Abstract
Reverse-transcription polymerase chain reaction (RT-PCR) of the PML/RARα fusion gene may predict relapse in acute promyelocytic leukemia (APL) patients in hematologic complete remission (CR). We have prospectively studied by RT-PCR 15 PML/RARα+ APL patients undergoing autologous bone marrow transplantation (ABMT) in second CR. The median time of first CR duration was 12 months (range, 6 to 40). All patients were reinduced with all-trans retinoic acid (ATRA), followed in 12 of 15 cases by mitoxantrone and Ara-C as consolidation. Fourteen patients received the BAVC (BCNU, Ara-C, m-AMSA, and VP-16) schedule as conditioning regimen. Unpurged marrows were collected immediately before conditioning treatment, analyzed by RT-PCR, and reinfused at median of 2 months (range, 2 to 7) from the achievement of second CR. Seven patients were PCR+ and eight PCR− for PML/RARα in their pretransplant marrows. All seven patients of the former group remained PCR+ during the follow-up and relapsed at a median time of 5 months (range, 2 to 9) from ABMT and 9 months (range, 4 to 14) from second CR. Of the eight PCR− patients, all remained PCR− during the follow-up controls. One patient relapsed at 10 months from ABMT, one died of a secondary (PML/RARα−) leukemia, and six are in hematologic and molecular remission at a median time of 28 months (range, 15 to 60) after ABMT and 32 months (range, 17 to 62) from second CR. Our results indicate that, in APL patients in second CR, ABMT with PML/RARα− marrow cells is likely to result in prolonged clinical and molecular remissions. Conversely, patients who test PCR+ after reinduction necessitate the use of alternative aggressive approaches, including unrelated allogeneic transplant.
OVER THE PAST few years, both basic and clinical investigations have allowed important advances in the management of acute promyelocytic leukemia (APL).1,2 The identification of a specific genetic lesion (ie, the PML/RARα fusion gene) by the reverse-trascription polymerase chain reaction (RT-PCR) in APL cells has proved extremely useful for rapid diagnosis, prediction of response to all-trans retinoic acid (ATRA), and sensitive detection of minimal residual disease (MRD).3-6 Modern treatment protocols for newly diagnosed patients, including various combinations of ATRA and conventional chemotherapy, have resulted in a better control of the APL-associated coagulopathy, extremely high remission rates, and improved disease-free survival.7-12
With regard to relapsed APL, most patients are usually reinduced into second complete remission (CR) by chemotherapy and/or ATRA. Although no consensus exists as to the optimal postremission treatment, allogeneic bone marrow transplantation (allo-BMT) is generally considered the treatment of choice for patients with an HLA-identical sibling. However, with the exception of a single retrospective analysis,13 no study has so far compared allo-BMT with autologous BMT (ABMT) in APL in second CR.
We have previously reported that ABMT after BAVC conditioning in acute myeloid leukemia (AML) in second CR is well tolerated and results in prolonged disease-free survival in a high proportion of cases (including APL).14 Because the analysis on a longer follow-up has confirmed these observations,15 we have kept using this strategy for all AML patients without HLA-identical donors who have achieved a second hematologic CR.
Several groups, including ours, have emphasized the relevance of RT-PCR studies of PML/RARα in the management of APL. In particular, there is general agreement that persistently positive tests during remission are strong predictors of subsequent hematologic relapse, whereas patients in long-term survival are PCR−.3-6 16-19
We report here clinical and MRD molecular monitoring data on a series of 15 consecutive APL cases in second CR treated by BAVC and ABMT at our institution. The results show that RT-PCR analysis of pretransplant BM is a reliable indicator of clinical outcome and should always be considered to better address further treatment.
MATERIALS AND METHODS
Patients.Since 1984, a total of 21 APL patients in second CR were treated by ABMT at the Hematology Institute of the University “La Sapienza” of Rome, Italy. Prospective RT-PCR studies of the PML/RARα fusion gene were initiated in 1991 and performed in 15 consecutive cases which are included in this study. Patients' characteristics, duration of first CR, treatments received for initial disease and first relapse, and time interval from second CR to ABMT are reported in Table 1. Thirteen patients received anthracycline-based chemotherapy as first-line treatment, whereas two were treated by combined ATRA and idarubicin according to the all-trans retinoic acid and idarubicin (AIDA) regimen.11,12 Patient no. 4 was resistant to chemotherapy and was induced into CR with ATRA. Three polychemotherapy cycles were administered to all patients as consolidation of first CR as previously reported.12 Second CR was reinduced in all patients with oral ATRA given at the standard dose of 45 mg/m2/d. Because of increasing white blood cell (WBC) counts after 4 days of ATRA, patient no. 12 also received chemotherapy with cytosine arabinoside and mitoxantrone. With the exception of patients no. 1 through 3, who did not receive consolidation treatment after ATRA, the other patients were consolidated with intravenous (IV) cytosine arabinoside 1 g/m2 days 1 through 4 and IV mitoxantrone 6 mg/m2 days 1 through 4.20
PCR Results, Treatments, and Clinical Outcome of the 15 APL Patients
| Pt. No. . | Age/Sex . | PML/RARα Junction Type . | Initial Therapy/Consolid. . | CR1 Length (mo) . | Therapy of Relapse/Consolid. . | Time to ABMT (mo from CR2) . | Pre-ABMT PCR* . | CR2 Length (mo post-ABMT) . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50/F | bcr3 | IDA/yes | 12 | ATRA/no | 2 | Positive | 2 | Relapsed/dead |
| 2 | 38/M | bcr1 | DNR/yes | 16 | ATRA/no | 2 | Positive | 5 | Relapsed/dead |
| 3 | 17/F | bcr3 | IDA/yes | 10 | ATRA/no | 2 | Positive | 7 | Relapsed/dead |
| 4 | 43/M | bcr1 | IDA + AraC/ATRA/yes | 20 | ATRA/yes | 2 | Positive | 3 | Relapsed/dead |
| 5 | 40/M | bcr2 | IDA/yes | 40 | ATRA/yes | 3 | Positive | 9 | Relapsed/dead |
| 6 | 39/F | bcr1 | IDA/yes | 6 | ATRA/yes | 6 | Positive | 4 | Relapsed/dead |
| 7 | 10/M | bcr3 | LAM 8704 | 6 | ATRA/yes | 7 | Positive | 7 | Relapsed/dead |
| 8 | 46/F | bcr1 | IDA + AraC/yes | 18 | ATRA/yes | 2 | Negative | 60+ | A & W† |
| 9 | 23/F | bcr3 | IDA + AraC/yes | 12 | ATRA/yes | 2 | Negative | 44+ | A & W |
| 10 | 27/M | bcr1 | IDA/yes | 12 | ATRA/yes | 2 | Negative | 27+ | A & W |
| 11 | 45/F | bcr1 | IDA + AraC/yes | 22 | ATRA/yes | 3 | Negative | 10 | Relapsed/dead |
| 12 | 31/M | bcr1 | IDA + AraC/yes | 13 | ATRA/Mitox + AraC/yes | 4 | Negative | 36 | Dead‡ |
| 13 | 35/F | bcr3 | AIDA/yes | 13 | ATRA/yes | 2 | Negative | 15+ | A & W |
| 14 | 53/F | bcr1 | AIDA/yes | 11 | ATRA/yes | 3 | Negative | 20+ | A & W |
| 15 | 32/M | bcr1 | IDA + AraC/yes | 11 | ATRA/yes | 5 | Negative | 30+ | A & W |
| Pt. No. . | Age/Sex . | PML/RARα Junction Type . | Initial Therapy/Consolid. . | CR1 Length (mo) . | Therapy of Relapse/Consolid. . | Time to ABMT (mo from CR2) . | Pre-ABMT PCR* . | CR2 Length (mo post-ABMT) . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50/F | bcr3 | IDA/yes | 12 | ATRA/no | 2 | Positive | 2 | Relapsed/dead |
| 2 | 38/M | bcr1 | DNR/yes | 16 | ATRA/no | 2 | Positive | 5 | Relapsed/dead |
| 3 | 17/F | bcr3 | IDA/yes | 10 | ATRA/no | 2 | Positive | 7 | Relapsed/dead |
| 4 | 43/M | bcr1 | IDA + AraC/ATRA/yes | 20 | ATRA/yes | 2 | Positive | 3 | Relapsed/dead |
| 5 | 40/M | bcr2 | IDA/yes | 40 | ATRA/yes | 3 | Positive | 9 | Relapsed/dead |
| 6 | 39/F | bcr1 | IDA/yes | 6 | ATRA/yes | 6 | Positive | 4 | Relapsed/dead |
| 7 | 10/M | bcr3 | LAM 8704 | 6 | ATRA/yes | 7 | Positive | 7 | Relapsed/dead |
| 8 | 46/F | bcr1 | IDA + AraC/yes | 18 | ATRA/yes | 2 | Negative | 60+ | A & W† |
| 9 | 23/F | bcr3 | IDA + AraC/yes | 12 | ATRA/yes | 2 | Negative | 44+ | A & W |
| 10 | 27/M | bcr1 | IDA/yes | 12 | ATRA/yes | 2 | Negative | 27+ | A & W |
| 11 | 45/F | bcr1 | IDA + AraC/yes | 22 | ATRA/yes | 3 | Negative | 10 | Relapsed/dead |
| 12 | 31/M | bcr1 | IDA + AraC/yes | 13 | ATRA/Mitox + AraC/yes | 4 | Negative | 36 | Dead‡ |
| 13 | 35/F | bcr3 | AIDA/yes | 13 | ATRA/yes | 2 | Negative | 15+ | A & W |
| 14 | 53/F | bcr1 | AIDA/yes | 11 | ATRA/yes | 3 | Negative | 20+ | A & W |
| 15 | 32/M | bcr1 | IDA + AraC/yes | 11 | ATRA/yes | 5 | Negative | 30+ | A & W |
Pre-ABMT PCR, performed in hematologic CR.
A & W, alive and well, in hematologic and molecular CR.
Died of secondary leukemia.
BM processing and preparative regimen.In all cases, the BM was obtained in second CR immediately before conditioning therapy and cryopreserved according to established procedures.21 No purging techniques were used. The median time interval from the achievement of second CR to ABMT was 2 months (range, 2 to 7). In 14 patients, the conditioning regimen was based on the BAVC protocol (BCNU, ARA-C, m-AMSA, and VP-16),14 while patient no. 12 received busulfan and cyclophosphamide according to the schedule reported by Tutschka et al.22
Supportive care.All patients had a central venous catheter placed for chemotherapy, fluids, and blood products administration. Oral ciprofloxacin was given as antibacterial prophylaxis and IV acyclovir (15 mg/kg/d) was started at day +1 to prevent herpesvirus infection. Broad-spectrum antibiotics were administered in case of fever higher than 38°C whenever the neutrophil counts were less than 500/μL. Patients with documented fungal infection or persistent fever after 72 to 96 hours of antibiotic therapy received IV amphotericin B. All blood products were irradiated with 20 Gy before infusion to prevent graft-versus-host reactions.
Molecular studies.BM samples for RT-PCR analyses were collected in all cases at the time of first hematologic relapse, immediately before ABMT, and at regular intervals during the follow-up after ABMT. Mononuclear cell fractions obtained by centrifugation on a Ficoll-Hypaque density gradient (Nycomed Pharma, Oslo, Norway) were washed using RNAase-free disposable materials and stored in 4 mol/L guanidium isothiocyanate at −20°C until RNA extraction. Total RNA was prepared according to the method of Chomczynsky and Sacchi.23 Before RT-PCR analysis, the integrity of RNAs was assessed by running the samples on a formaldehyde minigel. The protocol and the oligoprimers used for RT-PCR of the PML/RARα hybrid gene have been reported elsewhere.4 18 Amplification of an RARα cDNA fragment including exon 2 and exon 3 sequences was performed in all cases at the same time as the amplification of PML/RARα to further assess RNA integrity, as well as the efficiency of the RT step. To assess the sensitivity of our assay, total RNA isolated from a diagnostic APL sample with 100% blast infiltration was serially diluted by mixing it with the t(15; 17)-negative myeloid cell line GF-D8 RNA. Our RT-PCR method allowed us to detect a PML/RARα-specific band in the presence of 0.1 ng total RNA, ie, a final dilution of 10−4 PML/RARα+ cells.
RESULTS
The PML/RARα fusion gene was identified in BM leukemia cells at first relapse in all cases. The types of PML breakpoints detected in each case are reported in Table 1. The conditioning regimen was well tolerated and no major complications were observed during the posttransplant phase of BM aplasia. The median time to attain PMN counts >0.5 × 109/L and platelet counts >50 × 109/L was 28 days (range, 11 to 35) and 53 days (range, 13 to 468), respectively. No correlations were observed between the number of nucleated BM cells or granulocyte-macrophage colony-forming cells reinfused and the rate of hematologic recovery.
The results of pre-ABMT PCR tests performed in second hematologic CR, second CR duration, and clinical outcome are also reported in Table 1. Seven of the 15 cases analyzed in the pretransplant marrows were PCR+ (nos. 1 through 7), whereas eight were PCR− (nos. 8 through 15). The comparison of pre-ABMT PCR-positive and -negative groups showed no significant differences regarding median first CR duration, type of PML breakpoint, and median WBC counts at diagnosis or at first relapse.
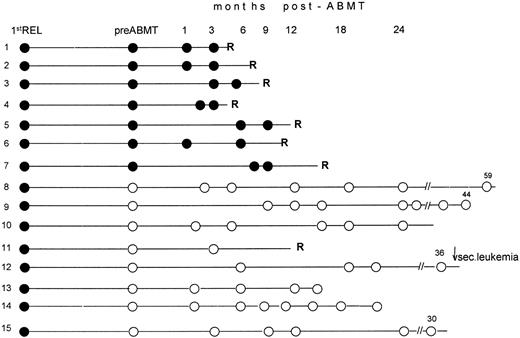
Eight patients (nos. 1 through 7, and 11) relapsed with APL within 2 to 10 months after transplant and died of their disease. All but one (no. 11) had tested PCR+ in pre-ABMT specimens collected in second hematologic CR. Patient no. 12, who had tested PCR− pre-ABMT, developed a secondary leukemia 36 months after transplant while in clinical and molecular remission from APL. In this case, all marrow samples collected during the post-ABMT monitoring, as well as the blast cells studied at the time of secondary leukemia diagnosis, were PCR− for PML/RARα (Fig 1). Both morpho-cytochemical characterization and karyotypic examination of blasts, which revealed a monosomy of chromosome 7 as the sole abnormality, contributed to rule out a recurrence of APL and were consistent with a diagnosis of therapy-related leukemia.
Longitudinal RT-PCR monitoring of the PML/RARα fusion gene in BM samples collected before and after ABMT. Following RT-PCR characterization of relapse, all pretransplantation (pre-ABMT) and posttransplantation (post-ABMT) tests were performed while patients were in hematologic remission. Black and white circles indicate positive and negative RT-PCR tests, respectively. 1st REL, first relapse; R, hematologic relapse; ↓ sec. leukemia, this patient developed at 36 months a secondary (or therapy-related) leukemia whose blasts tested RT-PCR− for the PML/RARα hybrid. Numbers above the white (open) circles indicate the last follow-up control, in months, after achievement of second remission.
Longitudinal RT-PCR monitoring of the PML/RARα fusion gene in BM samples collected before and after ABMT. Following RT-PCR characterization of relapse, all pretransplantation (pre-ABMT) and posttransplantation (post-ABMT) tests were performed while patients were in hematologic remission. Black and white circles indicate positive and negative RT-PCR tests, respectively. 1st REL, first relapse; R, hematologic relapse; ↓ sec. leukemia, this patient developed at 36 months a secondary (or therapy-related) leukemia whose blasts tested RT-PCR− for the PML/RARα hybrid. Numbers above the white (open) circles indicate the last follow-up control, in months, after achievement of second remission.
Six patients (nos. 8, 9, 10, 13, 14, and 15) remain in hematologic and molecular remission at a median time of 28 months (range, 15 to 60) from AMBT and 32 months (range, 17 to 62) from the achievement of second CR. All were PCR− pre-ABMT. A diagram showing the longitudinal PCR results for all patients is illustrated in Fig 1.
The correlation analysis of pretransplant MRD status, assessed by RT-PCR, and occurrence of APL relapse showed a statistically significant difference between PCR+ and PCR− cases (P < .001), as reported in Table 2.
Correlation Analysis Between Pretransplant PCR of PML/RARα and Occurrence of APL Relapse
| . | Relapsed <14 mo . | CCR* >14 mo . | |
|---|---|---|---|
| Pre-ABMT PCR +ve | 7 | 0 | |
| Pre-ABMT PCR −ve | 1 | 7 | P < .001† |
| . | Relapsed <14 mo . | CCR* >14 mo . | |
|---|---|---|---|
| Pre-ABMT PCR +ve | 7 | 0 | |
| Pre-ABMT PCR −ve | 1 | 7 | P < .001† |
Continuous clinical and molecular remission of APL, thus including patient no. 12 who developed a secondary leukemia.
By Fisher's exact test.
DISCUSSION
With the advent of ATRA and its use in combination with anthracycline-based chemotherapy (CHT), up to 70% of newly diagnosed APL patients treated with these schemes are now estimated to be potentially cured.7-9,11,12 An important contribution to these clinical results has been offered by improved diagnosis through the identification of the specific PML/RARα genetic lesion. In fact, no ATRA (or ATRA + CHT)–resistant cases have been documented in patients with a molecularly confirmed diagnosis of APL.8,11,12 Moreover, this genetic lesion has proved to be an ideal marker for MRD monitoring and its detection by RT-PCR during remission correlates with subsequent hematologic relapse.1-9,12 16-19 Thus, it is hoped that early treatment of PCR-detected MRD might further improve the cure rate in this disease.
Unlike patients with other AML subtypes, relapsed APL still have a high probability of long-term second CR and potential cure.1 For this latter purpose, the choice of the most appropriate postreinduction therapy is of paramount relevance. In this setting, the results of our study provide important indications.
All seven patients who were submitted to ABMT with persistent PCR-detectable MRD in their BM cells relapsed within 9 months after transplant. These data confirm the value of PCR positivity during remission as a predictor of relapse in APL. Among these seven cases, three had not received consolidation after reinduction with ATRA (cases 1 through 3). Although they are not comparable to the others as concerning the intensity of treatment received, these three patients are informative for the purpose of establishing the predictive value of PCR during hematologic CR, and were therefore included in the analysis. These patients were treated in the early 1990s. Following general awareness that ATRA-induced remissions are short-lived and do not result in PCR negativity, all other patients of this study (nos. 4 through 15) were consolidated with intensive chemotherapy. Nonetheless, four patients tested after consolidation by RT-PCR remained positive before ABMT and relapsed thereafter (nos. 4 through 7). Based on these observations, we decided to avoid the use of ABMT for patients who remain PCR+ after reinduction and consolidation.
As to the origin of relapse, from the present study we are not able to establish whether the residual leukemic burden, the reinfusion of contaminated marrow, or both had a major role in disease recurrence.
Of the eight patient undergoing AMBT with a PCR− marrow, only one relapsed with APL. As reported by several groups, the achievement of PCR negativity for the PML/RARα is no guarantee of cure and some patients do occasionally relapse during the follow-up.3-6,16-19 These findings are probably related to the limited sensitivity of the RT-PCR technique for the PML/RARα, which is notoriously lower than the one obtained by amplifying other fusion genes.3-6 16-19 Despite its poor sensitivity, our method enabled us to predict seven of eight relapses in the present series.
With regard to the patient who developed a secondary leukemia, it is important to observe that this was the only patient who received a conditioning regimen different from BAVC, and that no cases of secondary leukemia were recorded in an updated report of 60 AML cases treated by BAVC and ABMT recently published by our group.15 Because he had tested PCR− pretransplant and remained 36 months in CR from APL, this case adds significance to the correlation study of pre-ABMT PCR status and outcome (Table 2).
Finally, we comment on the efficacy of ABMT in the six cases (nos. 8, 9, 10, 13, 14, and 15) who persist in hematologic and molecular CR after transplant. As shown in Table 1, in these six patients the duration of second CR is already much longer than that of first CR.
In conclusion, accurate determination of postremission MRD by RT-PCR of the PML/RARα fusion gene is mandatory to better decide further consolidation therapy in APL patients in second CR. ABMT should be avoided and alternative approaches (including allogeneic BMT from unrelated donor) should be considered for patients with persistent MRD in their marrows. On the contrary, patients who convert to a PCR− status in second CR might be spared the risk of an allogeneic BMT, because they still have a high likelihood of long-term survival with a less aggressive ABMT. Further improvement of the sensitivity of the RT-PCR assay for the PML/RARα hybrid gene is needed to identify all patients at risk of relapse.
Supported in part by a grant from CNR (Consiglio Nazionale delle Ricerche) contratto ACRO n. 96.00613.PF39, MPI 40%, and by ROMAIL (Associazione Italiana contro le Leucemie, Sezione di Roma).
Address reprint requests to Francesco Lo Coco, MD, Hematology, Department of Human Biopathology, University “La Sapienza,” Via Benevento 6, 00161 Rome, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal