Abstract
Ligneous conjunctivitis is a rare and unusual form of chronic pseudomembranous conjunctivitis that usually starts in early infancy. The disease may be associated with pseudomembranous lesions of other mucous membranes in the mouth, nasopharynx, trachea, and female genital tract. We examined two unrelated Turkish girls both suffering from ligneous conjunctivitis and occlusive hydrocephalus. Both children exhibited a severe plasminogen deficiency. Genomic DNA from both patients as well as from clinically healthy family members were screened for mutations in the plasminogen gene by polymerase chain reaction, single-strand conformation polymorphism (SSCP) analysis, and DNA sequencing. In the first girl with ligneous conjunctivitis a homozygous G → A point mutation was identified in plasminogen exon 7 at position 780 leading to an amino acid exchange (Arg216 → His). Her healthy sister and her healthy parents were heterozygous for this mutation. The second patient revealed a homozygous G → A point mutation in plasminogen exon 15 at position 1924 which leads to a stopcodon (Trp597 → Stop). The healthy parents were shown to be heterozygous for this mutation. In addition, the father's second allele revealed another mutation in the same codon (Trp597 → Cys) (compound heterozygosity). In conclusion, certain homozygous mutations in the plasminogen gene may cause ligneous conjunctivitis.
LIGNEOUS CONJUNCTIVITIS is an unusual and rare form of chronic conjunctivitis characterized by chronic tearing and redness of the conjunctivae. Initially, pseudomembranes form on the palpebral surfaces which then progress to thick nodular masses that replace the normal mucosa. The first report of ligneous conjunctivitis in a 46-year-old man with bilateral pseudomembraneous conjunctivitis was published as early as 1847.1 Because the pseudomembranes have a woodlike consistency, the disease was termed later “ligneous” conjunctivitis.2
In many patients, additional similar pseudomembranous lesions are observed in the mucosa of the mouth, tongue, nasopharynx, tracheobronchial tree, and female genital tract.3-7 Most affected children are healthy at birth and develop ligneous conjunctivitis during the first weeks or months of life. It has been suggested that minor trauma or infection of the conjunctival epithelium may initiate the development of this disorder.8 Histological examination of pseudomembranes from affected eyes or mucosa tissue from the genital tract exhibits a disrupted epithelium that is replaced by a massive deposition of fibrin and amorphous hyalinlike eosinophilic material and accompanied by an inflammatory cellular infiltration. Surviving epithelium is found only at the edges of these deposits.7,9-11 Ligneous conjunctivitis occurs sporadically and in general is found more often in girls than in boys.12 However, in some families this disease was clearly shown to be inherited in an autosomal-recessive manner.9,13 14
Some children with ligneous conjunctivitis suffer also from congenital occlusive hydrocephalus.9,11 15-18 The pathogenesis of this complication is still unclear.
Ligneous conjunctivitis has been described not only in humans but also in different animal species such as Doberman pinschers19 and canines (D.T. Ramsey, Michigan State University, East Lansing; personal communication, April 1997).
Recently, Mingers et al15 have for the first time shown severe type I plasminogen deficiency (plasminogen activity and antigen both extremely low) to be present in three unrelated girls who all suffered from ligneous conjunctivitis.
Here were report different homozygous mutations in the plasminogen gene in two unrelated girls both suffering from ligneous conjunctivitis and occlusive hydrocephalus. The data suggest that certain homozygous plasminogen mutations may cause ligneous conjunctivitis.
MATERIALS AND METHODS
Patients
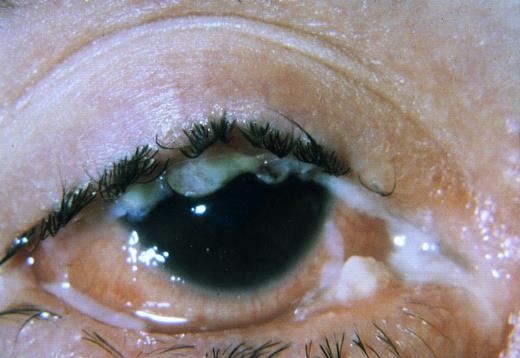
Patient 1.The clinical findings and the course of disease of the first patient with ligneous conjunctivitis (see Fig 2, II-1), a Turkish girl currently 16 years old, have already been reported.16 The family history is unremarkable, and both parents and an older sister are clinically healthy. Episodes of venous thrombosis were never reported in any member of the family. Starting at 4 months of age, the patient gradually developed chronic bilateral conjunctivitis with subsequent undulating swelling of both upper and lower eye lids. At 2 years of age macrocephalus was noted. At the age of 25 months, the child suddenly became comatose and exhibited generalized hypotonia. On hospital admission a decompensated occlusive hydrocephalus internus was diagnosed. Head circumference was 53.5 cm (2.5 cm > 97th percentile), length 93 cm (97th percentile), and weight 12.4 kg (50th percentile). A ventriculo-atrial shunt was implanted, and the girl's condition rapidly improved. During the hospital stay the child revealed chronic pseudomembranous lesions on both conjunctivae (Fig 1) and gingival hyperplasia. At 3 years of age pseudomembranes from both eyes were surgically removed. Histological examination showed ligneous conjunctivitis. The disease frequently recurred, necessitating surgical intervention on several occasions. A severe deterioration of ligneous conjunctivitis at 8 years of age rapidly improved after local treatment with hyaluronidase-containing eyedrops. Extensive studies of the hemostasis system were performed at the age of 15 years 8 months.
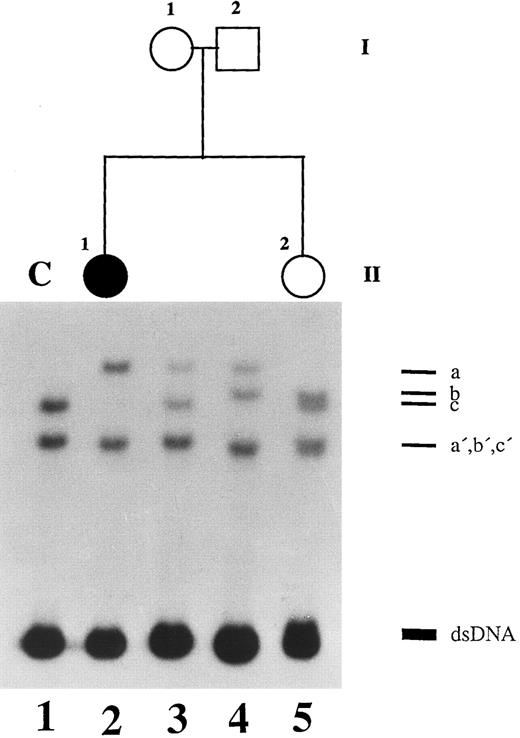
SSCP analysis of plasminogen gene exon 7 in patient 1, her healthy parents, and her healthy sister. Radiolabeled single-stranded PCR products of plasminogen exon 7 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, patient 1 with ligneous conjunctivitis (II-1); lane 3, healthy mother of patient 1 (I-1); lane 4, healthy father of patient 1 (I-2); lane 5, healthy sister of patient 1 (II-2). dsDNA, double-stranded DNA.
SSCP analysis of plasminogen gene exon 7 in patient 1, her healthy parents, and her healthy sister. Radiolabeled single-stranded PCR products of plasminogen exon 7 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, patient 1 with ligneous conjunctivitis (II-1); lane 3, healthy mother of patient 1 (I-1); lane 4, healthy father of patient 1 (I-2); lane 5, healthy sister of patient 1 (II-2). dsDNA, double-stranded DNA.
Ligneous conjunctivitis with thickened everted eyelids (patient 1).
Ligneous conjunctivitis with thickened everted eyelids (patient 1).
Patient 2.The clinical and hemostaseologic findings have already been published in part elsewhere.15 This female patient is the second child of consanguinous Turkish parents (second cousins). A female cousin living in Turkey was reported to suffer from ligneous conjunctivitis. Episodes of venous thrombosis were not observed in any member of the family.
Pregnancy of the mother was uneventful. In the 34th gestational week ultrasound examinations showed congenital macrocephalus and hydrocephalus of the fetus. At 36 gestational weeks a female baby (see Fig 3, II-1) was born by cesarean section. The Apgar score was 9 and 10, the birth weight 2,900 g (25th percentile), the length 51 cm (50th percentile), and the head circumference 40 cm (>97th percentile). Clinical examination of the patient was normal except for a bulging fontanelle. Cranial ultrasound at the first day of life showed enlargement of all four ventricles and the cisterna magna, and hypoplasia of the cerebellum (Dandy-Walker malformation). At the second day of life a Rickham reservoir was transiently implanted which was then replaced 2 weeks later by a ventriculo-peritoneal shunt. Up to the age of 18 months eight shunt revisions (ventriculo-atrial and -peritoneal) were necessary because of repeated thrombotic occlusions of the shunt system. Abdominal shunt revisions showed extensive fibrin deposits on the surface of the peritoneum. After occlusion of a ventriculo-atrial shunt at the age of 18 months a large thrombus was found at the tip of the catheter within the right atrium and was surgically removed. Fibrin-rich thrombotic material was found also on the surface of two pericardial drainage tubes, which were removed 2 weeks after the operation. Thereafter, a shunt implantation from the ventricles into the gallbladder was successful and functionally intact for more than 2 years.
SSCP analysis of plasminogen gene exon 15 in patient 2, her healthy sister, and her parents. Radiolabeled single-stranded PCR products of plasminogen exon 15 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, patient 2 with ligneous conjunctivitis (II-1); lane 3, healthy mother of patient 2 (I-1); lane 4, healthy father of patient 2 (I-2); lane 5, healthy sister of patient 2 (II-2). dsDNA, double-stranded DNA.
SSCP analysis of plasminogen gene exon 15 in patient 2, her healthy sister, and her parents. Radiolabeled single-stranded PCR products of plasminogen exon 15 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, patient 2 with ligneous conjunctivitis (II-1); lane 3, healthy mother of patient 2 (I-1); lane 4, healthy father of patient 2 (I-2); lane 5, healthy sister of patient 2 (II-2). dsDNA, double-stranded DNA.
Repeated abdominal ultrasound examinations revealed increasing thickening of the gallbladder wall, which may possibly reflect thrombotic deposits at the inner surface of this organ.
Since the age of 4 weeks the patient developed tarsal pseudomembranous conjunctivitis of both the upper and lower eyelids; she also suffered from repeated tracheobronchitis. The bulbar conjunctivae and the cornea were less affected. At the age of 2 years histological examination of pseudomembranes of the eyes exhibited massive exsudation of fibrin with an inflammatory cellular infiltration, a disrupted epithelium, and areas containing an amorphous, eosinophilic, hyaline material. These findings confirmed the diagnosis of ligneous conjunctivitis in this patient. Repeated surgical excisions of conjunctival pseudomembranes and topical administration of steroids, cyclosporine, hyaluronidase, and cromolyn (disodium chromoglycate) resulted in only transient and short-term improvement. The motor and mental development was markedly delayed: At the age of 2 years 10 months the patient could sit by herself and could pull to a standing position; walking with and without support was not possible. The child never spoke any words. At the age of 3 years the girl suffered from severe bronchopneumonia. At the age of 3 years the child was admitted to hospital in a comatose state because of sudden shunt occlusion. The girl died 2 days later because of irreversible brain damage. Autopsy was denied by the parents.
Hemostasis Parameters
Quantitative determination of plasminogen antigen in citrated plasma of patients was performed by 1% agarose immunoelectrophoresis20 using a polyclonal rabbit anti-plasminogen antiserum (OSCB; Behringwerke AG, Marburg, Germany). Using this method the lower detection limit of plasminogen antigen in human plasma is 0.4 mg/dL.
Plasminogen functional activity in citrated plasma of patients was determined by activation with streptokinase using a chromogenic assay with p-nitroaniline as substrate (Berichrom Plasminogen; Behringwerke AG). The lower detection limit of this assay is 5% plasminogen functional activity.
Quantitative determination of tissue-type plasminogen activator (t-PA) in citrated plasma was done by enzyme-linked immunosorbent assay (ELISA) (Asserachrom t-PA; Boehringer Mannheim, Mannheim, Germany). Plasminogen activator inhibitor type 1 (PAI-1) activity in citrated plasma was determined in a chromogenic assay (Baxter Diagnostics AG, Düdingen, Switzerland).
Mutation Analysis
Genomic DNA was prepared from peripheral blood samples of both girls with ligneous conjunctivitis (Fig 2, subject II-1; Fig 3, subject II-1) as well as from healthy family members (parents, sisters). Thereafter, DNA samples were amplified by polymerase chain reaction (PCR) using a set of primer pairs flanking all 19 exons including intron boundaries of the human plasminogen gene.21 Primer sequences and PCR conditions were as described previously.22 To screen for plasminogen gene mutations, we used the method of single-strand conformation polymorphism (SSCP) analysis, which allows the detection of point mutations and other sequence alterations according to a variant migration pattern of the mutant versus the wild-type DNA fragment in gel electrophoresis similarly as described previously.23 Amplified and subsequently denatured PCR segments were electrophoresed in a nondenaturing 5% polyacrylamide gel (Roth, Karlsruhe, Germany) containing 5% glycerol in 0.5× TBE buffer at room temperature for 7 hours at 10 W. The gel was dried and exposed to Kodak XAR-5 films (Eastman Kodak, Rochester, NY). Variant bands of single-stranded DNA fragments which differed in their migration pattern were excised from dried gels, dissolved in 1× TE buffer, purified with MicroSpin columns (Pharmacia, Freiburg, Germany), and reamplified. Amplified PCR products were again purified with MicroSpin columns and directly cycle-sequenced by the dideoxy-termination method using 35S-dATP and the Exo(-)Pfu Cyclist DNA Sequencing Kit (Stratagene, Heidelberg, Germany) according to the manufacturer's protocol. Samples were electrophoresed on 6% polyacrylamide/8.3 mol/L urea sequencing gels (Roth), dried, and exposed to Kodak XAR-5 films.
Direct Analysis of Plasminogen Mutation by Amplification Mutagenesis
For direct detection of plasminogen mutation in exon 7 (patient 1), genomic DNA from the patient, healthy family members, and healthy controls was amplified by PCR with the following primer pair flanking the mutation in exon 7: 5′ AACCTGAAGAAGAATTACTGTG-3′ (forward) and 5′-CGGGATCCTGGAATCTGGGTGTGCATCATAC-3′ (reverse). The first primer has a single sequence mismatch at position 779 (C → G) that results in the introduction of an artificial BstEII restriction site into the wild-type exon 7 allele only. Amplified PCR products were digested with BstEII (GIBCO, Eggenstein, Germany), electrophoresed in a 3% agarose gel and visualized by ethidium bromide staining. The wild-type BstEII-digested PCR fragment has a size of 119 bp, the mutant (uncut) PCR fragment has a size of 141 bp.
For direct detection of plasminogen mutation in exon 15 (patient 2), genomic DNA from the patient and relatives as well as from healthy controls was amplified by PCR with the following primer pair flanking the mutation in exon 15: 5′-CGGAATTCGGAGCAGAACAAAGTATCAATTTAAC-3′ (forward) and 5′-GCAGTGGGCAGCAGTCAAGA-3′ (reverse). The second primer has a single sequence mismatch at position 1927 (C → G) that results in the introduction of an artificial HinfI restriction site into the mutant exon 15 allele only. Amplified PCR products were digested with HinfI (Pharmacia, Freiburg, Germany), electrophoresed in a 2.5% agarose gel, and visualized by ethidium bromide staining. The mutant HinfI-digested PCR fragment has a size of 107 bp and the wild-type (uncut) PCR fragment has a size of 129 bp.
Hemostasis Parameters and Molecular Genetic Findings in Two Families With Ligneous Conjunctivitis
| Subjects . | Fibrinogen (mg/dL)* . | t-PA (ng/mL)† . | PAI-1 (U/mL)‡ . | Plasminogen Activity (%)ρ . | Plasminogen Antigen . | Mutations in the Plasminogen Gene . |
|---|---|---|---|---|---|---|
| . | . | . | . | . | (mg/dL)1-154 . | First Allele/Second Allele . |
| Family 1 | ||||||
| Patient 1 (II-1) | 346 | 5.0 | 10.6 | 6.0 | <0.4 | Arg216 → His/Arg216 → His |
| Mother (I-1) | 249 | 3.0 | 3.5 | 66 | 2 | Arg216 → His/Wild type |
| Father (I-2) | 306 | 6.2 | 3.0 | 60 | 7 | Arg216 → His/Wild type |
| Sister (II-2) | 289 | 3.6 | 8.0 | 76 | 7.5 | Arg216 → His/Wild type |
| Family 2 | ||||||
| Patient 2 (II-1) | 400 | 2.0 | 3.1 | <5 | <0.4 | Trp597 → Stop/Trp597 → Stop |
| Mother (I-1) | 250 | 3.7 | 3.1 | 60 | 5.2 | Trp597 → Stop/Wild type |
| Father (I-2) | 240 | 1.1 | 3.1 | 15 | 1.2 | Trp597 → Stop/Trp597 → Cys |
| Sister (II-2) | 330 | 2.0 | 0.89 | 66 | 6.9 | Wild type/Trp597 → Cys |
| Subjects . | Fibrinogen (mg/dL)* . | t-PA (ng/mL)† . | PAI-1 (U/mL)‡ . | Plasminogen Activity (%)ρ . | Plasminogen Antigen . | Mutations in the Plasminogen Gene . |
|---|---|---|---|---|---|---|
| . | . | . | . | . | (mg/dL)1-154 . | First Allele/Second Allele . |
| Family 1 | ||||||
| Patient 1 (II-1) | 346 | 5.0 | 10.6 | 6.0 | <0.4 | Arg216 → His/Arg216 → His |
| Mother (I-1) | 249 | 3.0 | 3.5 | 66 | 2 | Arg216 → His/Wild type |
| Father (I-2) | 306 | 6.2 | 3.0 | 60 | 7 | Arg216 → His/Wild type |
| Sister (II-2) | 289 | 3.6 | 8.0 | 76 | 7.5 | Arg216 → His/Wild type |
| Family 2 | ||||||
| Patient 2 (II-1) | 400 | 2.0 | 3.1 | <5 | <0.4 | Trp597 → Stop/Trp597 → Stop |
| Mother (I-1) | 250 | 3.7 | 3.1 | 60 | 5.2 | Trp597 → Stop/Wild type |
| Father (I-2) | 240 | 1.1 | 3.1 | 15 | 1.2 | Trp597 → Stop/Trp597 → Cys |
| Sister (II-2) | 330 | 2.0 | 0.89 | 66 | 6.9 | Wild type/Trp597 → Cys |
Fibrinogen (normal range, 180-450 mg/dL).
Tissue-type plasminogen activator (normal range, 1-12 ng/mL).
Plasminogen activator inhibitor type 1 (normal, <10 U/mL).
ρ Plasminogen activity (normal range, 80%-120%).
Plasminogen antigen (normal range, 6-25 mg/dL).
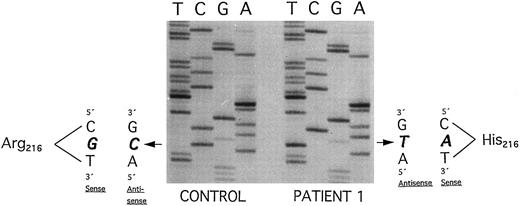
Plasminogen exon 7 sequence analysis in family 1 (noncoding strand is shown in the autoradiography). Single-stranded DNA fragments with a variant migration pattern in SSCP analysis (Fig 2, bands a, a′, b, b′) were excised from polyacrylamide gel and sequenced as described in Materials and Methods. The DNA of patient 1 with ligneous conjunctivitis exhibited a homozygous point mutation (G → A) at position 780, leading to an amino acid exchange at position 216 (Arg216 → His) (right). The healthy sister and the healthy parents (Fig 2, II-1, I-1, and I-2) are heterozygous for this mutation. Sequencing of band b and b′ exhibited the wild-type sequence (left; CONTROL). Numbering of nucleotides and amino acids is rendered according to Forsgren et al52 and Petersen et al,21 respectively.
Plasminogen exon 7 sequence analysis in family 1 (noncoding strand is shown in the autoradiography). Single-stranded DNA fragments with a variant migration pattern in SSCP analysis (Fig 2, bands a, a′, b, b′) were excised from polyacrylamide gel and sequenced as described in Materials and Methods. The DNA of patient 1 with ligneous conjunctivitis exhibited a homozygous point mutation (G → A) at position 780, leading to an amino acid exchange at position 216 (Arg216 → His) (right). The healthy sister and the healthy parents (Fig 2, II-1, I-1, and I-2) are heterozygous for this mutation. Sequencing of band b and b′ exhibited the wild-type sequence (left; CONTROL). Numbering of nucleotides and amino acids is rendered according to Forsgren et al52 and Petersen et al,21 respectively.
RESULTS
Hemostasis Parameters in Patient 1 and Other Members of Family 1
Plasminogen functional activity in plasma of patient 1 was only 6% (normal range, 80% to 120%) and the level of plasminogen antigen was <0.4 mg/dL (normal range, 6 to 25 mg/dL). Other hemostasis parameters (eg, fibrinogen, factor XII, t-PA, and PAI-1) were normal in the patient and all other family members tested so far (Table 1).
Molecular Genetic Findings in Patient 1 and Other Members of Family 1
Altered single-strand band patterns in genomic DNA of patient 1 (II-1) were found by SSCP analysis only when primers specific for plasminogen exon 7 were used (Fig 2). Direct sequencing of both exon 7 single strands (Fig 2; band a and a′) from patient 1 (II-1) showed a (homozygous) point mutation at position 780 (G → A) leading to an amino acid substitution at position 216 (Arg216 → His)(Fig 4, right). This mutation is located in the kringle 2 domain of plasminogen just beneath the intrakringle disulfite bridge Cys215 -Cys238 .21 The healthy sister (Fig 2, II-2, lane 5) and the healthy parents (Fig 2, I-1 and I-2, lanes 3 and 4, respectively) were shown to be heterozygous for this mutation by direct sequencing of their allele a. The second allele (band b) contained only the wild-type sequence of plasminogen exon 7 (Fig 4, left). The exon 7 mutation could also be differentiated from the wild-type allele by amplification mutagenesis. The parents and the sister were shown to be heterozygous for the (larger) mutant allele (Fig 5, lanes 2+, 3+ and 4+, respectively), whereas patient 1 was homozygous for the mutant allele (Fig 5, lane 1+). Examination of 50 healthy controls by this method revealed only the wild-type allele (data not shown).
Direct detection of plasminogen exon 7 mutation in family 1 by amplification mutagenesis. A part of plasminogen exon 7 containing the mutation (G → A at position 780) was amplified by PCR as described in Materials and Methods. The first primer has a single sequence mismatch that results in the introduction of an artificial BstEII restriction site into the wild-type allele only. Amplified PCR products were digested with BstEII, electrophoresed in a 2.5% agarose gel, and visualized by ethidium bromide staining. The wild-type BstEII-digested PCR fragment has a size of 119 bp, the mutant (uncut) PCR fragment has a size of 141 bp. −, No BstEII added to PCR product; +, BstEII added to PCR product. Lane M, 100-bp ladder; lane 1, patient 1; lane 2, mother of patient 1; lane 3, father of patient 1; lane 4, sister of patient 1; lane 5, healthy control. The larger (mutant) allele is found after BstEII-digestion in patient 1 (lane 1+), both parents (lanes 2+ and 3+, heterozygotes) and the sister (lane 4+, heterozygote), but not in the healthy control (lane 5+).
Direct detection of plasminogen exon 7 mutation in family 1 by amplification mutagenesis. A part of plasminogen exon 7 containing the mutation (G → A at position 780) was amplified by PCR as described in Materials and Methods. The first primer has a single sequence mismatch that results in the introduction of an artificial BstEII restriction site into the wild-type allele only. Amplified PCR products were digested with BstEII, electrophoresed in a 2.5% agarose gel, and visualized by ethidium bromide staining. The wild-type BstEII-digested PCR fragment has a size of 119 bp, the mutant (uncut) PCR fragment has a size of 141 bp. −, No BstEII added to PCR product; +, BstEII added to PCR product. Lane M, 100-bp ladder; lane 1, patient 1; lane 2, mother of patient 1; lane 3, father of patient 1; lane 4, sister of patient 1; lane 5, healthy control. The larger (mutant) allele is found after BstEII-digestion in patient 1 (lane 1+), both parents (lanes 2+ and 3+, heterozygotes) and the sister (lane 4+, heterozygote), but not in the healthy control (lane 5+).
In this family a homozygous mutation in the plasminogen gene (Arg216 → His) led to plasminogen antigen concentrations below the detection limit of the assay and markedly decreased plasminogen functional activity in patient 1 (Table 1). In the father and the sister, who are heterozygous for this mutation, plasminogen antigen (7 and 7.5 mg/dL, respectively) and functional activity (60% and 76%, respectively) were reduced to almost the same extent and were within or slightly above the theoretically predicted heterozygous range (antigen, 3 to 13 mg/dL, functional activity, 40% to 60%, respectively). In the mother, who is also heterozygous for this mutation (Arg216 → His), plasminogen functional activity was slightly above the theoretically predicted heterozygous range (functional activity, 40% to 60%), whereas her plasminogen antigen concentration (2 mg/dL) was below the heterozygous range (3 to 13 mg/dL). The reason for this discrepancy between the three heterozygous family members is not known.
Hemostasis Parameters in Patient 2 and Other Members of Family 2
When the hemostasis system of patient 2 was examined at the age of 2 years, no plasminogen functional activity was found (<5%; normal range, 80% to 120%). In addition, plasminogen antigen levels were below the detection limit of the assay used (<0.4 mg/dL; normal range, 6 to 25 mg/dL). Fibrinogen, t-PA, and PAI-1) of the patient and all healthy family members tested so far were within the normal range (Table 1). Plasminogen functional activities in the mother (Fig 3, I-1), father (Fig 3, I-2), and the 4-year-old sister (Fig 3, II-2) were 60%, 15%, and 66%, respectively. Plasminogen antigen concentrations were 5.2, 1.2, and 6.9 mg/dL, respectively (Table 1).
Molecular Genetic Findings in Patient 2 and Other Members of Family 2
An altered SSCP band pattern was found in this affected family only when primers specific for plasminogen exon 15 were used (Fig 3). Direct sequencing of both single strands of plasminogen exon 15 from patient 2 (Fig 3, lane 2; band a and a′) showed a homozygous point mutation (G → A) at position 1924, leading to a stopcodon (TGA) at position 597 (Trp597 → Stop) (Fig 6a). This mutation lies in the β chain of plasminogen, 36 amino acids downstream of the Arg561 -Val562 cleavage site that is necessary for the conversion of plasminogen to plasmin.21 By SSCP analysis (Fig 3, lane 3, I-1; lane 4, I-2) and direct sequencing of band a both parents were shown to be heterozygous for this mutation. The second allele from the mother (Fig 3, lane 3, band c) contains the wild-type sequence of plasminogen exon 15.
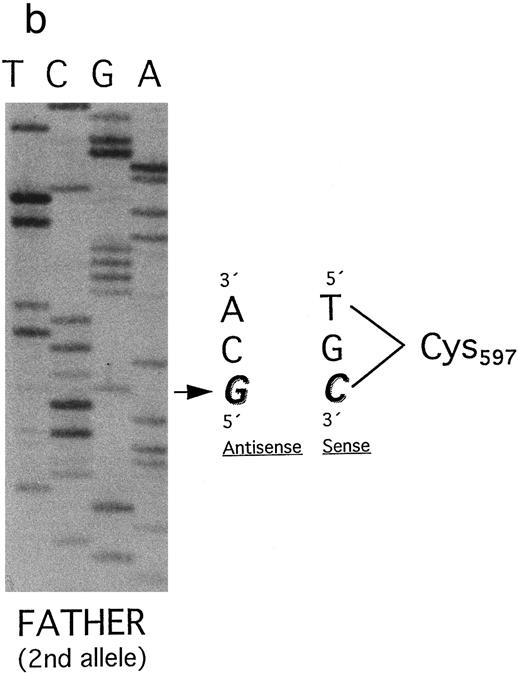
(a) Plasminogen exon 15 sequence analysis in family 2 (noncoding strand is shown). Single-stranded DNA fragments with a variant migration pattern in SSCP analysis (Fig 3, bands a, b, and c) were excised from polyacrylamide gel and sequenced as described in Materials and Methods. The DNA of the patient 2 (band a in Fig 3) exhibited a homozygous point mutation (G → A) at position 1924, leading to a stop codon at position 597 (Trp597 → STOP). The mother and the father (Fig 3, I-1 and I-2) are heterozygous for this mutation. Numbering of nucleotides and amino acids is rendered according to Forsgren et al52 and Petersen et al,21 respectively. (b) The second allele of the father (band b) contained a point mutation (G → C) at position 1924, leading to an amino acid exchange (Trp597 → Cys597 ). Numbering of nucleotides and amino acids is rendered according to Forsgren et al52 and Petersen et al,21 respectively.
(a) Plasminogen exon 15 sequence analysis in family 2 (noncoding strand is shown). Single-stranded DNA fragments with a variant migration pattern in SSCP analysis (Fig 3, bands a, b, and c) were excised from polyacrylamide gel and sequenced as described in Materials and Methods. The DNA of the patient 2 (band a in Fig 3) exhibited a homozygous point mutation (G → A) at position 1924, leading to a stop codon at position 597 (Trp597 → STOP). The mother and the father (Fig 3, I-1 and I-2) are heterozygous for this mutation. Numbering of nucleotides and amino acids is rendered according to Forsgren et al52 and Petersen et al,21 respectively. (b) The second allele of the father (band b) contained a point mutation (G → C) at position 1924, leading to an amino acid exchange (Trp597 → Cys597 ). Numbering of nucleotides and amino acids is rendered according to Forsgren et al52 and Petersen et al,21 respectively.
Surprisingly, by SSCP analysis the father's DNA showed a second band with an altered migration pattern (Fig 3, lane 4; band b). Direct sequencing of this single-stranded DNA segment revealed a point mutation at position 1924 (G → C) leading to an amino acid exchange at position 597 (Trp597 → Cys) (Fig 6b). Therefore, the father is compound heterozygous for two different point mutations in the same codon of plasminogen exon 15. The healthy sister of patient 2 was shown to be heterozygous for the latter mutation (Trp597 → Cys) (Fig 3, lane 5). Her second allele (band c) contains the wild-type sequence of exon 15.
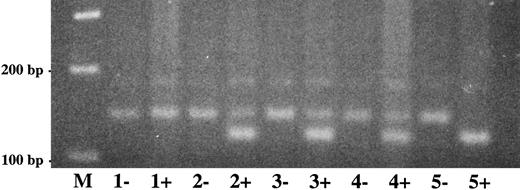
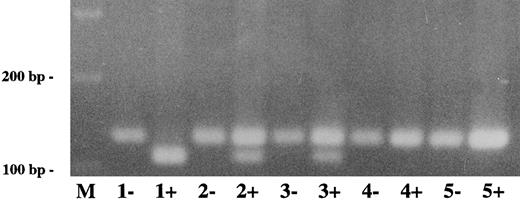
The exon 15 stop codon mutation (Trp597 → Stop) could be easily differentiated from the wild-type allele by amplification mutagenesis (Fig 7). The (shorter) mutant allele was found in the patient (lane 1+, homozygous) and her parents (lanes 2+ and 3+, heterozygotes) but not in the sister (lane 5+). Examination of 50 healthy controls by this method showed only the wild-type DNA fragment (data not shown).
Direct detection of plasminogen mutation in family 2 by amplification mutagenesis. A part of plasminogen exon 15 containing the stop codon mutation (G → A) at position 1924) was amplified by PCR as described in Materials and Methods. The reverse primer has a single sequence mismatch that results in the introduction of an artificial Hinf I restriction site into the mutant exon 15 allele only. Amplified PCR products were digested with Hinf I and electrophoresed in a 2.5% agarose gel and visualized by ethidium bromide staining. The mutant Hinf I-digested PCR fragment has a size of 107 bp, the wild-type (uncut) PCR fragment has a size of 129 bp. −, No Hinf I added to PCR product; +, Hinf I added to PCR product. Lane M, 100-bp ladder; lane 1, patient 2; lane 2, mother of patient 2; lane 3, father of patient 2; lane 4, healthy sister of patient 2; lane 5, healthy control. The (shorter) mutant Hinf I-digested allele is found in patient 1 (lane 1+) and the parents (lanes 2+ and 3+, heterozygotes), but not in the sister (lane 4+) and the healthy control (lane 5+).
Direct detection of plasminogen mutation in family 2 by amplification mutagenesis. A part of plasminogen exon 15 containing the stop codon mutation (G → A) at position 1924) was amplified by PCR as described in Materials and Methods. The reverse primer has a single sequence mismatch that results in the introduction of an artificial Hinf I restriction site into the mutant exon 15 allele only. Amplified PCR products were digested with Hinf I and electrophoresed in a 2.5% agarose gel and visualized by ethidium bromide staining. The mutant Hinf I-digested PCR fragment has a size of 107 bp, the wild-type (uncut) PCR fragment has a size of 129 bp. −, No Hinf I added to PCR product; +, Hinf I added to PCR product. Lane M, 100-bp ladder; lane 1, patient 2; lane 2, mother of patient 2; lane 3, father of patient 2; lane 4, healthy sister of patient 2; lane 5, healthy control. The (shorter) mutant Hinf I-digested allele is found in patient 1 (lane 1+) and the parents (lanes 2+ and 3+, heterozygotes), but not in the sister (lane 4+) and the healthy control (lane 5+).
In this family a homozygous nonsense mutation in the plasminogen gene (Trp597 → Stop), which removes the protease domain of plasmin, leads to undetectable plasminogen antigen and functional activity in patient 2. However, because of the limited sensitivities of the assays used we cannot totally exclude traces of plasminogen antigen and a very low residual functional activity in this subject (Table 1). In the mother, who is heterozygous for this mutation, plasminogen values (antigen concentration, 5.2 mg/dL; functional activity, 60%) are reduced by ∼50% (heterozygous range) when compared with normal subjects. Also, in the sister, who is heterozygous for another mutation of the same codon (Trp597 → Cys), plasminogen values (antigen concentration, 6.9 mg/dL; functional activity, 66%) are within or slightly above the heterozygous range (antigen concentration, 3 to 13 mg/dL; activity, 40% to 60%). The father, who is compound-heterozygous (first plasminogen allele: Trp597 → Stop; second plasminogen allele: Trp597 → Cys), showed markedly reduced plasminogen values (antigen concentration, 1.2 mg/dL; functional activity, 15%) when compared with “single” heterozygotes such as the mother or sister of patient 2 (Table 1).
DISCUSSION
Recently, Mingers et al15 demonstrated for the first time severe type I plasminogen deficiency (plasminogen antigen and functional activity both markedly reduced) in three unrelated girls with ligneous conjunctivitis, including patient 2 of this report. Here we report different homozygous inactivating mutations of the plasminogen gene (Arg216 → His in the kringle 2 domain and Trp597 → Stop in the β chain of plasminogen) in two unrelated girls both suffering from ligneous conjunctivitis and occlusive hydrocephalus. Both patients had severe type I plasminogen deficiency. It is possible that as a consequence of these mutations, mRNA transcription and/or the half-life of mRNA of the mutant plasminogen allele may be markedly decreased. It is also conceivable that the liver may synthesize a truncated plasminogen molecule that is rapidly cleared from the circulation. A third possibility is that secretion of mutant plasminogen molecules is impaired. This mechanism has been recently demonstrated in vitro by genetic engineering techniques for type I plasminogen deficiency caused by a Ser572 → Pro mutation.24
Plasmin is thought to contribute to the displacement of fibrin-containing extracellular matrix in healing human skin wounds.25 In addition, plasmin facilitates keratinocyte division, migration, and differentiation, and supports the closure of skin wounds.26 Subjects suffering from inflammation of the conjunctiva or the cornea exhibit elevated plasmin activity in the lacrimal fluid.27 28 These findings indicate that plasmin plays an important role in wound-healing processes of the eye.
In patients with ligneous conjunctivitis, wound healing in mucous membranes is markedly impaired; it seems to be arrested at the stage of granulation tissue formation.12 One major constitutent of pseudomembranes is fibrin.10 This points to a major deficiency of plasmin-mediated fibrinolysis in the extravascular compartment. Because amorphous deposits present in pseudomembranes also contain other plasma proteins such as albumin and immunoglobulins, it has been suggested that permeability of blood vessels in these lesions may be increased.29,30 Electron microscopic studies of pseudomembraneous lesions showed abnormal blood vessels with wide gaps between lining endothelial cells that were degenerated and surrounded by a thick and multilaminar basement membrane.30 It is not clear at present whether hyperpermeability of these blood vessels is primarily caused by severe plasmin(ogen) deficiency or is only the consequence of conjunctival inflammation.
Impaired healing of skin wounds of patients with ligneous conjunctivitis has not been reported in the literature. These apparently contradictory findings may be, at least in part, explained by the fact that the conjunctivae (and other mucous membranes) represent a “locus minoris resistentiae” when compared with normal intact skin. Predisposed children may develop ligneous conjunctivitis after minor trauma or infection of conjunctival mucous membranes.30 Surgical excision of pseudomembranes is frequently followed by rapid regrowth of the membranes and is believed to be by itself a trigger for recurrences.11,31-33 On the other hand, 6 of 17 patients with ligneous conjunctivitis showed spontaneous resolution after numerous recurrences.30
These findings suggest that external triggers such as injuries or infections may induce and exacerbate formation of pseudomembraneous in children with severe type I plasminogen deficiency and that these lesions may spontaneously resolve when “irritations” are absent. This implies that the capacity of wound healing in mucous membranes is not absent, but more or less impaired. In addition, it may well be that in patients with ligneous conjunctivitis the time of skin healing is also slightly prolonged. However, this will not become clinically apparent in most patients. In any case, the exact pathophysiologic mechanism underlying these differences has to be evaluated in further studies.
Our molecular genetic findings confirm observations by others9,13,14 that ligneous conjunctivitis may be inherited in an autosomal-recessive manner. In one case reported so far, ligneous conjunctivitis with additional gingival and peritoneal lesions developed as a serious side effect during treatment with tranexamic acid, an agent used in the treatment of menorrhagia.34 Tranexamic acid blocks the lysine binding sites on the kringle domains of plasminogen and leads to dissociation of the fibrin(ogen)-plasminogen complex.35 This observation gives further emphasis to the central role of functional plasmin(ogen) deficiency in the pathogenesis of this rare disease.
It is surprising that none of the homozygous and heterozygous members of both families reported here spontaneously developed any thrombotic events. (However, the risk of thrombotic occlusions of implanted catheters may be markedly increased, as shown in patient 2.) There are also no reports in the literature that patients with ligneous conjunctivitis ever suffered from venous thrombosis. Furthermore, two recent epidemiological studies suggest that heterozygous type I plasminogen deficiency by itself is not a risk factor for thrombosis.36,37 It has been suggested that symptomatic patients with heterozygous type I plasminogen deficiency may have additional risk factors such as protein C or S or antithrombin III deficiencies.37 The reason for why even homozygous type I plasminogen deficiency does not predispose to thrombosis is not yet clear. One may speculate that in these subjects the deficient intravascular plasmin-mediated fibrinolysis pathway is fully compensated by other serine proteases (such as elastase, produced by granulocytes38 ) or by increased fibrin(ogen) clearance by activated monocytoid cells via the integrin receptor Mac-1 (CD11b/CD18).39
In addition to the plasminogen mutations presented here (Arg216 → His, Trp597 → Stop, and Trp597 → Cys) two other (heterozygous) mutations in plasminogen exon 14 (Ser572 → Pro) and exon 17 (Ala675 → Thr) have been identified in Japanese families with type I plasminogen deficiency.22 40 It could well be that these mutations, if inherited as a homozygous trait, will lead to severe plasminogen deficiency with subsequent development of ligneous conjunctivitis.
Other distinct mutations of the plasminogen gene (Ala601 → Thr, Val355 → Phe) have been found in subjects with different types of dysplasminogenemias (normal or only slightly reduced levels of plasminogen antigen, plasminogen functional activity markedly decreased).41 In the Japanese and the Chinese Han populations the (Ala601 → Thr) mutation in the plasminogen gene is found in 2.2% and 2.9%, respectively, of healthy subjects.42,43 Only a small fraction of subjects with homozygous or heterozygous dysplasminogenemia become clinically symptomatic. It also seems that isolated dysplasminogenemia alone is not a risk factor for thrombosis.37
Mice with homozygously disrupted plasminogen genes (Plg−/− mice) have been shown to develop extravascular fibrin(ogen) depositions in liver, lung, and stomach, as well as ulcerated lesions in the gastrointestinal tract and rectal tissue as well as in the trachea, larynx, vagina and uterus.44-46 Healing of skin, vascular, and renal wounds is impaired in these animals.46-49
Ulcers in Plg−/− mice have a characteristic morphology with a necrotic surface epithelium covered by exsudate, necrotic underlying tissue with diffuse distribution of fibrin-rich fibrillar material, reactively altered hyperplasia of adjacent epithelium, and occasional vascular occlusions in the lamina propria.46 Similar histologic changes occur in artificial wounds in Plg−/− mice. The migration of keratinocytes from the wound edges is impaired leading to delayed reepithelialization.47
Although histological findings in wounds and ulcers of Plg−/− mice are somewhat similar to those observed in mucous membranes of patients with ligneous conjunctivitis, there are three significant differences between both groups: (1) Healing of skin wounds is markedly impaired only in Plg−/− mice. (2) Plg−/− mice, but not patients with ligneous conjunctivitis, are predisposed to severe thrombosis.44,45 (3) Plg−/− mice, but not patients with ligneous conjunctivitis, exhibit retarded postnatal growth.44 The reason for these differences between mice and humans is still unknown.
Similar clinical and histological findings as described in the Plg−/− mice have been also found in mice homozygously deficient for both the urokinase-type and the tissue-type plasminogen activator genes (u-PA−/−/t-PA−/− mice), but not in mice homozygously deficient for both the urokinase-receptor and the tissue-type plasminogen activator genes (u-PAR−/−/t-PA−/− mice).50 The animal data suggest that u-PA–mediated plasminogen activation is of primary importance for the resolution of fibrin–rich lesions in skin.50 In theory, ligneous conjunctivitis could result from either homozygous plasminogen or homozygous u-PA deficiency.
In conclusion, our findings show that certain homozygous mutations in the plasminogen gene may lead to severe type I plasminogen deficiency. It is mainly the extravascular plasmin-mediated fibrinolytic pathway that is impaired in affected subjects leading to deficient wound healing in mucous membranes. In predisposed children with homozygous type I plasminogen deficiency even minor trauma or local inflammation of mucous membranes (conjunctiva, trachea, mouth, nasopharynx, female genital tract) may initiate the formation of “ligneous” pseudomembranes.
Pathologic findings in humans with ligneous conjunctivitis resemble only to a certain extent those observed in Plg−/− mice. In these plasminogen-deficient animals, bolus administration of plasminogen was able to restore the intravascular fibrinolytic potential.51 It remains to be elucidated whether early intravenous plasminogen administration to children with homozygous type I plasminogen deficiency may prevent ligneous conjunctivitis and other associated complications or may even cure manifest disease.
ACKNOWLEDGMENT
We are indebted to Dr I. Johnston for critically reviewing the manuscript.
Supported by a grant (Schu 560/2-3) from the Deutsche Forschungsgemeinschaft (to V.S.)
Address reprint requests to Volker Schuster, MD, Children's Hospital, University of Würzburg, Josef-Schneider-Straβe 2, D-97080 Würzburg, Germany.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal