Abstract
Gene activation by translocation between an oncogene and an immunoglobulin heavy-chain gene, which leads to increased expression of the oncoprotein, is a well-known mechanism in the genesis of B-cell lymphomas. In contrast, the role of gene amplification in activation of oncogenes in non-Hodgkin's lymphomas is poorly characterized. To study the BCL2 amplification we performed comparative genomic hybridization (CGH), Southern blot hybridization, Western analysis, immunohistochemistry, metaphase fluorescence in situ hybridization, and chromosome analysis on 26 cases of diffuse large B-cell lymphoma (large noncleaved cell lymphoma). The gain or high-level amplification of 18q was found in eight tumors (31%) by CGH, and Southern analysis revealed BCL2 amplification in these cases, but not in the cases with normal chromosome 18 or t(14; 18)(q32; q21). Western immunoblot analysis and immunohistochemistry revealed a high-level expression of BCL2 protein in the cases with BCL2 amplification and t(14; 18)(q32; q21). However, translocation (14; 18)(q32; q21) was not detected in any of the cases with BCL2 amplification. Therefore, our results suggest that amplification of the BCL2 gene is an important mechanism for BCL2 protein overexpression in diffuse large B-cell lymphoma.
B CL2 ONCOGENE was first found because of its participation in translocation (14; 18)(q32; q21) in lymphomas of the follicular center B-cell origin.1,2 After the translocation, the BCL2 oncogene is subject to the regulatory elements of the immunoglobulin heavy-chain gene. This leads to constitutive activation and increased expression of BCL2, which has been shown to inhibit apoptosis.3-7 The translocation is found in 70% to 85% of follicular lymphomas and in 20% to 30% of diffuse large-cell lymphomas.8-10 In normal lymphatic cells the BCL2 protein is expressed at pre–B-cell stages, but the expression usually decreases upon differentiation.11 However, cells with the (14; 18) translocation express unusually high levels of the BCL2 protein. In addition to lymphomas with t(14; 18), increased BCL2 protein expression has been found in lymphomas lacking the translocation.12-14
In several tumors, gene amplification has been shown to occur in response to a selection pressure for increased gene expression.15 However, the role of gene amplification in non-Hodgkin's lymphomas is poorly known.16-18 In our previous study, we found a gain or a high-level amplification of the 18q region including BCL2 by comparative genomic hybridization (CGH) in 21% of the cases of the large noncleaved cell lymphoma subtype of diffuse large B-cell lymphoma.19 These results suggested that, in addition to t(14; 18), BCL2 amplification might be another mechanism for BCL2 protein overexpression. In the present study, we investigated the mechanism and frequency of BCL2 amplification in diffuse large B-cell lymphoma by Southern blot hybridization and by fluorescence in situ hybridization. BCL2 protein expression was studied by Western immunoblot analysis and immunohistochemistry.
MATERIALS AND METHODS
Patients.A total of 26 cases of diffuse large B-cell lymphoma classified according to the Revised European-American Classification of Lymphoid Neoplasms were chosen for the study.20 To keep the material as homogenous as possible we included in the series only the cases that fulfilled the criteria of large noncleaved cell lymphoma according to Lukes-Collins classification or of centroblastic lymphoma according to Kiel classification.21 22 The samples were obtained from the frozen tissue bank of the Pathology Laboratory of the Department of Oncology, Helsinki University Central Hospital (Helsinki, Finland), where they had been collected from 1989 to 1996. The tissue for freezing was selected to contain only tumor tissue by a pathologist (K.F.) experienced in lymphomas. In paraffin sections the proportion of nonneoplastic cells never exceeded 10% in the tumor area. The proportion of nonneoplastic cells was below 5% in 58% of the cases and in 27% of the lymphomas it was less than 2%. The clinical data are presented in Table 1. Twenty of the cases were primary tumors and six were recurrent tumors. In all of the recurrent lymphomas, the primary tumor had also been a large noncleaved cell lymphoma (Table 1). Eleven of the patients were men and 15 women, and age at diagnosis ranged from 25 to 84 years (median, 59 years). All lymphomas were immunoreactive for B-cell antigens CD19 and/or CD20, and often for immunoglobulin as well.
Clinical Data of 26 Patients With Diffuse Large B-Cell Lymphoma
| Case No. . | Age/Sex . | Ann Arbor Stage . | Primary/Recurrent . | Follow-up (mo) . | Survival Status . | Site of the Specimen . |
|---|---|---|---|---|---|---|
| . | . | (I-IV) . | . | . | . | . |
| 1 | 64/M | IV | P | 20 | DOI | Lymph node |
| 2 | 70/F | II | P | 31 | DOL | Mediastinum |
| 3 | 84/M | III | P | 2 | DOL | Lymph node |
| 4 | 50/M | I | P | 60 | NED | Pharyngeal tonsil |
| 5 | 77/F | II | R | 12 | DOL | Lymph node |
| 6 | 59/F | I | R | 125 | NED | Lymph node |
| 7 | 53/M | II | P | 3 | NED | Lymph node |
| 8 | 50/M | II | P | 10 | NED | Lymph node |
| 9 | 64/F | III | P | 36 | AWD | Lymph node |
| 10 | 60/F | III | P | 31 | NED | Lymph node |
| 11 | 47/F | I | P | 36 | NED | Lymph node |
| 12 | 25/M | IV | P | 29 | DOL | Spleen |
| 13 | 69/F | IV | P | 11 | DOL | Small bowel |
| 14 | 35/F | I | P | 54 | NED | Lymph node |
| 15 | 38/M | I | P | 51 | DOI | Lymph node |
| 16 | 59/F | I | R | 29 | DOI | Breast |
| 17 | 71/F | III | P | 1 | DOL | Lymph node |
| 18 | 47/M | IV | P | 8 | DOL | Mesentery |
| 19 | 59/F | I | R | 36 | DOL | Skin |
| 20 | 37/M | II | R | 10 | DOL | Lymph node |
| 21 | 81/F | I | R | 20 | DOI | Stomach |
| 22 | 70/M | IV | P | 0 | DOL | Mesentery |
| 23 | 51/F | IV | P | 59 | NED | Lymph node |
| 24 | 52/F | I | P | 26 | NED | Spleen |
| 25 | 62/F | IV | P | 0 | DOL | Lymph node |
| 26 | 54/M | II | P | 72 | NED | Lymph node |
| Case No. . | Age/Sex . | Ann Arbor Stage . | Primary/Recurrent . | Follow-up (mo) . | Survival Status . | Site of the Specimen . |
|---|---|---|---|---|---|---|
| . | . | (I-IV) . | . | . | . | . |
| 1 | 64/M | IV | P | 20 | DOI | Lymph node |
| 2 | 70/F | II | P | 31 | DOL | Mediastinum |
| 3 | 84/M | III | P | 2 | DOL | Lymph node |
| 4 | 50/M | I | P | 60 | NED | Pharyngeal tonsil |
| 5 | 77/F | II | R | 12 | DOL | Lymph node |
| 6 | 59/F | I | R | 125 | NED | Lymph node |
| 7 | 53/M | II | P | 3 | NED | Lymph node |
| 8 | 50/M | II | P | 10 | NED | Lymph node |
| 9 | 64/F | III | P | 36 | AWD | Lymph node |
| 10 | 60/F | III | P | 31 | NED | Lymph node |
| 11 | 47/F | I | P | 36 | NED | Lymph node |
| 12 | 25/M | IV | P | 29 | DOL | Spleen |
| 13 | 69/F | IV | P | 11 | DOL | Small bowel |
| 14 | 35/F | I | P | 54 | NED | Lymph node |
| 15 | 38/M | I | P | 51 | DOI | Lymph node |
| 16 | 59/F | I | R | 29 | DOI | Breast |
| 17 | 71/F | III | P | 1 | DOL | Lymph node |
| 18 | 47/M | IV | P | 8 | DOL | Mesentery |
| 19 | 59/F | I | R | 36 | DOL | Skin |
| 20 | 37/M | II | R | 10 | DOL | Lymph node |
| 21 | 81/F | I | R | 20 | DOI | Stomach |
| 22 | 70/M | IV | P | 0 | DOL | Mesentery |
| 23 | 51/F | IV | P | 59 | NED | Lymph node |
| 24 | 52/F | I | P | 26 | NED | Spleen |
| 25 | 62/F | IV | P | 0 | DOL | Lymph node |
| 26 | 54/M | II | P | 72 | NED | Lymph node |
Abbreviations: M, male; F, female; P, primary; R, recurrent; AWD, alive with disease; DOI, dead of intercurrent disease; DOL, dead of lymphoma; NED, no evidence of disease.
CGH.CGH was performed according to Kallioniemi et al23 with the modifications used in our laboratory as described in detail elsewhere.19 24
Fluorescence in situ hybridization (FISH).FISH was performed on archival G-banded slides of three lymphomas (nos. 1, 4, and 6) with a high-level amplification at 18q using a chromosome 18–specific probe. Furthermore, probes specific for chromosomes 16 and 19 were used for case 6. The protocol used in our laboratory has been described in detail elsewhere.25
Southern blot analysis.Seven and a half micrograms of DNA was digested with HindIII restriction enzyme, and Southern blot hybridization was performed as described previously.19 The BCL2 probe for the major breakpoint region (MBR) was used to detect amplification and translocation of the gene. DNAs extracted from a healthy male's blood were used as controls. Furthermore, the p105-153A probe, which was hybridized to the 5q11.2-13.3 region, was used as a control because this region did not show any copy number changes in most of these patients. Densitometric analysis was performed to evaluate the approximate BCL2 amplification level in the tumors. Intensities of the two bands were measured using LKB 2202 Ultro Scan Laser Densitometer (Bromma, Sweden). The peak heights of the signals were measured and the signal intensity ratios of the BCL2 band and the control band (p153-105A ) were calculated (t1/t2). The signal intensity ratio of the blood of a healthy individual was set at 1 for comparison with the others. If the ratio exceeded 1.4, the BCL2 gene was considered to be amplified.
Karyotype analysis.Chromosome analysis was done according to the standard protocols using the Giemsa staining method. A more detailed description of our protocol has been published previously.19
Polymerase chain reaction (PCR).One microgram of DNA extracted from a fresh frozen tissue sample was amplified for 25 cycles in a PCR reaction (93°C for 1 minute, 55°C for 2 minutes, and 72°C for 2 minutes). Techne PHC1 temperature cycler, Taq-polymerase, and reagents from Perkin-Elmer (Norwalk, CT) were used. The oligonucleotide primers were 5′ GCCTTGAAACATTGATGG 3′ for the MBR, 5′ GATGGCTTTGCTGAGAGGTAT 3′ for the minor cluster region (MCR), and 5′ ACCTGAGGAGACGGTGAC 3′ consensus for all Ig heavy-chain joining regions. The DNA fragments were fractionated in a 1.5% agarose gel and detected under ultraviolet light.
Western immunoblot.Lymphomas, from which fresh frozen tissue was available, were studied. Protein concentration was measured against standard curve by using BCA protein assay reagent (Pierce, Rockford, IL). Equal amounts of protein from each cell lysate were subjected to electrophoresis in 12.5% SDS-PAGE gels and blotted to nitrocellulose filters. The immobilized BCL2 protein was detected with the mouse monoclonal antibody 100 (kindly provided by Dr David Y. Mason, John Radcliffe Hospital, Oxford, UK) according to the standard procedures using enhanced chemiluminescence detection (Amersham, UK). The translocation t(14; 18) positive case was used as a positive control (case no. 10), whereas the case with no copy number changes in chromosome 18 (case no. 25) or the case with a loss of 18 (case no. 24) were used as negative controls. Additionally, a sample of normal reactive lymphoid tissue was loaded to two gels.
Immunohistochemistry.Paraffin section immunohistochemistry was performed on all the 26 lymphomas using the monoclonal antibody for BCL2 and the streptABComplex/HRP duct kit (DAKO, Glostrup, Denmark) according to the standard protocols with microwave pretreatment in 10 mmol/L citrate buffer, pH 6.0 (5 minutes 780 W followed by 2 × 5 minutes 480 W). The expression of the BCL2 protein was divided in three categories. If about 80% to 100% of the cells were stained, the expression was designated as ++ (Table 2). If 50% to 80% of the cells were BCL2 positive, the positivity was designated as +, and if less than 40% of the cells were stained or no immunostaining was present it was designated negative (−). In the cases classified as negative in the present series, the proportion of the stained cells never exceeded 10%.
BCL2 Gene Amplification, Densitometric Values of all the Cases (t1/t2), t(14; 18), BCL2 Gene Rearrangement, DNA Copy Number Changes at 18q, and BCL2 Protein Expression in 26 Diffuse Large B-Cell Lymphomas
| Case No. . | t(14; 18) Detected by Karyotype Analysis . | BCL2 Rearrangement by PCR . | BCL2 Amplification by Southern Blot Hybridization . | BLC2/p105-153A (t1/t2) . | DNA Copy Number Changes at 18q by CGH . | BCL2 Protein Expression WB/IH . |
|---|---|---|---|---|---|---|
| 1 | − | − | + | 1.6 | ++ | +/++ |
| 2 | − | − | + | 1.4 | + | +/++ |
| 3 | − | − | + | 1.4 | + | +/++ |
| 4 | − | − | + | 1.9 | ++ | +/++ |
| 5 | − | − | + | 1.6 | + | +/++ |
| 6 | − | − | + | 1.8 | ++ | +/++ |
| 7 | − | ns | + | 1.7 | ++ | +/++ |
| 8 | − | ns | + | 2.1 | ++ | +/++ |
| 9 | + | + | − | 1.1 | n | +/+ |
| 10 | + | − | − | 0.7 | n | +/++ |
| 11 | ns | + | − | 0.9 | n | +/++ |
| 12 | − | − | − | 1.0 | n | −/− |
| 13 | ns | − | − | 1.1 | n | +/++ |
| 14 | − | − | − | 1.0 | n | −/+ |
| 15 | − | − | − | 0.9 | n | −/+ |
| 16 | − | − | − | 1.3 | n | ns/++ |
| 17 | − | − | − | 1.1 | n | −/+ |
| 18 | ns | − | − | 1.2 | n | ns/++ |
| 19 | ns | − | − | 1.1 | n | −/+ |
| 20 | − | − | − | 1.0 | n | −/+ |
| 21 | ns | − | − | 1.3 | n | ns/− |
| 22 | − | − | − | 1.3 | n | −/− |
| 23 | − | − | − | 0.9 | n | −/− |
| 24 | − | − | − | 0.6 | − | −/− |
| 25 | ns | − | − | 0.8 | n | −/− |
| 26 | ns | − | − | 0.8 | n | −/− |
| Case No. . | t(14; 18) Detected by Karyotype Analysis . | BCL2 Rearrangement by PCR . | BCL2 Amplification by Southern Blot Hybridization . | BLC2/p105-153A (t1/t2) . | DNA Copy Number Changes at 18q by CGH . | BCL2 Protein Expression WB/IH . |
|---|---|---|---|---|---|---|
| 1 | − | − | + | 1.6 | ++ | +/++ |
| 2 | − | − | + | 1.4 | + | +/++ |
| 3 | − | − | + | 1.4 | + | +/++ |
| 4 | − | − | + | 1.9 | ++ | +/++ |
| 5 | − | − | + | 1.6 | + | +/++ |
| 6 | − | − | + | 1.8 | ++ | +/++ |
| 7 | − | ns | + | 1.7 | ++ | +/++ |
| 8 | − | ns | + | 2.1 | ++ | +/++ |
| 9 | + | + | − | 1.1 | n | +/+ |
| 10 | + | − | − | 0.7 | n | +/++ |
| 11 | ns | + | − | 0.9 | n | +/++ |
| 12 | − | − | − | 1.0 | n | −/− |
| 13 | ns | − | − | 1.1 | n | +/++ |
| 14 | − | − | − | 1.0 | n | −/+ |
| 15 | − | − | − | 0.9 | n | −/+ |
| 16 | − | − | − | 1.3 | n | ns/++ |
| 17 | − | − | − | 1.1 | n | −/+ |
| 18 | ns | − | − | 1.2 | n | ns/++ |
| 19 | ns | − | − | 1.1 | n | −/+ |
| 20 | − | − | − | 1.0 | n | −/+ |
| 21 | ns | − | − | 1.3 | n | ns/− |
| 22 | − | − | − | 1.3 | n | −/− |
| 23 | − | − | − | 0.9 | n | −/− |
| 24 | − | − | − | 0.6 | − | −/− |
| 25 | ns | − | − | 0.8 | n | −/− |
| 26 | ns | − | − | 0.8 | n | −/− |
Abbreviations: − denotes negative and + positive result for t(14;18), BCL2 rearrangement, amplification, or protein expression. In immunohistochemistry ++ denotes staining in 80-100% of the cells, + in 50% to 80% of the cells, and − denotes BCL2 expression in less than 10% of the cells. Cases not studied have been marked with ns. The cases with a gain or a high-level amplification of chromosome 18q in CGH analysis have been marked with + and ++, respectively. Normal chromosome 18 in a copy number karyotype assessed by CGH has been marked with n, and the loss of the chromosome with −. WB, Western Blot; IH, immunohistochemistry.
RESULTS
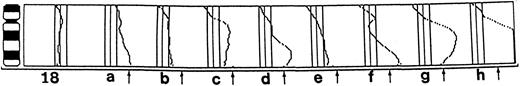
Overrepresentation of chromosome 18 and amplification of the BCL2 gene.A gain or a high-level amplification of 18q was detected by CGH in 8 patients out of 26 (case nos. 1 through 8; Fig 1). In five of the patients the 18q21-ter was found to be highly amplified (case nos. 1, 4, 6, 7, and 8). Three lymphomas (case nos. 1, 4, and 6) were studied by FISH, which revealed four or more labels when using the probe for chromosome 18. In case no. 1, both normal chromosomes were painted, but also two to three marker chromosomes were stained. In case no. 4 the probe was hybridized to one normal chromosome 18 as well as to three marker chromosomes. In case no. 6, in addition to the normal chromosomes, the label was seen to be translocated to 1q32 and to two marker chromosomes. The markers contained a part of 1q with segments of 18 translocated to chromosomes 16 and 19 (Fig 2). Case no. 7 presented 18q in six copies in the form of three isochromosomes i(18q), which were detected by chromosome analysis.
Overrepresentation of 18q in diffuse large B-cell lymphomas. CGH profiles of cases 1 to 8 are shown from (a) to (h), in which a gain or a high-level amplification in 18q was detected. The cutoff values for gains and losses are 1.17 and 0.85, respectively. The region was considered to be highly amplified if the profile exceeded the value of 1.5 (marked with an arrow). In case nos. 1, 4, 6, 7, and 8 (a, d, f, g, and h) the 18q was found to be highly amplified. Normal profile of a healthy individual is shown on the left.
Overrepresentation of 18q in diffuse large B-cell lymphomas. CGH profiles of cases 1 to 8 are shown from (a) to (h), in which a gain or a high-level amplification in 18q was detected. The cutoff values for gains and losses are 1.17 and 0.85, respectively. The region was considered to be highly amplified if the profile exceeded the value of 1.5 (marked with an arrow). In case nos. 1, 4, 6, 7, and 8 (a, d, f, g, and h) the 18q was found to be highly amplified. Normal profile of a healthy individual is shown on the left.
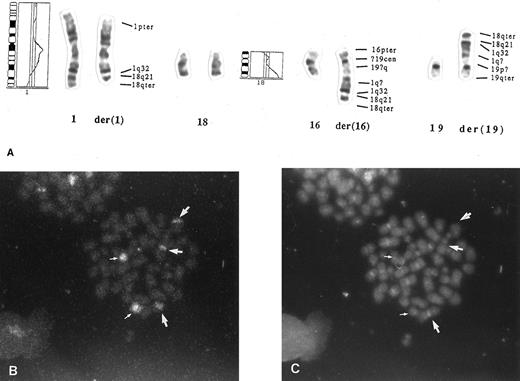
Mechanism for BCL2 amplification in a diffuse large B-cell lymphoma. (A) Chromosome 18q–derived sequences were translocated to chromosome 1q32, which was further translocated to chromosomes 16 and 19 (case no. 6; Table 1). CGH profiles of chromosomes 1 and 18 are also shown. The karyotype is 48,X,–X,der(1),+3,del(6)(q12q22),+7,−16,+der(16),−19,+der(19),+r(?)×2, but only the chromosomes taking part in the translocations are described in the figure. The interpretation of the marker chromosomes was confirmed by painting, using probes specific for chromosomes 16, 18, and 19. (B) Fluorescence in situ hybridization using a chromosome 18-specific probe shows three labels (large arrows) in addition to normal chromosomes (small arrows). This shows that 18-derived material was translocated to three chromosomes. (C) DAPI (4,6-diamidino-2-phenylindole) and propidium iodide staining of the metaphase in (B).
Mechanism for BCL2 amplification in a diffuse large B-cell lymphoma. (A) Chromosome 18q–derived sequences were translocated to chromosome 1q32, which was further translocated to chromosomes 16 and 19 (case no. 6; Table 1). CGH profiles of chromosomes 1 and 18 are also shown. The karyotype is 48,X,–X,der(1),+3,del(6)(q12q22),+7,−16,+der(16),−19,+der(19),+r(?)×2, but only the chromosomes taking part in the translocations are described in the figure. The interpretation of the marker chromosomes was confirmed by painting, using probes specific for chromosomes 16, 18, and 19. (B) Fluorescence in situ hybridization using a chromosome 18-specific probe shows three labels (large arrows) in addition to normal chromosomes (small arrows). This shows that 18-derived material was translocated to three chromosomes. (C) DAPI (4,6-diamidino-2-phenylindole) and propidium iodide staining of the metaphase in (B).
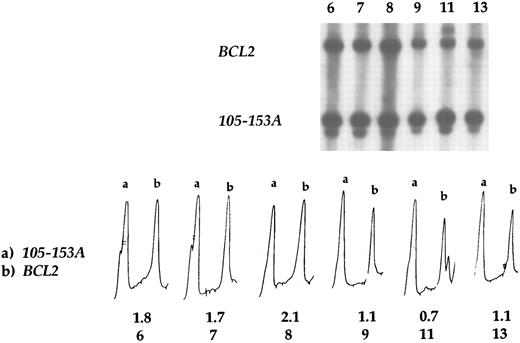
Southern blot analysis revealed the amplification of the BCL2 gene in all eight patients with a gain or a high-level amplification at 18q by CGH (case nos. 1 through 8; Table 2). Densitometric analysis showed that the relative intensity of the BCL2 and control (p105-153 ) bands in the tumors with 18q gain or high-level amplification varied from 1.4 to 2.1, whereas in the cases that displayed no gains or high-level amplifications of 18q by CGH, the signal intensity ratio varied from 0.6 to 1.3 (mean value 1.0; Table 2, Fig 3). Hence, there was a 100% concordance between the results obtained by CGH and the Southern blot analysis.
BCL2 amplification by Southern blot hybridization. In case nos. 6, 7, and 8 BCL2 amplification was detected, whereas tumors 9, 11, and 13 displayed no amplification. Case no. 9 is the t(14; 18) positive case as well as the case no. 11, in which the translocation occurred in the MBR region. Case no. 13 had a normal chromosome 18. Densitometric profiles show the amplification of BCL2 (b) in the first three cases, in which the signal intensity ratio varied from 1.7 to 2.1, whereas in the other cases it varied from 0.7 to 1.1. Probe 105-153A was used as a control, and the peak (a) corresponds to its intensity.
BCL2 amplification by Southern blot hybridization. In case nos. 6, 7, and 8 BCL2 amplification was detected, whereas tumors 9, 11, and 13 displayed no amplification. Case no. 9 is the t(14; 18) positive case as well as the case no. 11, in which the translocation occurred in the MBR region. Case no. 13 had a normal chromosome 18. Densitometric profiles show the amplification of BCL2 (b) in the first three cases, in which the signal intensity ratio varied from 1.7 to 2.1, whereas in the other cases it varied from 0.7 to 1.1. Probe 105-153A was used as a control, and the peak (a) corresponds to its intensity.
Translocation (14; 18)(q32; q21) and BCL2 rearrangement.Translocation t(14; 18) was detected in the karyotype analyses of two cases, and the BCL2 rearrangement by PCR appeared in two lymphomas (Table 2). In two of the cases the breakpoint was in the MBR region (case nos. 9 and 11), and in case no. 10 the breakpoint was not identified. In the cases with BCL2 amplification (nos. 1 through 8), neither the t(14; 18) nor a BCL2 rearrangement was detected.
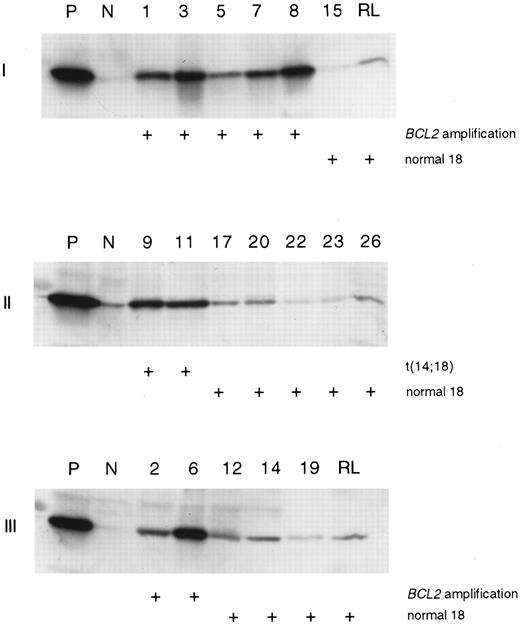
Expression of the BCL2 protein.Western immunoblot analysis was performed on 23 cases (Table 2). The protein was strongly expressed in all the cases where an amplification of the BCL2 gene or translocation (14; 18) was found, whereas the rest of the cases showed a very faint protein expression (Table 2, Fig 4). Similarly, in normal reactive lymphatic tissue the BCL2 protein was only expressed at a low level. One case (no. 13) expressed high levels of the protein in Western analysis, even though neither the amplification of the BCL2 gene nor the t(14; 18) was detected.
BCL2 protein expression in diffuse large B-cell lymphoma. A Western immunoblot analysis was performed using a BCL2-specific monoclonal antibody. Lymphoma with t(14; 18) translocation was used as a positive control (P) and cases with loss of 18 (gels I and II) and normal 18 (gel III) were used as negative controls (N). The samples loaded to each gel are marked on the top of the figures. Protein from reactive lymphatic tissue (RL) was added to two gels (I and III).
BCL2 protein expression in diffuse large B-cell lymphoma. A Western immunoblot analysis was performed using a BCL2-specific monoclonal antibody. Lymphoma with t(14; 18) translocation was used as a positive control (P) and cases with loss of 18 (gels I and II) and normal 18 (gel III) were used as negative controls (N). The samples loaded to each gel are marked on the top of the figures. Protein from reactive lymphatic tissue (RL) was added to two gels (I and III).
Immunohistochemistry showed strong positive staining (++) in all the cases with BCL2 amplification and in two of three lymphomas with (14; 18) translocation. Furthermore, high expression of the BCL2 protein (++) was seen in three cases (nos. 13, 16, and 18), which did not show either the translocation or amplification. Most of the cases in which both the Western immunoblot and immunohistochemistry were performed showed concordant results. However, in five cases (case nos. 14, 15, 17, 19, and 20) the Western blot technique showed negative staining, whereas immunohistochemistry showed moderate positivity (+) for the BCL2 protein (Table 2).
DISCUSSION
Increased expression of the BCL2 protein and inhibition of apoptosis is regarded as a major mechanism in the genesis of follicular lymphomas. Translocation t(14; 18), which leads to the increased expression of the BCL2 protein, is found in the majority of follicular lymphomas and in 20% to 30% of diffuse large-cell lymphomas.8-10 We found evidence for t(14; 18) either by karyotyping, PCR, or both in 12% of our cases of diffuse large B-cell lymphoma. Additionally, a gain or amplification of 18q was found in 31% of lymphomas by CGH, and the amplification of BCL2 was confirmed in all of these cases by Southern blot analysis. All of the eight cases with BCL2 amplification were shown by Western blotting technique and immunohistochemistry to overexpress BCL2 protein. In four of the cases with a high-level amplification, FISH or chromosome analysis confirmed the presence of several copies of chromosome 18–derived sequences. These data indicate that the amplification of 18q and BCL2 are common in diffuse large B-cell lymphoma and suggest that the amplification of BCL2 may be as common a mechanism for BCL2 protein overexpression as is t(14; 18) in diffuse large B-cell lymphoma.
The oncogenic potential of t(14; 18) coupled with BCL2 overexpression, which appears frequently in follicular lymphoma, has been shown in transgenic mice bearing a BCL2-immunoglobulin fusion gene.26,27 In our series t(14; 18) or BCL2 rearrangement was found in none of the lymphomas with BCL2 amplification. Overexpression of BCL2 protein, caused either by amplification of the BCL2 gene or by t(14; 18), may be important not only in the genesis of follicular lymphoma, but also in diffuse large B-cell lymphomas. The increased expression of BCL2 protein that Western blot technique revealed in one case, which lacked both the translocation and the BCL2 amplification, reflects the possibility that other additional mechanisms may cause overexpression of BCL2 protein in diffuse large B-cell lymphomas. Moreover, staining shown by immunohistochemistry was interpreted to be positive in 8 out of 14 cases with a normal chromosome 18. Although the results of strong positivity obtained by Western blot analysis and immunohistochemistry agreed well, a few cases with moderate staining in immunohistochemistry showed discrepancy, which may be caused by tissue fixation or other technical factors in the immunohistochemical analyses.28 The mechanism behind oncogene activation in cases without any rearrangement or amplification remains to be solved. Point mutations have been detected in the open reading frame of the BCL2 gene in follicular and diffuse large-cell lymphomas.29 Mutations in the open reading frame, those of the regulatory elements of the gene, or posttranslational changes may explain BCL2 overexpression in cases that lack t(14; 18) translocation or BCL2 gene amplification.
The role of gene amplification in non-Hodgkin's lymphomas has not been widely studied, probably because of the lack of proper genome-wide screening methods. In previous studies, a twofold BCL2 amplification was detected in two cases of non-Hodgkin's lymphoma by Southern blot analysis,16 and one non-Hodgkin's lymphoma cell line was found to have homogeneously staining regions containing many-fold copies of the BCL2 oncogene.30 Increased BCL2 protein expression has been found immunohistochemically in testicular non-Hodgkin's lymphoma, diffuse large cell non-Hodgkin's lymphoma, and in Hodgkin's disease without t(14; 18).13,14,31 Similarly, another study showed a high BCL2 protein level by Western immunoblot analysis in a human myeloma cell line that exhibited a fourfold amplification of the BCL2 gene but not the rearrangement of BCL2.32 Therefore, the previous data, albeit fragmentary, are in line with those obtained in the present study and provide further support to the hypothesis that amplification of the BCL2 gene may be of importance in the tumorigenesis of lymphomas. In our study, the highest value of BCL2 amplification in densitometric analysis was 2.1-fold as high as in the controls supporting the results obtained by CGH, which showed the high-level amplification of 18q in this tumor. The cases with 18q high-level amplification in CGH seemed to show a higher level of BCL2 amplification than the cases with a lower level copy number increase. CGH and Southern blot analysis are not, however, directly comparable, as CGH results depend not only on the amplification of the gene, but also on the size of the amplicon.33
In three cases where a high-level amplification of a region in 18q was detected by CGH, the presence of the amplification was confirmed by FISH. A novel type of translocation, t(1; 18)(q32; q?21), was detected in one of these cases. Furthermore, a part of the q-arm of this translocation was transferred to chromosomes 16 and 19. Therefore, the translocations detected between three and four chromosomes suggest that the terminal bands of chromosome 18 have been amplified in the translocated part of 1q. Overrepresentation of chromosome 18 has been detected to appear in 34% of diffuse large cell lymphomas and in 31% of follicular lymphomas, which also suggests that chromosome 18 contains genes important in the tumorigenesis of B-cell lymphomas.34 35
In conclusion, BCL2 oncogene amplification was found by Southern blot analysis to be present in 31% of diffuse large B-cell lymphomas, and in all lymphomas where a gain or a high-level amplification at 18q was detected by CGH. Moreover, a new mechanism for BCL2 amplification was detected by FISH. BCL2 amplification was found more frequently than t(14; 18). The two mechanisms for BCL2 overexpression may be mutually exclusive because none of these lymphomas showed both the amplification and the translocation. In addition to t(14; 18), BCL2 amplification appears to be an important mechanism for BCL2 overexpression, but other mechanisms are likely to exist as well. The clinical importance of BCL2 amplification in diffuse large B-cell lymphoma remains to be investigated in future studies.
Address reprint requests to Sakari Knuutila, PhD, Department of Medical Genetics, Haartman Institute, PO Box 21 (Haartmaninkatu 3), FIN-00014 University of Helsinki, Finland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal