Abstract
Bcl-2 and its homologue Bcl-XL are expressed in a variety of tumors and their expression modulates the sensitivity of tumor cells to a wide spectrum of chemotherapeutic agents and γ-irradiation. In the present report, we generated clones of FL5.12 lymphoid cells with similar levels of Bcl-2 and Bcl-XL using the Flag epitope to determine if these survival proteins could provide equivalent protection when challenged with chemotherapy or γ-irradiation. Using four M-phase specific chemotherapeutic agents, Bcl-XL and Bcl-2 provided similar protection against vincristine and vinblastine whereas Bcl-XL afforded as much as 50% greater cell viability than Bcl-2 against etoposide and teniposide-induced cell death. In addition, Bcl-XL provided significantly greater cell viability than Bcl-2 against methotrexate, fluorouracil, and hydroxyurea, three S-phase specific agents. In apoptosis induced by γ-irradiation and cisplatin, two antitumor treatments that are cell-cycle phase-nonspecific agents, both Bcl-XL and Bcl-2 conferred similar protection against γ-irradiation, but Bcl-XL provided better protection than Bcl-2 against cisplatin. These results indicate that Bcl-XL and Bcl-2 confer a differential ability to protect against chemotherapy-induced cell death, which appears to be dependent on the molecular mechanism targeted by the drug rather than its cell-cycle phase specificity.
APOPTOSIS, a morphologically distinguished type of programmed cell death, is critical not only during development but also in the pathogenesis of a variety of diseases including cancer, autoimmune disease, viral infection, and neurodegenerative disorders.1 The bcl-2 gene, the first member of a rapidly expanding family of genes that regulate apoptosis, was initially isolated from the t(14; 18) chromosomal translocation found in human B-cell lymphomas.2 Subsequently, Bcl-2 has been shown to repress cell death triggered by a diverse array of stimuli including chemotherapy and γ-irradiation.3 Relatively high levels of Bcl-2 protein have been detected in 20% of patients with acute myelogenous leukemia and in 70% with chronic lymphocytic leukemia.4,5 Moreover, immunohistochemical analysis of the Bcl-2 protein has indicated that alterations in either the levels or patterns of Bcl-2 expression, or both, can occur in a variety of solid tumors.6 Bcl-XL a functional and structural homologue of Bcl-2, also provides protection against a wide variety of chemotherapeutic agents.7 Interestingly, expression levels of the Bcl-2 family of proteins change as tumors become more malignant or after treatment suggesting that expression of these survival proteins is critical not only for tumor development, but also for tumor progression and resistance to therapy.8,9 The mechanism by which Bcl-2 and Bcl-XL provide chemotherapy resistance is unknown, but it is thought that these survival proteins act at a common final step in the cell death pathway induced by antitumor agents.9 10
Initial studies suggested that Bcl-XL protected better than Bcl-2 against apoptosis induced by the immunosuppressant drugs cyclosporin A, FK-506, and rapamycin.11 However, these studies were inconclusive in that levels of Bcl-2 and Bcl-XL could not be directly compared as they were determined using different antibodies. In the present studies, we transfected FL5.12 lymphoid cells with expression constructs that produce Flag-epitope tagged Bcl-2 and Bcl-XL and developed cell clones that express similar levels of these proteins as determined with a Flag-specific antibody. Our results show that Bcl-XL protected significantly better than Bcl-2 against cell death induced by several chemotherapy agents. However, the differential ability of Bcl-XL and Bcl-2 against chemotherapy-induced apoptosis was not universal and appeared to be dependent on the molecular mechanism or cellular target of the drug rather than its cell-cycle phase specificity. The implications of these findings for tumor therapy and chemotherapy-resistance are discussed.
MATERIALS AND METHODS
Cell culture and transfection.FL5.12 cells were cultured in interleukin-3 (IL-3) conditioned medium as described.12 FL5.12 were transfected by electroporation (200 V, 960 mF ) with 10 mg of the pSFFV-Neo plasmid containing either Flag-bcl-XL or Flag-bcl-2 in the sense orientation or control pSFFV-Neo plasmid as previously described.13 Individual cell clones were selected for growth in the presence of G418 (1.0 mg/mL) by limiting dilution. Similar levels of protein expression for Flag-Bcl-XL and Flag-Bcl-2 in single cell clones was analyzed by flow cytometry using anti-Flag monoclonal antibody (MoAb) (M2) (Scientific Imaging Systems, Rochester, NY) as described13 and confirmed by immunoblot analysis as described below. Multiple clones expressing similar levels of Flag-Bcl-XL and Flag-Bcl-2 were randomly selected for further study.
Immunoblot analysis.The expression of Flag-Bcl-XL and Flag-Bcl-2 was determined by immunoblot analysis as previously described.14 Briefly, 1 × 106 cells were lysed in NP-40 isotonic lysis buffer with freshly added protease inhibitors and electrophoresed through 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred to nitrocellulose, blocked with 5% nonfat dry milk (Nestle Food Co, Glendale, CA) and incubated with anti-Flag MoAb (1 mg/mL). Equal protein loading was assessed by immunoblotting with anti-β-tubulin Ab (clone TUB 2.1) (Sigma Chemicals, St Louis, MO) as described.15 The reaction was developed by enhanced chemiluminescence using the ECL kit (Amersham, Arlington Heights, IL) after incubation with horseradish peroxidase-conjugated goat antimouse secondary antibody (Jackson Laboratories, Inc, West Grove, PA) and exposed to film for 1 minute (Kodak, Rochester, NY) as described.14 For immunoblot analysis of endogenous expression of Bcl-XL, Bcl-2, Bax, and Bad at 24 hours of incubation in each chemotherapy drug, FL5.12 control cells (Neo) were lysed as above and 50 mg of total protein separated and transferred to a nitrocellulose membrane as above and incubated in anti-Bcl-X MoAb (1:2,000), hamster antimouse Bcl-2 MoAb (clone 3F11) (Pharmingen, San Diego, CA), rabbit polyclonal anti-Bax (1:1,000) (a gift of Dr Leber, McMaster University, Hamilton, Ontario) or rabbit polyclonal anti-Bad (N 20) (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) and the blots developed as above. Control FL5.12 (Neo) cells were seeded (1 × 105 cells/mL) in each chemotherapy drug at concentrations indicated in the figure legends for time-course experiments (10 μg/mL of fluorouracil, hydroxyurea, or cisplatin) and harvested for immunoblot analysis as described above.
Chemotherapy and radiation-induced cell death assays.Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and either vincristine (Eli Lilly and Co, Indianapolis, IN), vinblastine (Bedford Laboratories, Bedford, OH), etoposide (Gensia Laboratories, LTD, Irvine, CA), teniposide (Bristol Laboratories, Princeton, NJ), methotrexate (Lederle Parenterals, Inc, Carolina, Puerto Rico), fluorouracil (Hoffman-LaRoche, Inc, Nutley, NJ), cisplatin (Bristol Laboratories, Princeton, NJ) or hydroxyurea (Sigma Chemicals) at concentrations indicated in the figure legends. For radiation-induced cell death assays, 1 × 106 cells/mL were γ-irradiated using a Cobalt-60 source (dose rate: 215 cGy/min) and seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3. For long-term viability studies, cells were seeded at 1 × 105 cells/mL in either 10 μg/mL of etoposide or 1.0 μg/mL of methotrexate, washed after 3 days of continuous incubation in chemotherapy drug, and replated in IL-3 conditioned media in the absence of drug. Cell viability was measured using trypan blue exclusion. Statistical analysis was performed using STATISTICA software (Stats Soft, Tulsa, OK).
RESULTS
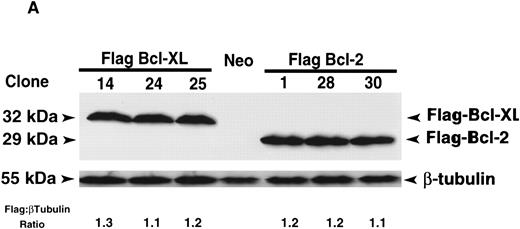
Bcl-XL and Bcl-2 provide similar protection from apoptosis induced by the M-phase specific vinca alkaloids, vincristine, and vinblastine.The IL-3–dependent murine lymphoid cell line, FL5.12, was transfected with human Flag-bcl-XL or Flag-bcl-2 and individual clones were isolated by limiting dilution. Multiple FL5.12 clones expressing similar levels of Flag-Bcl-XL and Flag-Bcl-2 were identified by immunoblot and FACScan analysis using the anti-Flag MoAb. Three representative clones of each, Flag-Bcl-XL and Flag-Bcl-2, with nearly identical levels of Flag expression as determined by immunoblotting with densitometric measurements and flow cytometric analysis were selected for further study (Fig 1A and B). Flow cytometric analysis also confirmed that the selected FL5.12 clones expressed Flag-Bcl-XL and Flag-Bcl-2 uniformly in all cells (Fig 1B).
Protein expression analysis of Flag-Bcl-2 and Flag-Bcl-XL in FL5.12 clones. (A) Immunoblot analysis of FL5.12 clones. Cell lysates from FL5.12 clones expressing Flag-Bcl-XL or Flag-Bcl-2 were electrophoresed through 12.5% SDS-polyacrylamide gels, transferred to nitrocellulose and incubated with anti-Flag MoAb (1 μg/mL). Relative ratios of Flag:β-tubulin protein expression determined by densitometric measurements following immunoblot analysis with anti-β–tubulin MoAb (clone TUB 2.1). Note that the Neo control does not contain Flag-Bcl-2 or Flag-Bcl-XL. The reaction was developed by enhanced chemiluminescence. (B) Fluorescence histograms of FL5.12 clones. Cell clones expressing Flag-Bcl-XL or Flag-Bcl-2 were analyzed for Flag-epitope expression by flow cytometry. Cells were stained with anti-Flag MoAb (1:50 dilution) and counter-stained with fluorescein isothiocyanate-conjugated Fc-specific antimouse IgG (1:50 dilution). Controls are FL5.12 clones expressing Flag-Bcl-XL (clone 14) or Flag-Bcl-2 (clone 1) stained with isotype-matched control IgG1 MoAb and counter-stained as above.
Protein expression analysis of Flag-Bcl-2 and Flag-Bcl-XL in FL5.12 clones. (A) Immunoblot analysis of FL5.12 clones. Cell lysates from FL5.12 clones expressing Flag-Bcl-XL or Flag-Bcl-2 were electrophoresed through 12.5% SDS-polyacrylamide gels, transferred to nitrocellulose and incubated with anti-Flag MoAb (1 μg/mL). Relative ratios of Flag:β-tubulin protein expression determined by densitometric measurements following immunoblot analysis with anti-β–tubulin MoAb (clone TUB 2.1). Note that the Neo control does not contain Flag-Bcl-2 or Flag-Bcl-XL. The reaction was developed by enhanced chemiluminescence. (B) Fluorescence histograms of FL5.12 clones. Cell clones expressing Flag-Bcl-XL or Flag-Bcl-2 were analyzed for Flag-epitope expression by flow cytometry. Cells were stained with anti-Flag MoAb (1:50 dilution) and counter-stained with fluorescein isothiocyanate-conjugated Fc-specific antimouse IgG (1:50 dilution). Controls are FL5.12 clones expressing Flag-Bcl-XL (clone 14) or Flag-Bcl-2 (clone 1) stained with isotype-matched control IgG1 MoAb and counter-stained as above.
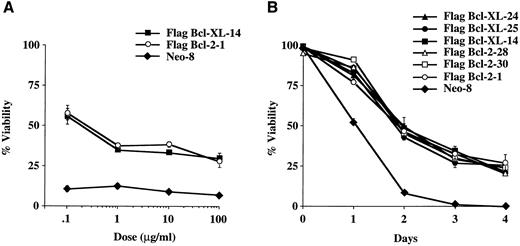
Vincristine and vinblastine, two inhibitors of tubulin polymerization, are widely used for the treatment of acute leukemias, Hodgkin's disease, non-Hodgkin's lymphomas, and neuroblastomas as well as in the treatment of many solid tumors.16 To evaluate the ability of Bcl-XL and Bcl-2 to protect cells from vincristine-induced apoptosis, we performed dose-response experiments using two representative cell clones that express similar levels of Flag-Bcl-XL and Flag-Bcl-2. At 72 hours of treatment, Bcl-XL and Bcl-2 conferred similar protection against vincristine induced apoptosis (Fig 2A). Time-course experiments using multiple Flag-Bcl-XL and Flag-Bcl-2 clones confirmed that Bcl-XL and Bcl-2 were able to confer similar protection against vincristine-induced cell death (Fig 2B). In addition, analysis of the same FL5.12 clones showed that Bcl-XL and Bcl-2 conferred a similar level of protection against vinblastine in dose-response as well as time-course experiments (Fig 2C and D). We conclude that Bcl-XL and Bcl-2 provide similar protection from apoptosis induced by the M-phase specific vinca alkaloids, vincristine, and vinblastine.
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after vinca alkaloid treatment. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of vincristine. Cells were lysed at 72 hours in a hypotonic buffer containing propidium iodide (PI) (0.01 mg/mL) and the percent of nuclei with fragmented DNA was determined by flow cytometric analysis. Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each concentration of drug (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. (B) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 10 μg/mL of vincristine. Cells were seeded at 1 × 105 cells/mL in triplicate wells and lysed in a hypotonic buffer containing propidium iodide (0.01 mg/mL). Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. (C) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of vinblastine. Cells were lysed at 72 hours in a hypotonic buffer containing propidium iodide (0.01 mg/mL) and the percent of nuclei with fragmented DNA was determined by flow cytometric analysis. Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each concentration of drug (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. (D) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 10 μg/mL of vinblastine. Cells were seeded at 1 × 105 cells/mL in triplicate wells and lysed in a hypotonic buffer containing propidium iodide (0.01 mg/mL). Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. The percentage of apoptotic nuclei was determined at different time points in triplicate cultures by nuclear PI staining followed by flow cytometric analysis. In this assay, the nuclei of apoptotic cells exhibit a sub-G0 profile characteristic of DNA fragmentation. Percentage of nonapoptotic nuclei is expressed as viability. Analysis was performed using Lysis II software (Becton Dickinson, San Jose, CA) on a FACScan flow cytometer. Results were based on the analysis of at least 5 × 104 events from each triplicate culture.
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after vinca alkaloid treatment. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of vincristine. Cells were lysed at 72 hours in a hypotonic buffer containing propidium iodide (PI) (0.01 mg/mL) and the percent of nuclei with fragmented DNA was determined by flow cytometric analysis. Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each concentration of drug (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. (B) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 10 μg/mL of vincristine. Cells were seeded at 1 × 105 cells/mL in triplicate wells and lysed in a hypotonic buffer containing propidium iodide (0.01 mg/mL). Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. (C) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of vinblastine. Cells were lysed at 72 hours in a hypotonic buffer containing propidium iodide (0.01 mg/mL) and the percent of nuclei with fragmented DNA was determined by flow cytometric analysis. Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each concentration of drug (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. (D) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 10 μg/mL of vinblastine. Cells were seeded at 1 × 105 cells/mL in triplicate wells and lysed in a hypotonic buffer containing propidium iodide (0.01 mg/mL). Viability (percent of cells with nonapoptotic nuclei) is shown as the mean ± SD of triplicate cultures. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point (P < .05, Student's t-test). The graph shown is representative of at least two separate experiments. The percentage of apoptotic nuclei was determined at different time points in triplicate cultures by nuclear PI staining followed by flow cytometric analysis. In this assay, the nuclei of apoptotic cells exhibit a sub-G0 profile characteristic of DNA fragmentation. Percentage of nonapoptotic nuclei is expressed as viability. Analysis was performed using Lysis II software (Becton Dickinson, San Jose, CA) on a FACScan flow cytometer. Results were based on the analysis of at least 5 × 104 events from each triplicate culture.
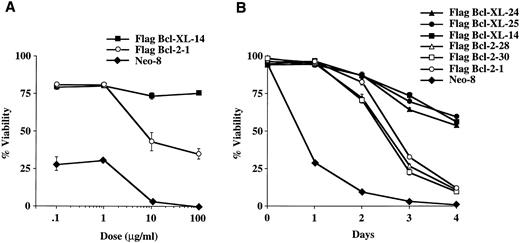
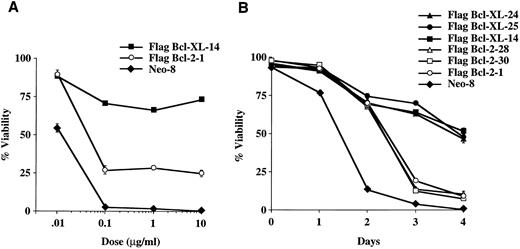
Bcl-XL and Bcl-2 provide differential protection from apoptosis induced by the M-phase specific epipodophyllotoxins, etoposide, and teniposide.To determine if protection from chemotherapy-induced cell death provided by Bcl-XL and Bcl-2 was related to the cellular target or the cell-cycle activity of the chemotherapy drug, we performed experiments using etoposide (VP-16), which is an inhibitor of topoisomerase II and like vincristine and vinblastine, M-phase specific.17 As seen in Fig 3A, a dose-response experiment showed that Bcl-XL was better able to protect FL5.12 cells from VP-16–induced cell death than Bcl-2. Time-course experiments using several clones confirmed that Bcl-XL could protect cells significantly better than Bcl-2 from etoposide-induced apoptosis (Fig 3B). In addition, similar experiments using another topoisomerase II inhibitor, teniposide, confirmed that Bcl-XL could afford better protection than Bcl-2 due to epipodophyllotoxin-induced cell death (Fig 3C and D). These results indicate that Bcl-XL and Bcl-2 can differentialy inhibit cell death induced by M-phase specific chemotherapy drugs dependent on cellular target of the drug rather than by its cell-cycle phase specificity.
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after epipodophyllotoxin treatment. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of etoposide. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 10 and 100 mg/mL of drug (P < .001, Student's t-test). (B) Cell viability was determined each time point following continuous incubation in media containing IL-3 and 10 μg/mL of etoposide. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at Days 3 and 4 (P < .001, Student's t-test). (C) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of teniposide. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 1, 10, and 100 μg/mL of drug (P < .001, Student's t-test). (D) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 10 μg/mL of teniposide. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at Days 2, 3, and 4 (P < .001, Student's t-test). Graphs shown in A, B, C, and D are the mean ± SD of triplicate cultures and representative of at least two separate experiments.
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after epipodophyllotoxin treatment. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of etoposide. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 10 and 100 mg/mL of drug (P < .001, Student's t-test). (B) Cell viability was determined each time point following continuous incubation in media containing IL-3 and 10 μg/mL of etoposide. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at Days 3 and 4 (P < .001, Student's t-test). (C) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of teniposide. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 1, 10, and 100 μg/mL of drug (P < .001, Student's t-test). (D) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 10 μg/mL of teniposide. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at Days 2, 3, and 4 (P < .001, Student's t-test). Graphs shown in A, B, C, and D are the mean ± SD of triplicate cultures and representative of at least two separate experiments.
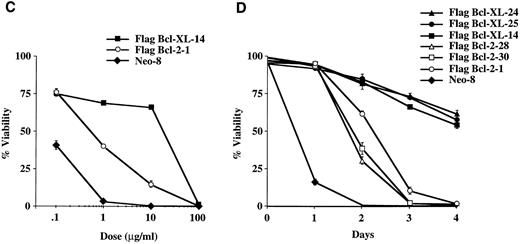
Bcl-XL provides better protection than Bcl-2 from apoptosis induced by the S-phase specific chemotherapy agents, methotrexate, fluorouracil, and hydroxyurea.Methotrexate, fluorouracil, and hydroxyurea are S-phase specific chemotherapy agents used to treat a variety of tumors. Methotrexate inhibits the enzyme, dihydrofolate reductase that is required for thymidine synthesis18 while fluorouracil potently inhibits thymidylate synthase also resulting in a decrease in thymidine synthesis as well as incorporates into RNA inhibiting its function.19 Hydroxyurea, on the other hand, inhibits the ribonucleotide reductase system that is required for conversion of ribonucleotides to deoxyribonucleotides needed in DNA synthesis and repair.20 As seen in Fig 4A and 4B, Bcl-XL protected significantly better the cells from methotrexate-induced cell death than Bcl-2 as determined in dose-response and time-course experiments. Additional dose-response experiments showed that Bcl-XL could also afford better protection than Bcl-2 against cell death induced by fluorouracil and hydroxyurea (Fig 4C and D).
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after treatment with S-phase specific chemotherapy drugs. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of methotrexate. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 0.1, 1, and 10 mg/mL of drug (P < .001, Student's t-test). (B) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 1.0 μg/mL of methotrexate. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at Days 3 and 4 (P < .001, Student's t-test). (C) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of fluorouracil. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each concentration of drug (P < .001, Student's t-test). (D) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of hydroxyurea. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 10, 100, and 1,000 μg/mL of drug (P < .001, Student's t-test). Graphs shown in A, B, C, and D are the mean ± SD of triplicate cultures and representative of at least two separate experiments.
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after treatment with S-phase specific chemotherapy drugs. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of methotrexate. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 0.1, 1, and 10 mg/mL of drug (P < .001, Student's t-test). (B) Cell viability was determined as above at each time point following continuous incubation in media containing IL-3 and 1.0 μg/mL of methotrexate. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at Days 3 and 4 (P < .001, Student's t-test). (C) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of fluorouracil. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each concentration of drug (P < .001, Student's t-test). (D) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 and increasing concentrations of hydroxyurea. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at 10, 100, and 1,000 μg/mL of drug (P < .001, Student's t-test). Graphs shown in A, B, C, and D are the mean ± SD of triplicate cultures and representative of at least two separate experiments.
Bcl-XL and Bcl-2 provide differential protection from apoptosis induced by the DNA damaging agents γ-irradiation and cisplatin.γ-Irradiation and cisplatin are used clinically to treat many tumors. These agents induce apoptosis in all phases of the cell-cycle by causing nonspecific DNA damage.21 Dose-response and time-course experiments revealed that Bcl-XL and Bcl-2 provided similar protection at each dose of γ-irradiation (Fig 5A and B). Bcl-XL, however, conferred increased survival compared to Bcl-2 when the cells were treated with cisplatin (data not shown). Interestingly, γ-irradiation induces DNA strand breaks through generation of oxygen free radicals while cisplatin acts as an alkylator.21 Therefore, Bcl-XL and Bcl-2, once again, provided divergent abilities to protect against chemotherapy but similar protection against γ-irradiation induced cell death confirming that the differential protection provided by Bcl-XL and Bcl-2 is more dependent on the specific molecular target rather than cell-cycle phase specificity of the antitumor treatment modality.
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after treatment with γ-irradiation. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 following increasing doses of γ-irradiation. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each dose of γ-irradiation (P < .05, Student's t-test). (B) Cell viability was determined as above at each time point following γ-irradiation. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point (P < .05, Student's t-test). Graphs shown in A and B are the mean ± SD of triplicate cultures and representative of at least two separate experiments.
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after treatment with γ-irradiation. (A) Cells were seeded at 1 × 105 cells/mL in triplicate wells in media containing IL-3 following increasing doses of γ-irradiation. Cells were lysed at 72 hours and analyzed by flow cytometry as described in Fig 2. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each dose of γ-irradiation (P < .05, Student's t-test). (B) Cell viability was determined as above at each time point following γ-irradiation. Cells were seeded at 1 × 105 cells/mL in triplicate wells and analyzed by flow cytometry as described in Fig 2. No statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point (P < .05, Student's t-test). Graphs shown in A and B are the mean ± SD of triplicate cultures and representative of at least two separate experiments.
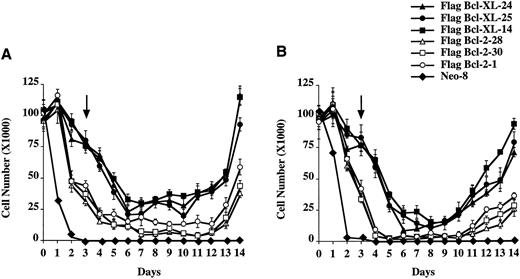
Bcl-XL and Bcl-2 provide differential long-term protection from etoposide and methotrexate induced cell death after removal of the chemotherapy drug.To determine if Bcl-XL and Bcl-2 could provide long-term protection from chemotherapy-induced cell death, Fl5.12 clones expressing Flag-Bcl-XL or Flag-Bcl-2 were washed out of drug after 3 days of continuous incubation in either etoposide or methotrexate and replated in media containing IL-3. As seen in Fig 6A and B, cells expressing Bcl-XL survived longer and, thus, were better able than cells expressing Bcl-2 to repopulate following removal of the chemotherapy drug. In additional experiments, FL5.12 cells expressing Bcl-2 failed to repopulate chemotherapy-free culture media following 4 days of continuous incubation in 10 mg/mL etoposide and 5 days in 1.0 μg/mL of methotrexate. Bcl-XL expressing cells, however, were still able to repopulate chemotherapy-free culture media following 6 days of incubation in etoposide and 8 days in methotrexate (data not shown). No cell-cycle changes were noted following removal of the drug compared to untreated cells expressing Flag-Bcl-XL or Flag-Bcl-2 (data not shown).
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after treatment with etoposide or methotrexate. (A) 1 × 105 cells were seeded in triplicate wells in media containing IL-3 and 10 mg/mL of etoposide. At each time point, cells were counted using trypan blue exclusion to assess viability. After 3 days of continuous incubation in etoposide, the cells were washed twice and replated in triplicate at the viable cell number for Day 3 in media containing IL-3. Arrow indicates time point at which the cells were washed and replated in drug-free media containing IL-3. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point after Day 1 (P < .01, Student's t-test). (B) 1 × 105 cells were seeded in triplicate wells in media containing IL-3 and 1.0 mg/mL of methotrexate. At each time point, cells were counted using trypan blue exclusion to assess viability. After 3 days of continuous incubation in methotrexate, the cells were washed twice and replated in triplicate at the viable cell number for Day 3 in media containing IL-3. Arrow indicates time point at which the cells were washed and replated in drug-free media containing IL-3. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point after Day 1 (P < .01, Student's t-test).
Viability of FL5.12 clones expressing Flag-Bcl-2 or Flag-Bcl-XL after treatment with etoposide or methotrexate. (A) 1 × 105 cells were seeded in triplicate wells in media containing IL-3 and 10 mg/mL of etoposide. At each time point, cells were counted using trypan blue exclusion to assess viability. After 3 days of continuous incubation in etoposide, the cells were washed twice and replated in triplicate at the viable cell number for Day 3 in media containing IL-3. Arrow indicates time point at which the cells were washed and replated in drug-free media containing IL-3. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point after Day 1 (P < .01, Student's t-test). (B) 1 × 105 cells were seeded in triplicate wells in media containing IL-3 and 1.0 mg/mL of methotrexate. At each time point, cells were counted using trypan blue exclusion to assess viability. After 3 days of continuous incubation in methotrexate, the cells were washed twice and replated in triplicate at the viable cell number for Day 3 in media containing IL-3. Arrow indicates time point at which the cells were washed and replated in drug-free media containing IL-3. A statistical significance was found between Flag-Bcl-2 and Flag-Bcl-XL clones at each time point after Day 1 (P < .01, Student's t-test).
DISCUSSION
Susceptibility of a cell to apoptotic signals appears to be regulated in part by the relative levels and competing dimerizations between Bcl-2 family members as well as competition for other cellular factors involved in the cell death pathway.14,22-24 Many neoplastic cells express either Bcl-2 or Bcl-XL and these apoptosis-suppressing proteins as well as other Bcl-2 family members have been implicated in relative resistance and susceptibility to antitumor treatment. In this report, we assessed if similar levels of Bcl-2 and Bcl-XL expression could provide equal protection against a variety of chemotherapy agents and γ-irradiation. We found that Bcl-XL provided enhanced protection versus Bcl-2 upon treatment with the M-phase specific chemotherapy agents, etoposide and teniposide, the S-phase specific antitumor agents, methotrexate, fluorouracil, and hydroxyurea and the cell-cycle nonspecific agent, cisplatin. However, Bcl-2 and Bcl-XL afforded similar protection against cell death on treatment with vincristine, vinblastine, and γ-irradiation. Several possibilities may explain these findings. First, Bcl-XL and Bcl-2 are thought to regulate a common cell death pathway. If all of the antitumor agents shown use a final common pathway, these results suggest that other cellular factors may be required for Bcl-XL and Bcl-2 to modulate this pathway since Bcl-XL and Bcl-2 did not provide equal protection upon treatment with all of these antitumor agents. These cellular factors could differentially regulate Bcl-XL and Bcl-2. Second, apoptotic stimuli including chemotherapy agents are known to activate a family of ICE/CED-3 cysteine proteases, which are thought to serve as executioners of the death pathway.25 Bcl-2 and Bcl-XL appear to act upstream and inhibit the activation of the death cysteine proteases.26 Therefore, it is plausible that certain chemotherapy agents activate different proteases which may be differentially blocked by Bcl-XL and Bcl-2. Another possibility is that chemotherapy agents differentially modify the expression/activity of Bcl-XL and Bcl-2 or other proteins that regulate the function of these survival proteins. Although we found no substantial change in levels of Bcl-2 family members including Bcl-2, Bcl-XL, Bax, and Bad in FL5.12 cells after treatment with these chemotherapy drugs (data not shown), we cannot exclude that other Bcl-2 family members or other regulatory proteins yet to be discovered could be altered by chemotherapy agents and could differentially affect the protective ability of Bcl-XL and Bcl-2. Additionally, it is possible that certain chemotherapy agents could biochemically modify Bcl-2 or Bcl-XL. In this regard, chemotherapy agents, such as taxol and fluorouracil, have been shown to induce posttranslational modification of Bcl-2 through phosphorylation,27 which has been suggested to modulate the anti-apoptotic activity of Bcl-2.28,29 Therefore, it is possible that certain chemotherapy drugs differentially regulate phosphorylation or dephosphorylation of Bcl-2 and Bcl-XL and selectively affect their ability to inhibit apoptosis. Finally, Bcl-2 has been shown to slow entry of cells from G0 into S.30 31 The Flag-Bcl-XL and Flag-Bcl-2 expressing FL5.12 clones used in these studies, however, did not show cell-cycle differences compared to the Neo control and untransfected FL5.12 cells while continuously cycling in culture media containing IL-3 and after removal of chemotherapy drugs (data not shown).
The expression of Bcl-2 and Bcl-XL has been implicated in the resistance of tumors to therapy. Unfortunately, treatment resistance and recurrence still remain formidable obstacles to the cure of most cancers. Since chemotherapy drugs and γ-irradiation destroy cancer cells by activating the cell death pathway, our studies suggest that the choice of antitumor agents should take into account the levels of Bcl-XL and Bcl-2 expressed by the tumor cells. Tumor cells that escape chemotherapy-induced cell death may continue to proliferate resulting in a relapse of the cancer. The fact that Bcl-XL was better able than Bcl-2 to protect FL5.12 cells from cell death induced by certain chemotherapy drugs suggests that Bcl-XL expressing tumor cells may be more difficult to completely destroy than cancer cells expressing Bcl-2. Clinically, chemotherapy drugs are inactivated or excreted from the body. Therefore, tumor cells that express Bcl-XL may be more likely to escape initial destruction by certain chemotherapy treatments than neoplasms expressing Bcl-2 increasing the likelihood of relapse. Our studies were performed in FL5.12 progenitor B cells so it is unclear if they similarly apply to cancer cells derived from different lineages. Future studies will determine if more powerful treatment stategies can be developed based on Bcl-XL or Bcl-2 expression and their divergent abilities to protect against certain chemotherapy-induced cell deaths in different cancers.
ACKNOWLEDGMENT
The authors thank Valerie Castle, Michael Clarke, Maribel Gonzalez-Garcia, J. Rebecca Liu, and Max Wicha for their critical review of this manuscript.
Supported by Grant No. CA-64556 from the National Institutes of Health (NIH); National Research Service Award, National Institute on Aging, NIH (P.L.S.); Fellowship from the Swiss National Science Foundation (D.A.M.G.); and Research Career Development Award CA-64421 from the NIH (G.N.).
Address reprint requests to Gabriel Nuñez, MD, Department of Pathology, University of Michigan Medical School, Ann Arbor, MI 48109.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal