearly, exposure of purified DC to RANTES during phase I led to more complete blocking of HIV-1 Yu-2 entry as compared with addition of eotaxin.
Effect of β chemokines on the replication of the M-tropic HIV-1 strain Yu-2 in the DC-T cell coculture model. DC were pretreated with the respective chemokine (500 ng/mL) for 60 minutes, followed by exposure to Yu-2 (3 ng of p24) for 2 hours in the continued presence of chemokines. The cells were then washed five times to remove virus and cocultured with PHA-induced, uninfected syngeneic T cells in medium containing IL-2. No further chemokine treatment was performed during this phase II. The medium was changed every 2 days.
Effect of β chemokines on the replication of the M-tropic HIV-1 strain Yu-2 in the DC-T cell coculture model. DC were pretreated with the respective chemokine (500 ng/mL) for 60 minutes, followed by exposure to Yu-2 (3 ng of p24) for 2 hours in the continued presence of chemokines. The cells were then washed five times to remove virus and cocultured with PHA-induced, uninfected syngeneic T cells in medium containing IL-2. No further chemokine treatment was performed during this phase II. The medium was changed every 2 days.
Next, we examined other CC and CXC chemokines for their ability to suppress p24 production by DC when present during phases I + II. In addition to RANTES (data not shown), MIP-1α and MIP-1β inhibited the Yu-2 strain effectively (Table 1), lowering p24 production 15-fold, whereas eotaxin resulted in a fourfold decrease. MCP-1 and MCP-4 yielded a twofold decrease (Table 2). Viral replication was not inhibited by two CXC chemokines, IP-10 or IL-8. IP-10 is a ligand for CXCR3, which is expressed on activated T cells under the culture conditions used during phase II.
Effect of C-C and CXC Chemokines on HIV-1 Replication When Present Throughout Phases I + II
| Chemokine . | p24 Antigen Detection (ng/mL) (days postinfection) . | |||
|---|---|---|---|---|
| . | 3 . | 6 . | 9 . | 12 . |
| Eotaxin | 0.089 | 0.099 | 5.79 | 18 |
| MIP-1α | 0.088 | 0.274 | 0.347 | 0.401 |
| MIP-1β | 0.097 | 0.211 | 0.327 | 0.597 |
| MCP-1 | 0.113 | 1.904 | 7.859 | 29.13 |
| MCP-4 | 0.092 | 2.611 | 18.7 | 39.8 |
| IP10 | 0.308 | 33.4 | 40 | 154.6 |
| None | 0.18 | 4.045 | 25.1 | 76.6 |
| Chemokine . | p24 Antigen Detection (ng/mL) (days postinfection) . | |||
|---|---|---|---|---|
| . | 3 . | 6 . | 9 . | 12 . |
| Eotaxin | 0.089 | 0.099 | 5.79 | 18 |
| MIP-1α | 0.088 | 0.274 | 0.347 | 0.401 |
| MIP-1β | 0.097 | 0.211 | 0.327 | 0.597 |
| MCP-1 | 0.113 | 1.904 | 7.859 | 29.13 |
| MCP-4 | 0.092 | 2.611 | 18.7 | 39.8 |
| IP10 | 0.308 | 33.4 | 40 | 154.6 |
| None | 0.18 | 4.045 | 25.1 | 76.6 |
Purified DC (1 × 105 cells) from a single donor were preincubated with chemokines (250 ng/mL) for 60 minutes. The cells were then pulsed with HIV-1 (Yu-2, M-tropic virus; 3 ng/mL of p24 antigen) for 60 minutes. The cells were washed five times and cocultivated with activated, autologous T cells and chemokines.
Effect of CC and CXC Chemokines on HIVYu-2 Replication in the DC-T-Cell Coculture System When Present During Phase I Only or During Phases I + II
| Chemokine . | p24 Production (ng/mL) in the Presence of Chemokines . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | (A) Chemokines Present During Phase I Only . | (B) Chemokines Present Throughout Phases I + II . | . | . | . | . | . | ||||||
| . | . | (days postinfection) . | (days postinfection) . | . | . | . | . | . | ||||||
| . | . | 3 . | 5 . | 7 . | 10 . | 3 . | 5 . | 7 . | 10 . | . | . | . | . | . |
| RANTES | 0.00 | 0.17 | 1.4 | 8.6 | 0.00 | 0.02 | 0.00 | 0.097 | ||||||
| MIP-1α | 0.2 | 0.5 | 3.35 | 19.6 | 0.02 | 0.23 | 0.569 | 1.92 | ||||||
| MIP-1β | 0.00 | 0.36 | 2.94 | 20.6 | 0.00 | 0.12 | 0.86 | 17.5 | ||||||
| MCP-1 | 0.00 | 0.62 | 4.8 | 69 | 0.00 | 0.3 | 2.89 | 16.9 | ||||||
| IP10 | 0.00 | 0.24 | 2.9 | 106 | 0.00 | 0.55 | 4.69 | 54.8 | ||||||
| IL-8 | 0.00 | 0.785 | 6.5 | 54 | 0.00 | 0.49 | 4.63 | 61 | ||||||
| None | 0.2 | 0.58 | 5.49 | 77 | 0.2 | 0.58 | 5.49 | 77 | ||||||
| Chemokine . | p24 Production (ng/mL) in the Presence of Chemokines . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | (A) Chemokines Present During Phase I Only . | (B) Chemokines Present Throughout Phases I + II . | . | . | . | . | . | ||||||
| . | . | (days postinfection) . | (days postinfection) . | . | . | . | . | . | ||||||
| . | . | 3 . | 5 . | 7 . | 10 . | 3 . | 5 . | 7 . | 10 . | . | . | . | . | . |
| RANTES | 0.00 | 0.17 | 1.4 | 8.6 | 0.00 | 0.02 | 0.00 | 0.097 | ||||||
| MIP-1α | 0.2 | 0.5 | 3.35 | 19.6 | 0.02 | 0.23 | 0.569 | 1.92 | ||||||
| MIP-1β | 0.00 | 0.36 | 2.94 | 20.6 | 0.00 | 0.12 | 0.86 | 17.5 | ||||||
| MCP-1 | 0.00 | 0.62 | 4.8 | 69 | 0.00 | 0.3 | 2.89 | 16.9 | ||||||
| IP10 | 0.00 | 0.24 | 2.9 | 106 | 0.00 | 0.55 | 4.69 | 54.8 | ||||||
| IL-8 | 0.00 | 0.785 | 6.5 | 54 | 0.00 | 0.49 | 4.63 | 61 | ||||||
| None | 0.2 | 0.58 | 5.49 | 77 | 0.2 | 0.58 | 5.49 | 77 | ||||||
DC were pretreated with 500 ng of the respective chemokine for 60 minutes and exposed to Yu-2 (6 ng/mL) for 60 minutes at 37°C. Chemokine-treated, virus-pulsed DC were washed four times with phosphate-buffered saline and treated with trypsin for 10 minutes. The cells were then washed twice and cocultivated with PHA-stimulated, uninfected autologous T cells. (A) chemokines were added only one time during phase I to pretreat DC; (B) the cultures were maintained in the continuous presence of the respective chemokine, phases I + II. Both sets of experiments were performed simultaneously on DC obtained from the same single donor. Therefore, one untreated control was used for both phase I and phase II chemokine treatment.
In subsequent experiments, we systematically compared the inhibitory effects of CC and CXC chemokines when present during phase I alone versus phases I + II (Table 2). In the continuous presence of chemokines, significant inhibition of M-tropic HIV-1 was noted again with RANTES and MIP-1α; in addition, MIP-1β blocked Yu-2 infection by greater than 90% and 78%, respectively (Table 2). Consistent with our previous observation, these β-chemokines were effective in lowering p24 production even when present solely during phase I, although less so than when present throughout the experiment. However, MCP-1 was not effective when present only during phase I; its effect appears to consist of blocking virus spread to activated lymphocytes.
To evaluate the role of β-chemokines on other HIV-1 strains, we compared the inhibitory effect of RANTES on p24 production by NL4-3 (T-tropic) and HIV-ADA (M-tropic). The chemokines were present during experimental phases I + II. Whereas RANTES reduced p24 production by HIV-ADA by greater than 90%, it did not block the entry of the T-tropic isolate (data not shown). Based on this differential effect of RANTES, we conclude that different coreceptors are used by M- and T-tropic strains to enter blood DC.
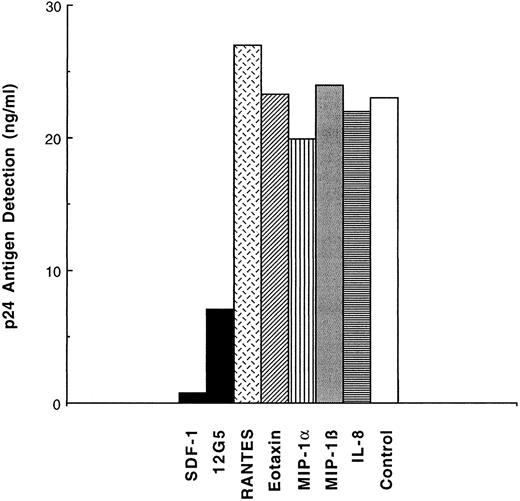
Inhibition of T-cell tropic virus in to DC by SDF-1 or by antifusin MoAb (12G5).To test whether entry of T-tropic NL4-3 could be blocked, a CXCR4 ligand, SDF-1, and an MoAb specific for this chemokine receptor, 12G5, were added to purified skin-derived LC during phase I only (Fig 3). SDF-1 reduced p24 production approximately 10-fold and 12G5 fourfold. As expected, both agents are only active against T-tropic HIV-1 strains that use the CXCR4 coreceptor, because neither SDF-1 nor 12G5 showed significant inhibition of Yu-2 entry in blood DC, in contrast to RANTES and MIP-1α (data not shown).
Inhibition of LC infection with the T-tropic NL4-3 by a CXCR4/fusin ligand, SDF-1, and by an anti-CXCR4/fusin MoAb (12G5). Purified LC were pretreated with SDF-1 (125 nmol/L), 12G5 (10 μg/mL), or the chemokines indicated and pulsed with NL4-3 (6 ng/mL of p24) and incubated for 16 hours, washed five times, trypsinized, washed two times, and cocultivated with heterologous, activated T cells. SDF-1, 12G5, or chemokines were no longer added during this phase II. The level of p24 released into the medium was measured on day 4 of cocultivation.
Inhibition of LC infection with the T-tropic NL4-3 by a CXCR4/fusin ligand, SDF-1, and by an anti-CXCR4/fusin MoAb (12G5). Purified LC were pretreated with SDF-1 (125 nmol/L), 12G5 (10 μg/mL), or the chemokines indicated and pulsed with NL4-3 (6 ng/mL of p24) and incubated for 16 hours, washed five times, trypsinized, washed two times, and cocultivated with heterologous, activated T cells. SDF-1, 12G5, or chemokines were no longer added during this phase II. The level of p24 released into the medium was measured on day 4 of cocultivation.
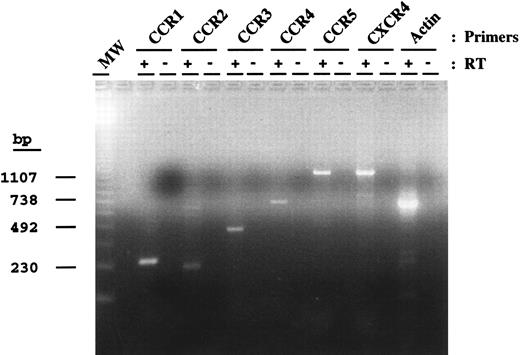
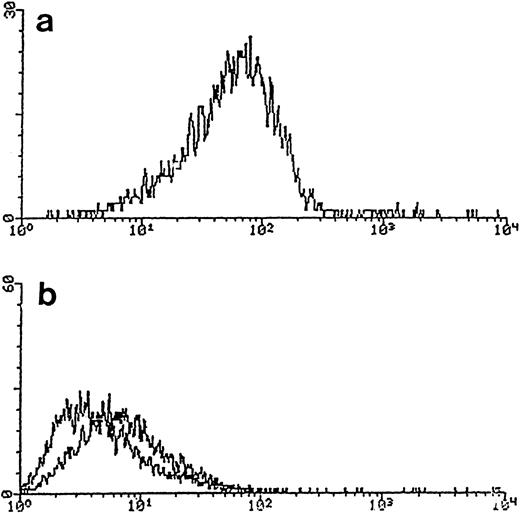
Expression of chemokine receptors in DC/LC.To investigate the expression of chemokine receptors by blood DC, RT-PCR was performed (Fig 4); RNA transcripts for multiple chemokine receptors were detected, namely for CCR1, CCR2, CCR3, CCR4, and CCR5, as well as for CXCR4. Because many chemokine receptors are intronless, our negative controls always included PCR amplification of the DNase-treated RNA preparation before RT-PCR to exclude contamination with genomic DNA. As mentioned previously, no contaminating T cells or monocytes were observed when purified DC were tested by RT-PCR for the expression of TcR receptor or for a monocyte marker (CD64). The expression of CXCR4 was confirmed further by staining DC with the MoAb 12G5 and analyzed by flow cytometry (Fig 5). We show here that purified blood DC can be stained with 12G5, indicating the abundant expression of CXCR4 on the surface of these cells.
Chemokine receptor RNA expression by DC. Equivalent amounts of DNase-treated RNA before (−) and after (+) RT were used as a template for PCR using primers specific for the indicated chemokine receptors. PCR products for CCR1 (272 bp), CCR2 (230 bp), CCR3 (444 bp), CCR4 (688 bp), CCR5 (1,117 bp), fusin (1,050 bp), and actin (658 bp) are shown. Molecular weight markers (MW) and their size in basepairs are indicated on the left.
Chemokine receptor RNA expression by DC. Equivalent amounts of DNase-treated RNA before (−) and after (+) RT were used as a template for PCR using primers specific for the indicated chemokine receptors. PCR products for CCR1 (272 bp), CCR2 (230 bp), CCR3 (444 bp), CCR4 (688 bp), CCR5 (1,117 bp), fusin (1,050 bp), and actin (658 bp) are shown. Molecular weight markers (MW) and their size in basepairs are indicated on the left.
Expression of CXCR4/fusin on blood DC using MoAb 12G5. Purified blood DC were stained with 12G5 or an isotype (IgG2a)-matched control antibody and analyzed by flow cytometry (see the Materials and Methods). (a) 12G5 (anti-CXCR4/fusin); (b) negative controls without primary antibody (left curve) or with an unrelated, isotype-specific IgG2a (curve on right).
Expression of CXCR4/fusin on blood DC using MoAb 12G5. Purified blood DC were stained with 12G5 or an isotype (IgG2a)-matched control antibody and analyzed by flow cytometry (see the Materials and Methods). (a) 12G5 (anti-CXCR4/fusin); (b) negative controls without primary antibody (left curve) or with an unrelated, isotype-specific IgG2a (curve on right).
DISCUSSION
In this study, we show that multiple chemokine receptors could serve as coreceptors for HIV-1 entry into DC. The CC-chemokines RANTES, MIP-1α, and MIP-1β had a profound effect on DC infection by M-tropic HIV-1. Eotaxin and to a lesser extent MCP-1 resulted in reduced p24 production also. MCP-4 only affected the Yu-2 isolate at the concentrations tested. RANTES was the most effective inhibitor in our assay system, as in those of others involving other cell types.18-22 This may be due to the fact that RANTES can block more than one CC-chemokine receptor, ie, CCR1, CCR3, and CCR5. However, the replication of T-tropic HIV-1 was not affected by the presence of RANTES or MCP-4, indicating that virus strains of different tropism vary in their coreceptor usage on DC. Although similar results have been reported for RANTES and MIP-1α–mediated inhibition of HIV-1 entry into DC differentiated in vitro from PBMC,29 we have also observed that MIP-1β and eotaxin block the entry of M-tropic HIV-1 isolates into blood DC. The differences between the two results could be attributed either to differences in the source of DC (in vitro differentiated PBMC v purified blood DC), to the stage of DC activation and differentiation, or to differences in virus strains (Ba-L v Yu-2).
A recent study on coreceptor usage in microglial cells showed that the CCR3 ligand, eotaxin, inhibited Yu-2 entry into target cells.35 The effect seen for MCP-4 against the Yu-2 virus could be attributed to the capacity of this chemokine to bind to CCR2 and CCR3, whereas the effect of MCP-1 could be attributed to its binding to CCR2.36 Two CXC-chemokines tested, IP-10 and IL-8, showed no inhibition when tested against M-tropic viruses. To the contrary, we have repeatedly observed an enhancement of p24 production in cultures maintained in the continuous presence of IP-10. Although the mechanism of this enhancement remains to be elucidated, a similar observation has been made using cultures of purified differentiated macrophages exposed to primary HIV-1 isolates,37 in which β-chemokines were shown to increase p24 production. These data, together with our own observation that IP-10 stimulates p24 production, suggest that ligand activation of a chemokine receptor that is not involved in viral entry can stimulate virus replication. This upregulation could be due to indirect effects arising from cellular activation.
Entry into DC by T-tropic HIV-1 may occur through CXCR4 or closely related molecules, because virus exposure of these cells in the presence of either a receptor ligand or an antireceptor antibody resulted in lower virus replication as measured by p24 production in phase II. In this context, it is interesting to speculate on the tropism of the HIV-1 strain that infected an individual homozygous for the 32-bp deletion in the CCR5 gene.28 This infected individual listed only homosexual receptive/insertive anal and oral intercourse as risk factors. Because tissue DC were among the first SIV target cells after mucosal inoculation,7 it is possible that the person with the homozygous deletion in the CCR5 gene may have been infected by a T-tropic strain via CXCR4-mediated virus entry into DC. The high level of CXCR4 expression on blood DC is intriguing and was seen also on LC isolated from skin (data not shown). It would be of interest to examine tissues directly for the level of CXCR4 expression because the extensive purification required to isolate cells from either blood or skin may alter chemokine receptor expression.
In summary, we have shown that either CXCR4 or various other CC chemokine receptors can serve as coreceptors on DC for T- or M-tropic strains of HIV-1, respectively. The permissiveness of DC for a wide spectrum of HIV-1 strains, coupled with their mobility and their close interaction with key elements of the immune system, could put cells of this lineage at the center of virus dissemination. We postulate that DC and LC play a pivotal role in the evolution of virus quasispecies during the course of HIV-1 infection and disease.
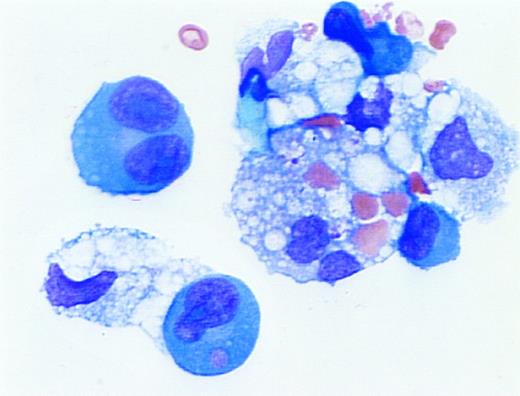
Erythrophagocytosis in myelomatous pleural effusion. A 59-year old woman with advanced multiple myeloma (IgG,κ) and ischemic heart disease presented with congestive heart failure and pancytopenia. A pleural effusion was noted on initial examination and persisted despite diuresis. A diagnostic thoracentesis was performed. The cytospin preparation demonstrated erythrophagocytosis by dysplastic plasma cells and macrophages. (Wright's stain; original magnification ×1,000.) (Courtesy of Larry L. Frase, MD, Joseph Newman, PhD, Martin L. Hurst, MD, and Marvin J. Stone, MD, Baylor-Sammons Cancer Center, Dallas, TX 75246.)
Erythrophagocytosis in myelomatous pleural effusion. A 59-year old woman with advanced multiple myeloma (IgG,κ) and ischemic heart disease presented with congestive heart failure and pancytopenia. A pleural effusion was noted on initial examination and persisted despite diuresis. A diagnostic thoracentesis was performed. The cytospin preparation demonstrated erythrophagocytosis by dysplastic plasma cells and macrophages. (Wright's stain; original magnification ×1,000.) (Courtesy of Larry L. Frase, MD, Joseph Newman, PhD, Martin L. Hurst, MD, and Marvin J. Stone, MD, Baylor-Sammons Cancer Center, Dallas, TX 75246.)
ACKNOWLEDGMENT
The authors thank A. DiSorbo for the preparation of this manuscript, S. Biancacalana for help with the SDF-1 synthesis, and Dr S. Kalams for providing TcR-specific primers.
Supported in part by National Institutes of Health (NIH) Grants No. R01-AI32330 and R01-AI34266 to R.M.R. and R01-CA69212 and R01-AI40618 to A.D.L. The work was also aided by the Center for AIDS Research (CFAR) Core Grant No. IP3028691 awarded to the Dana-Farber Cancer Institute and by the Harvard Skin Disease Research Center at Brigham and Women's Hospital (NIH Grants No. R01 AI 25082, R01 AR40214, and P30 AR 43629 to T.S.K.). S.A. is supported by the AIDS training grant and E.A.G.-Z. is supported by a Fogarty International Fellowship. A.D.L. is also supported by a Cancer Research Institute/Benjamin Jacobson Family Investigator Award.
Address reprint requests to Ruth M. Ruprecht, MD, PhD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal