Abstract
CD44 is described to be an activation molecule in a number of different cell types. We investigated the role of CD44 on human endothelial cells (EC) and in tumor angiogenesis. Using flow cytometry we showed that EC from the vasculature of human solid tumors display an enhanced expression of CD44 as compared to EC from normal tissue. This finding was confirmed by immunohistochemical studies on frozen tissue sections. Because tumors are dependent on angiogenesis, the role of angiogenic stimuli in the enhanced CD44 expression was investigated. We found that basic fibroblast growth factor (bFGF ) and vascular endothelial growth factor were able to efficiently upregulate CD44 expression on cultured human EC. The upregulation reached maximal levels after treatment for 3 days with 10 ng/mL bFGF. The physiological impact of this upregulation was shown by the enhanced binding of EC to hyaluronate after pretreatment with bFGF. In a next set of studies that were designed to unravel the regulation of CD44 expression on EC we concluded that CD44 is an activation antigen on human EC since (1) human umbilical vein derived endothelial cells, which in vivo do not express CD44, begin to express CD44 when plated and cultured, (2) CD44 expression is enhanced after subculture of confluent cultures, (3) CD44 is predominantly expressed on the BrdU incorporating subset of cultured EC. The specific expression of CD44 on activated and tumor EC prompted us to study the usefulness of CD44 as an endothelial target for therapy with immunotoxins. In vitro experiments showed that EC are efficiently killed after targeting immunotoxin to CD44.
SPROUTING OF new microvasculature from pre-existing blood vessels, a process called angiogenesis, is occurring in tumors and wound healing.1,2 Outgrowth of solid tumors is dependent on the process of angiogenesis. Basic fibroblast growth factor (bFGF ) and vascular endothelial cell growth factor (VEGF ) are the most extensively investigated angiogenic factors that play a role in tumor angiogenesis. Recent research has been focused on the phenotype and growth characteristics of tumor associated endothelial cells (EC). Although altered expression of certain endothelium associated antigens in tumors has been described,3-6 insight in regulation of endothelial adhesion molecule expression and endothelial functions induced by angiogenic factors is largely lacking.
CD44 is a broadly distributed family of glycoproteins now generally believed to be a cell adhesion molecule with proposed functions in extracellular matrix binding, leukocyte homing and activation, and metastasis formation.7-11 CD44 has been shown to display a great molecular heterogeneity owing both to alternative exon use and a variegated posttranslational modification. Earlier studies on the cellular distribution of CD44 are discrepant concerning the expression on EC. In a number of reports human endothelium has been cited to lack this marker12,13 while other studies showed expression of CD44 on endothelium.14,15 In a recent report it was observed that EC abundantly expressed the standard CD44 isoform (CD44s) and that even after additional activation with tumor necrosis factor-α (TNF-α) EC do not express larger alternatively spliced CD44 molecules. Binding and presentation of heparin binding growth factors like bFGF by exon v3 containing isoforms, which might be of advantage for leukocyte infiltration in inflamed tissue,15 was therefore excluded. CD44 has been described as the principle cell surface receptor for hyaluronate (HA).7 Binding of CD44 to HA is dependent on activational processes16,17 and the glycosylation state.18,19 It has been shown that CD44 and its ligand HA mediate rolling of lymphocytes under physiologic flow conditions, which potentiates a novel lymphocyte-EC adhesion pathway.20 Recently the effects of CD44 ligation on cell activation have been clarified. CD44 signaling involves tyrosine kinases and is therefore able to contribute directly to cell activation.9
The aim of the present study was to investigate the regulated expression and functional characteristics of CD44 on human endothelium. We quantitatively studied endothelial CD44 expression in human solid tumors. Regulation of CD44 expression by angiogenic factors was investigated in vitro both at the mRNA and protein level using umbilical vein and dermis derived EC. Evidence is provided that CD44 is an activation molecule on human EC and that splice variants of CD44 are expressed on EC. The enhanced expression of CD44 on tumor associated vessels suggests that the molecule is involved in tumor angiogenesis.
MATERIALS AND METHODS
Monoclonal antibodies and cytokines.Anti-CD44 antibodies NKI-P1,21 515,22 and HERMES-323 were kind gifts of Dr C. Figdor (Nijmegen, The Netherlands), Dr G. Kansas (Iowa City, IA), and Dr S. Jalkanen (Helsinki, Finland), respectively. VFF8 anti-CD44v5, VFF7 anti-CD44v6, VFF9 anti-CD44v7, and VFF14 anti-CD44v10 antibodies were obtained from Bender (Vienna, Austria). EN4 EC-marker was obtained from Sanbio (Uden, The Netherlands). The cytokines bFGF and VEGF were purchased from Intergen (Purchase, NY).
Cells and cultures.Human umbilical vein derived endothelial cells (HUVEC) were harvested from normal human umbilical cords by perfusion with 0.125% trypsin/EDTA as described previously.24 Cells were cultured in fibronectin (FN) coated tissue culture flasks in culture medium (RPMI-1640 with 20% human serum [HS], supplemented with 2 mmol/L glutamine and 100 U/mL penicillin and 0.1 mg/mL streptomycin). Confluent HUVEC cultures were subcultured 1:3. After reaching confluence in the second passage EC were seeded in 6-well tissue culture plates (2 × 105 cells/well) in culture medium for cytokine treatment and subsequent immunofluorescence experiments.
Immunofluorescence.Immunofluorescence using indirect phycoerythrin (PE)-conjugated reagents required three separate incubations. 1 × 105 EC (procured by incubation in 0.125% trypsin in phosphate-buffered saline [PBS]) were washed, fixed for 1 hour in 1% paraformaldehyde, resuspended in 20 μL appropriately diluted monoclonal antibody (MoAb) and incubated for 1 hour on ice. Subsequently, cells were washed two times in PBS/bovine serum albumin (BSA) (0.1%) and incubated for another 30 minutes with biotinylated rabbit-antimouse Ig (Dako, Glostrup, Denmark). After another 2 washings, cells were incubated with streptavidin-phycoerythrin conjugate (Dako). Stained cells were analyzed on a FACScan flowcytometer. Of each sample, forward scatter (FSC), side scatter (SSC), green fluorescence (525/20 nm) and orange fluorescence (640/20 nm) signals of 5,000 cells were recorded. Data analysis was performed using PClysys software (Becton Dickinson, Mountain View, CA). Statistical significance of observed differences was determined using the Student's t-test.
Flow cytometric analysis of tumor derived endothelial cells.Tumor and normal tissues, obtained from surgical procedures as first treatment of renal cell carcinoma, were processed immediately after resection under sterile conditions. An ethical review committee approved informed consent procedure was used to obtain the relevant materials. EC were isolated as described previously.25,26 In short, normal and tumor tissue was minced and treated for 1 hour with 1 mg/mL collagenase and 2.5 U/mL dispase II (both from Boehringer Mannheim, Mannheim, Germany) in PBS at 37°C. Tissue was subsequently sieved and single cells were washed and resuspended in culture medium. Cells were cultured briefly (1 hour) in an FN coated culture flask. Then, nonadhering cells and debris were decanted and adhering cells were stained with antiadhesion molecule antibodies and fluorescein isothiocyanate (FITC)-conjugated goat antimouse Ig (Dako). Identification of EC was performed by subsequent indirect staining with EC specific antibody EN4-biotin and streptavidin-PE. EN4 expressing cells were also positive for platelet endothelial cell adhesion molecule (PECAM)/CD31, CD34, endoglin/CD105, and intercellular adhesion molecule-2 (ICAM-2).25 Additional identification of EC was performed on the basis of EC-specific FSC/SSC characteristics. Previous studies indicated that this procedure identified the cells that stain after DiI-acetylated-LDL incubation.25 27
Immunohistochemistry.Immunoperoxidase staining was performed on normal and pathological tissues, by fixation of cryostat sections for 10 minutes in aceton and subsequent preincubation with normal goat serum (10% in PBS). Sections were incubated for 1 hour with the primary antibody. Before incubation with the secondary antibody (rabbit antimouse coupled to peroxidase, 1 hour, Dako) endogenous peroxidases were blocked with 0.3% H2O2 in methanol. Then, sections are incubated in peroxidase coupled swine antirabbit antibody (Dako). All antibodies were titrated to give optimal staining results. For detection, sections were incubated in 3,3-amino-9-ethyl carbazole (Sigma Immunochemicals, St Louis, MO) for 10 to 20 minutes and counterstained with hematoxilin.
Proliferation measurement and BrdU double staining.EC proliferation was measured using a [3H]thymidine incorporation assay. EC were seeded in flatbottomed 96-well tissue culture plates (5,000 cells/well) and grown for 3 days, in culture medium. In some cultures the proliferation of EC was enhanced by incubation with 10 ng/mL bFGF. During the last 6 hours of the assay, the culture was pulsed with 0.5 μCi [methyl-3H]thymidine/well. Results are expressed as the arithmetic mean counts per minute (cpm) of quadruplicate cultures. Dividing cells were visualized by detection of 5-bromo-2′-deoxyuridine (BrdU; Boehringer Mannheim) incorporation. Subconfluent EC cultures were incubated for 16 hours with 20 μmol/L BrdU.28 Cells were procured and fixed with 70% ethanol 4°C for 30 minutes, washed with PBS/BSA, and incubated in 2 mol/L HCl for 10 minutes. Next, four volumes of 0.1 mol/L Na2B4O7 were added and cells were centrifugated and washed again with PBS/BSA. Subsequently cells were resuspended in 20 μL antiadhesion molecule antibodies and incubated for 1 hour on ice. Cells were washed 2 times with PBS/BSA and incubated in 20 μL diluted rabbit antimouse Ig (Dako) for 30 minutes on ice. After another 2 washings cells were incubated with streptavidine-phycoerythrin conjugate (Dako) and FITC-conjugated anti BrdU MoAb (Becton Dickinson) (in the presence of 10% normal mouse serum to prevent cross reactivity with rabbit antimouse Ig). Cells were then washed and flow cytometrically analyzed as described above.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis.Total RNA was isolated from untreated and bFGF-treated (10 ng/mL) HUVEC. CD44 splice variants were detected using RT-PCR as described earlier.29 Briefly, 1 μg of total RNA was transcribed using a CD44-specific primer and reverse transcriptase Superscript Plus (GIBCO-BRL, Paisley, UK). CD44 cDNA (the equivalent of 100 ng reverse transcribed RNA) was amplified by PCR (35 cycles) using CD44 exon-specific oligonucleotides and Taq DNA polymerase (Pharmacia, Uppsala, Sweden). PCR products were analyzed on a 1.25% agarose gel with ethidium bromide.
Cell adhesion to hyaluronate.Binding of soluble HA was performed by treatment of paraformaldehyde fixed cells with HA conjugated to FITC (a gift from Dr J. Lesley, San Diego, CA). Control and bFGF pretreated EC were incubated for 2 hours at room temperature with appropriately diluted HA-FITC. Cells were subsequently washed and analyzed flow cytometrically. Specificity of adherence was determined by blocking with excess soluble unlabeled HA (2.5 mg/mL) and with 1 μg/mL 515 anti-CD44 antibody (1 hour, RT), before fixation.
To study the adhesion of EC to immobilized hyaluronate (HA), 96-well plates were coated with 5 mg/mL HA in H2O (ICN Biochemicals, Costa Mesa, CA), overnight at RT. Aspecific binding was blocked by incubating the wells with 10% BSA in PBS for 1 hour. After washing the cells, control and pretreated EC were added to the wells (2 × 104 cells/well, in culture medium) and incubated for 15 minutes at 37°C. Nonadhering cells were removed by a standardized wash procedure. Adherence to HA was shown by blocking with excess soluble HA and adherence by CD44 was shown by prior incubation of EC with the blocking 515 anti-CD44 antibody. The number of adhering cells was scored using an inverted microscope.
Indirect immunotoxin assay.HUVEC and the human CD44 expressing keratinocyte cell line HaCaT were used as target cells in the indirect immunotoxin assay. Five thousand cells were seeded in a 96-well plate and cultured overnight. Subsequently cultures were incubated with NKI-P1 anti-CD44 and OKT3 anti-CD3 (both 10 mg/mL) at RT. After 1 hour the wells were washed and a mixture of equal concentrations of specific antibody and saporin-conjugated goat antimouse Ig antibody (both 0.5 mg/mL) was added to the wells. The cells were then cultured for 3 days and subsequently protein synthesis was determined by measurement of incorporation of 3H-leucine (0.5 μCi/well, 6 hours). Results are expressed as the arithmetic mean cpm of quintuplicate cultures. In control cultures 1 mmol/L cycloheximide was added to obtain a positive inhibition of protein synthesis. Percentage killing by an antibody Ab is calculated as follows: % killing = 100 − (100 × [cpmAb − cpmcyclo / cpmcontrol − cpmcyclo ]) where cpmAb = cpm measured in the presence of the antibody of interest, cpmcontrol = cpm measured in the presence of control antibody, and cpmcyclo = cpm measured after addition of cycloheximide.
RESULTS
Endothelial CD44 expression is enhanced in human tumors.In a survey to investigate the phenotype of tumor endothelial cells, using flow cytometric analysis of tumor endothelial cells isolated from renal cell carcinoma (RCC), we found an enhanced expression of CD44 on tumor associated endothelium as compared to EC obtained from normal renal tissue from the same patient. This upregulated CD44 expression on tumor EC contrasted the suppressed expression of ICAM-1 and ICAM-2 and CD34 on these cells described earlier,25,26,30 and was found using both NKI-P1 and HERMES-3 anti-CD44 antibodies (Fig 1). A clear augmented expression of CD44 was found for 12 out of 14 different RCC (Table 1). Mean expression of CD44 on tumor EC was 384% of the expression observed for EC from normal tissue (SEM 71%, n = 14, P < .001). In these experiments no differences were found in EC expression of PECAM (CD31) and HLA class-I antigens.25 Similar results were obtained for CD44 expression on EC from ovarian carcinoma (in two experiments 440% and 707% of dermis derived normal EC). The results presented were obtained with CD44 antibodies recognizing the standard domain (CD44s) and therefore all isoforms of the CD44 molecule. To investigate whether the CD44 expression on EC from normal tissue is due to the isolation procedure, in three experiments cells were stained without the adhesion step for enrichment of EC. The results for CD44 expression of normal and tumor tissue derived EC were (presented as mean fluorescence intensity ± SD) 23 ± 6 and 134 ± 47, respectively, where the background fluorescence was 17 ± 7. Normal tissue derived EC are therefore considered CD44 negative. Apparently the isolation procedure affected CD44. This has been addressed in subsequent studies (see below). Variant CD44 molecules (CD44v) could be demonstrated on freshly isolated tumor derived EC using antibodies recognizing the domains encoded by exon v5 and v10. The expression of these variant molecules, however, was very low and inconsistent between different tumors. It was therefore not possible to distinguish tumor EC at the level of CD44v expression. Antibodies directed against v6 and v7 encoded regions were not reactive with EC (not shown).
CD44 expression on normal renal tissue and RCC derived endothelial cells. Freshly isolated adherent cells of normal renal tissue (A and B) and RCC (C and D) were analyzed flow cytometrically and gated on EC FSC/SSC. Cells were stained with the NKI-P1 anti-CD44 (horizontal axis) antibody and counterstained with the EN4 EC-marker (vertical axis). Left panels represent conjugate controls. Mean fluorescence intensities of the endothelial cell subset is given in the upper right corner. The data of this patient are also given as RCC #7 in Table 1.
CD44 expression on normal renal tissue and RCC derived endothelial cells. Freshly isolated adherent cells of normal renal tissue (A and B) and RCC (C and D) were analyzed flow cytometrically and gated on EC FSC/SSC. Cells were stained with the NKI-P1 anti-CD44 (horizontal axis) antibody and counterstained with the EN4 EC-marker (vertical axis). Left panels represent conjugate controls. Mean fluorescence intensities of the endothelial cell subset is given in the upper right corner. The data of this patient are also given as RCC #7 in Table 1.
Elevated CD44 Expression on Tumor EC*
| Experiment No. . | Normal . | Tumor . |
|---|---|---|
| RCCs | 200 | 474 |
| 1 | ||
| 2 | 30 | 249 |
| 3 | 310 | 447 |
| 4 | 200 | 1,162 |
| 5 | 172 | 1,187 |
| 6 | 67 | 180 |
| 7† | 70 | 556 |
| 8 | 74 | 287 |
| 9 | 126 | 301 |
| 10 | 25 | 14 |
| 11 | 39 | 78 |
| 12 | 98 | 273 |
| 13 | 72 | 31 |
| 14 (VHL) | 359 | 2,300 |
| Ovarium carcinomas | 57‡ | 403 |
| 15 | ||
| 16 | 90‡ | 396 |
| Experiment No. . | Normal . | Tumor . |
|---|---|---|
| RCCs | 200 | 474 |
| 1 | ||
| 2 | 30 | 249 |
| 3 | 310 | 447 |
| 4 | 200 | 1,162 |
| 5 | 172 | 1,187 |
| 6 | 67 | 180 |
| 7† | 70 | 556 |
| 8 | 74 | 287 |
| 9 | 126 | 301 |
| 10 | 25 | 14 |
| 11 | 39 | 78 |
| 12 | 98 | 273 |
| 13 | 72 | 31 |
| 14 (VHL) | 359 | 2,300 |
| Ovarium carcinomas | 57‡ | 403 |
| 15 | ||
| 16 | 90‡ | 396 |
Abbreviation: VHL; renal cell carcinoma of a patient with Von Hippel-Lindau syndrome.
Mean CD44 (NKI-P1 antibody) fluorescence intensity not corrected for background (<20) values and counterstained with EN4-PE.
The data of this RCC are also given in Fig 1.
Values of ovarium carcinomas are compared to CD44 expression of normal dermis derived EC.
Immunohistochemistry on frozen tissue sections revealed that EC present in normal tissues are unreactive with anti-CD44 antibodies. This holds true for umbilical vein EC (HUVEC, Fig 2B) and for capillaries, small arterioles and venules present in dermis, skeletal muscle and kidney (both glomerular capillaries and intertubular capillaries, Fig 2D). Staining of frozen tissue sections of renal and ovarian cancer with anti-CD44s antibodies showed reactivity with tumor associated EC. Expression of CD44 was present in the majority of small vessels while the expression decreases with increasing vessel diameter (Fig 2). Besides the variety of staining intensities of different vessels a large heterogeneity among EC within one vessel was observed as is shown in the insert of Fig 2F. In placenta tissue, where angiogenic stimulation of EC is also highly expected, the majority of vessels also stained with anti-CD44 antibodies (not shown). Although in some cases present on the tumor cells, endothelial variant exon containing isoforms of CD44 (CD44v) could not be detected by immunohistochemical techniques.
Immunohistochemical peroxidase staining of CD44 on normal tissue and RCC associated EC. Frozen tissue sections of human umbilical vein (A and B), normal renal tissue (C and D), and RCC (E and F ) were stained for EC-marker EN4 (A, C, and E) and CD44 (NKI-P1 antibody, B, D, and F ). The arrows in (A and B) identify the EC layer. In (C and D) the arrows identify the glomeruli and the arrowheads mark the EC in the larger vessels. From the RCC sections one vessel is magnified (insets). Bars represent 50 μm, in (A) for (A and B), in (C) for (C through F ), insets same magnitude.
Immunohistochemical peroxidase staining of CD44 on normal tissue and RCC associated EC. Frozen tissue sections of human umbilical vein (A and B), normal renal tissue (C and D), and RCC (E and F ) were stained for EC-marker EN4 (A, C, and E) and CD44 (NKI-P1 antibody, B, D, and F ). The arrows in (A and B) identify the EC layer. In (C and D) the arrows identify the glomeruli and the arrowheads mark the EC in the larger vessels. From the RCC sections one vessel is magnified (insets). Bars represent 50 μm, in (A) for (A and B), in (C) for (C through F ), insets same magnitude.
CD44 expression is affected by confluence and proliferation.The specific expression of CD44 on tumor associated endothelium prompted us to study the regulation of expression on cultured EC. As expected from immunohistochemical data, HUVEC stained directly after isolation do not express CD44 at the cell surface as determined with various anti-CD44s and anti-CD44v antibodies. Culture of these cells in 20% human serum resulted in the appearance of expression of CD44 on a subset of EC in a time dependent manner. Already after 16 hours of culture CD44 expression appeared on a number of EC. After 3 days of culture this CD44 expressing subset reached 20% to 60% of cells (Fig 3A). The absence of CD44 on EC immediately after isolation was not due to the isolation procedure since the same technique applied to subcultured EC did not result in loss of CD44.
CD44 is an activation marker on human EC. HUVEC do not express CD44 immediately after isolation. Plating and culture of these cells results in the expression of CD44 on a subset of EC (A). Cells grown to confluence lose their membrane CD44 while plating them at low confluence induces CD44 expression again (B).
CD44 is an activation marker on human EC. HUVEC do not express CD44 immediately after isolation. Plating and culture of these cells results in the expression of CD44 on a subset of EC (A). Cells grown to confluence lose their membrane CD44 while plating them at low confluence induces CD44 expression again (B).
The appearance of endothelial CD44 expression after starting culture suggested that CD44 is an activation marker of EC expressed on growing cells. Two more lines of evidence support this assumption. First, culture of HUVEC at increasing confluences resulted in a decrease of CD44 expression (Fig 3B). The diminished expression of CD44 on contact inhibited EC could be enhanced again by subculture of the cells to a lower confluence. Second, when low confluence cultures were pulsed with BrdU for 16 hours, and subsequently doublestained for CD44 and BrdU, a threefold to sixfold higher CD44 expression was found on cells that incorporated BrdU during this period (Table 2). As a control CD34 did not show any significant differences between resting and BrdU-incorporating EC (Table 2).
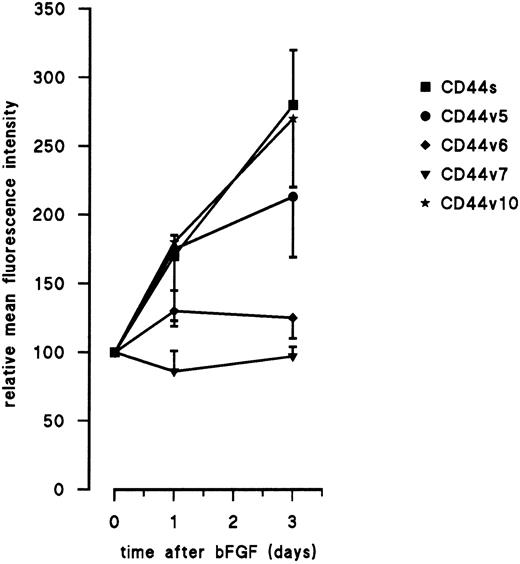
Angiogenic factors upregulate CD44 expression on EC.Since tumors are dependent on the formation of new blood vessels, factors that stimulate angiogenesis are produced. Hence, tumor infiltrating EC are exposed to high levels of these angiogenic factors. The augmented expression of CD44 on endothelial cells obtained from tumor tissue and the earlier finding that CD44 expression is elevated in actively dividing cells,12 31 led to the hypothesis that as a result of tumor angiogenesis expression of CD44 is induced. To test this hypothesis the angiogenic factors bFGF and VEGF were screened for their ability to influence CD44 expression. Cultured HUVEC were treated with 10 ng/mL bFGF, which induced a mitogenic response (on time-points 0, 1, and 3 days after stimulation, proliferation was 22,400 ± 761, 33,200 ± 1,693, and 82,880 ± 3,415 cpm, respectively). CD44 was monitored in time after initiation of the culture. We found an elevation of CD44 that coincided proliferation and could be measured 24 hours after treatment with bFGF. This upregulation was twofold to threefold at day 3 of culture (Fig 4). The upregulation of CD44 was transient since longer incubation periods of EC with bFGF resulted in lowered CD44 expression levels. The transient characteristic of this regulation is caused by the fact that cells reach confluence at this timepoint; subculture of the cells during the bFGF treatment resulted in prolonged upregulation of CD44. Variant CD44 molecules could be detected using antibodies recognizing exons v5 and v10. Reactivity of these antibodies was also enhanced twofold to threefold after treatment of EC with 10 ng/mL bFGF. CD44v expressing domains encoded by v5 and v6 were not detectable and were not induced by bFGF (Fig 4). The same results were obtained after treatment of EC with VEGF, which is another potent angiogenic factor. Also microvascular EC isolated from the dermis regulated the surface CD44 expression similarly (not shown).
Upregulation of CD44 isoforms by bFGF. Cultured HUVEC were treated with 10 ng/mL bFGF for several time periods. Isoforms are stained for FACS-analysis by the identified antibodies. Mean fluorescence intensity (MFI) of five independent experiments (± SEM) is given as the percentage of untreated cells cultured for the same time without bFGF. The absolute levels of expression (MFI ± SEM) were 185 ± 45, 44 ± 8, 13 ± 2 (negative), 8 ± 2 (negative), 26 ± 5 for CD44s and CD44 exon v5, v6, v7, and v10, respectively (conjugate control 8 ± 1).
Upregulation of CD44 isoforms by bFGF. Cultured HUVEC were treated with 10 ng/mL bFGF for several time periods. Isoforms are stained for FACS-analysis by the identified antibodies. Mean fluorescence intensity (MFI) of five independent experiments (± SEM) is given as the percentage of untreated cells cultured for the same time without bFGF. The absolute levels of expression (MFI ± SEM) were 185 ± 45, 44 ± 8, 13 ± 2 (negative), 8 ± 2 (negative), 26 ± 5 for CD44s and CD44 exon v5, v6, v7, and v10, respectively (conjugate control 8 ± 1).
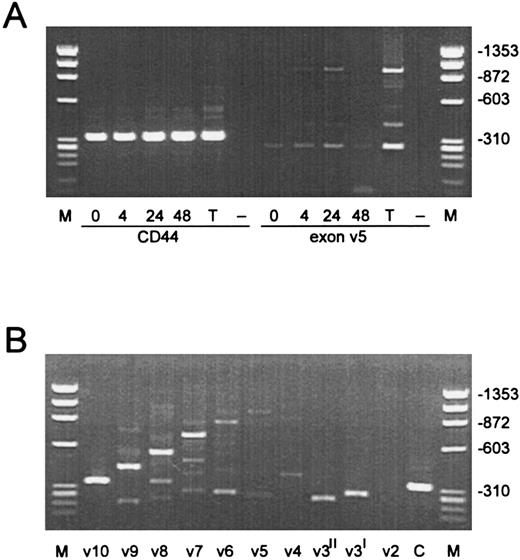
RT-PCR analysis shows regulated expression of CD44 splice variants.RT-PCR analysis was performed on EC RNA to determine the expression of different isoforms of CD4429 after stimulation of EC with angiogenic factors. RNA, prepared from resting EC and EC treated for 4, 24, and 48 hours with bFGF, was subjected to RT-PCR amplification using primers corresponding to 5′ and 3′ standard domains and to the variant exons. We found that CD44s are expressed in resting EC at the mRNA level (Fig 5). Also at least two different exon 5-containing mRNAs were found. In contrast to CD44s, these exon v5 expressing variant CD44 molecules on EC were upregulated on stimulation with 10 ng/mL bFGF. These results indicate that changes in expression of CD44 protein are regulated at the RNA level. Highest expression of exon 5-containing mRNA was found 24 hours after treatment of EC with bFGF. As a positive control CD44 variant expression on PHA activated peripheral blood T lymphocytes is shown (Fig 5A). We also analyzed expression of the other variant exons in the bFGF treated EC. These experiments showed that several different CD44 splice variants are present on activated EC, among which are variants expressing v5-v10 and variants expressing only v4 or v3. Exon v2 is not expressed in activated EC (Fig 5B).
RT-PCR amplification of cDNA with CD44 specific primers. (A) RNA prepared from resting EC and EC treated for 4, 24, and 48 hours with bFGF was amplified with 5′ and 3′ constant exon primers (CD44s) and with an exon v5 specific primer. The lane identified with “T” represents RNA from CD3 activated peripheral blood T lymphocytes, which serves as a positive control. Lanes identified with “–” represents RNA from a CD44− cell line. “M” represents the molecular weight markers, which are identified on the right. (B) RNA from bFGF activated (10 ng/mL, 24 hours) is amplified with specific primers for all variant exons. The lane identified with “C” represents the amplification with 3′ and 5′ constant exon primers (CD44s).
RT-PCR amplification of cDNA with CD44 specific primers. (A) RNA prepared from resting EC and EC treated for 4, 24, and 48 hours with bFGF was amplified with 5′ and 3′ constant exon primers (CD44s) and with an exon v5 specific primer. The lane identified with “T” represents RNA from CD3 activated peripheral blood T lymphocytes, which serves as a positive control. Lanes identified with “–” represents RNA from a CD44− cell line. “M” represents the molecular weight markers, which are identified on the right. (B) RNA from bFGF activated (10 ng/mL, 24 hours) is amplified with specific primers for all variant exons. The lane identified with “C” represents the amplification with 3′ and 5′ constant exon primers (CD44s).
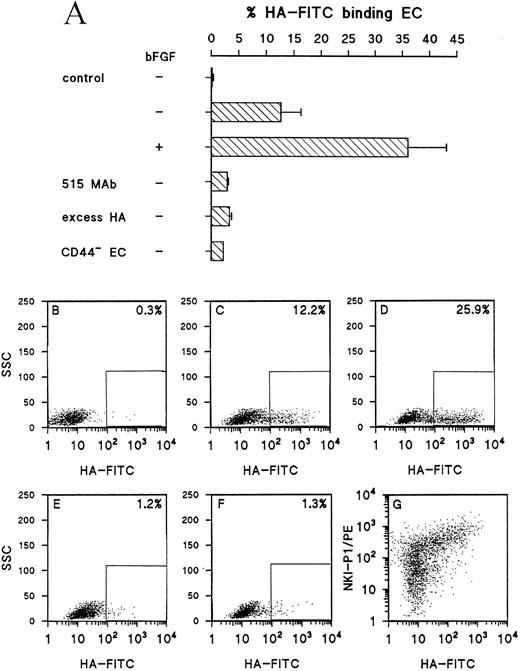
bFGF treatment of EC results in enhanced HA binding.The results described above indicate that CD44 can be considered as an activation marker on EC and that the molecule plays a role in tumor angiogenesis. Upregulation of adhesion molecules on EC enables, next to adhesion and extravasation of leukocytes, adhesion to extracellular matrix components which might facilitate sprouting of new blood vessels. To assess whether the enhanced CD44 expression on activated EC has a functional impact, the binding of control and bFGF treated EC to HA was measured. Treatment of EC with bFGF, which causes an augmented expression of CD44 as determined by FACS-analysis, resulted in a threefold enhanced binding of EC to HA-FITC. This binding was inhibited by excess soluble unlabeled HA and by pretreatment of EC with the blocking 515 anti-CD44 antibody (Fig 6A through F ). Figure 6G shows that all HA-FITC binding cells are CD44+ and that no CD44− cells bound HA-FITC. Next to binding of HA-FITC, the adhesion to immobilized HA was measured. bFGF pretreated cells displayed a more than twofold enhanced adhesion to HA coated wells. This binding could also be inhibited by either excess soluble HA or 515 antibody (data not shown). It was also found that freshly isolated HUVEC that do not express CD44 (see also Fig 2B) do not bind to either immobilized HA or soluble HA-FITC.
bFGF induces an enhanced binding of EC to HA. (A) Control and bFGF (10 ng/mL) pretreated HUVEC were incubated with FITC-conjugated HA. Specificity of binding was determined by pretreatment of cells with 1 μg/mL 515 anti-CD44 antibody and by addition of excess (2.5 mg/mL) soluble HA. Results are expressed as the mean percentage of HA-binding cells of three experiments (± SEM). CD44− EC represent HUVEC directly used after isolation. The bar identified with “control” represents HUVEC that were not incubated with HA-FITC. (B) through (F) show the FACS-plots of one experiment in which green fluorescence (HA-FITC) is plotted against SSC. The percentage HA-FITC binding cells is indicated in the upper right corner.
bFGF induces an enhanced binding of EC to HA. (A) Control and bFGF (10 ng/mL) pretreated HUVEC were incubated with FITC-conjugated HA. Specificity of binding was determined by pretreatment of cells with 1 μg/mL 515 anti-CD44 antibody and by addition of excess (2.5 mg/mL) soluble HA. Results are expressed as the mean percentage of HA-binding cells of three experiments (± SEM). CD44− EC represent HUVEC directly used after isolation. The bar identified with “control” represents HUVEC that were not incubated with HA-FITC. (B) through (F) show the FACS-plots of one experiment in which green fluorescence (HA-FITC) is plotted against SSC. The percentage HA-FITC binding cells is indicated in the upper right corner.
Use of endothelial CD44 as a target for immunotoxins.The upregulation of CD44 on activation of EC and the rather specific expression of endothelial CD44 molecules on tumor associated vessels, combined with the high accessibility of EC, prompted us to study the possibility to use endothelial CD44 as a target for anticancer therapy with immunotoxins. The approach of cancer treatment by the destruction of tumor associated vessels has been extensively studied and validated in earlier reports.32 To study the usefulness of CD44 as a target molecule in this approach, anti-CD44 antibodies were tested for the in vitro killing of EC in the indirect immunotoxin assay.33 HUVEC and the human CD44-expressing keratinocyte cell line HaCaT, were cultured in a 96-well culture plate. Cells were then treated with control and anti-CD44 antibodies. Subsequently, cells were cultured with saporin-conjugated goat antimouse antibodies.34 This resulted in high levels of cell killing whereas cells cultured in control antibody were not killed (Table 3). These experiments suggest that CD44 is internalized by EC and may therefore in this regard serve as a target for treatment with immunotoxins.
EC Killing in an Indirect Immunotoxin Assay by NKI-P1 anti-CD44 Antibody
| . | Experiment 1 . | Experiment 2 . | Experiment 3 . | |
|---|---|---|---|---|
| EC | HaCaT | |||
| Control Ab (OKT3/CD3) | 2,953* ± 143 | 1,328 ± 340 | 980 ± 105 | 3,295 ± 384 |
| Anti-CD44 (NKI-P1) | 1,717 ± 201 | 617 ± 80 | 759 ± 54 | 1,894 ± 171 |
| Cycloheximide | 200 | 279 ± 78 | 41 ± 4 | 176 ± 25 |
| Inhibition of protein synthesis | 45%3-151 | 68% | 34% | 45% |
| By NKI-P1 anti-CD44 | ||||
| . | Experiment 1 . | Experiment 2 . | Experiment 3 . | |
|---|---|---|---|---|
| EC | HaCaT | |||
| Control Ab (OKT3/CD3) | 2,953* ± 143 | 1,328 ± 340 | 980 ± 105 | 3,295 ± 384 |
| Anti-CD44 (NKI-P1) | 1,717 ± 201 | 617 ± 80 | 759 ± 54 | 1,894 ± 171 |
| Cycloheximide | 200 | 279 ± 78 | 41 ± 4 | 176 ± 25 |
| Inhibition of protein synthesis | 45%3-151 | 68% | 34% | 45% |
| By NKI-P1 anti-CD44 | ||||
Protein synthesis measured in counts per minute after incorporation of 3H-leucine.
Percentage of control Ab values after subtraction of cycloheximide values.
DISCUSSION
In the present report we showed that CD44 expression on tumor associated vessels is augmented as compared to vasculature in the normal tissue. It is shown that CD44 expression on EC is regulated during activational processes. For endothelial cells the activation dependent expression may provide an intricate regulation of both angiogenesis by interaction with the extracellular matrix and leukocyte infiltration in tumors by regulation of leukocyte rolling and adhesion. Immunohistochemical studies indicate that there is little or no expression of CD44 on vessels in normal tissue. Flow cytometric measurements indicate a low expression of CD44 on these EC. This difference is probably caused by the quantitative and more sensitive characteristics of flow cytometry. It can also be envisaged that, since CD44 is an inducible molecule on EC, the low expression of CD44 on EC from normal renal tissue appeared during the isolation procedure of EC from tissue, which includes an adhesion step on an FN coat. Isolation dependent artifacts might influence the level of CD44 expression, however, both normal and tumor tissues were treated similarly and synchronically and have a major quantitative difference in expression. This and the fact that other molecules are expressed at the same level (CD31 and HLA class I) strengthen the finding that CD44 expression is enhanced on tumor EC.
In the present report the upregulation of CD44 molecules on cultured EC by bFGF has been shown at the RNA and the protein level. Antibody binding studies with both antistandard (CD44s) and antivariant (CD44v) antibodies revealed upregulation of CD44 upon stimulation with the endothelial mitogen bFGF. Since antibodies against CD44s recognize all CD44 isoforms, these experiments were not conclusive on the upregulation of the CD44s isoform. PCR-analysis revealed that (1) the majority of CD44 on EC is CD44s, (2) expression of CD44s is not influenced by bFGF and (3) isoforms containing the variant exon v5 are upregulated upon stimulation with bFGF. These results show that the upregulation of CD44 is largely due to the variant isoforms. This resembles the situation in lymphocyte activation, where the upregulation of CD44s on antigen stimulation is moderate as compared to the profound upregulation of variant exon containing isoforms.35 These results suggest functions for alternatively spliced CD44 molecules in the process of angiogenesis.
The immunohistochemical data and also the FACS analysis of isolated tumor derived EC show that CD44 is usually expressed on a subset of cells. For example, not all vessels in RCC are positive for CD44 and a clear subset of CD44 expressing EC can be discriminated both in isolated tumor EC and in cultured HUVEC. The difference in CD44 expression is likely to reflect the activation state of the EC. A large heterogeneity among the EC within one vessel could be observed. It is feasible that the signals necessary to form a new vessel from a preexisting one, should not affect all EC in one compartment. Heterogeneity in susceptibility for growth signals may provide the required specificity.
Although alternative splicing generates an enormous heterogeneity of CD44, it is now recognized that posttranscriptional modification of CD44 contributes as much to the generation of diversity.18,36 37 Western blot analysis of EC lysates, prepared from resting and bFGF activated EC did not reveal a shift of the molecular mass of the CD44s molecule, suggesting similar glycosylation patterns in activated and unactivated EC. Also treatment of EC with bFGF did not result in a molecular weight shift of the larger isoforms of CD44 (Griffioen et al, unpublished observation, March 1996). These results indicate that the bFGF regulation of CD44 function does not involve posttranslational modification of CD44. This suggests that bFGF only increases the number of receptors on the EC membrane leading to enhanced ligand binding.
In previous studies of our laboratory we described that one of the mechanisms that tumors use to escape from immune surveillance is the downregulation of endothelial adhesion molecules.25,26,30 The upregulation of CD44 contrasts these findings more since recently CD44 has been described to mediate lymphocyte rolling via a novel mechanism involving hyaluronate.20 These studies have been performed using EC sources that do not express selectins. In the in vivo situation, however, EC especially in certain tumors may express E-selectin as we (unpublished observation)10 and others38 have shown. In addition, tumor EC express CD34 which involves rolling via L-selectin on the leukocyte. Therefore, the in vivo contribution of CD44 in leukocyte rolling might be moderate.
The mechanism of tumor metastasis formation might involve vascular CD44. The association of CD44 expression with poor prognosis is generally believed to be the result of the facilitated interaction with extracellular matrix components resulting in dissemination. It is described that tumor patients have high levels of circulating tumor cells. The adhesion of tumor cells to the vasculature and the induced CD44 expression by angiogenic factor production of the tumor cell might lead to homotypic CD44 adhesion (involving HA) to EC and facilitated extravasation.
The role of CD44-hyaluronate interactions may be more important for migration and differentiation including tube formation, of EC during angiogenesis.39 In this context, it is of importance to mention the bFGF mediated regulation of ICAM-1.25 Although prolonged treatment of EC with angiogenic factors results in suppression of ICAM-1, during the first 24 hours of this treatment ICAM-1 is significantly upregulated. Therefore, the function of ICAM-1 to act as a receptor for carbohydrate antigens or even hyaluronate40 may also contribute to the facilitated formation of new blood vessels.
CD44 has been described to play a role in cell activation and poor prognosis in cancer in a variety of different systems.9,12,31,35,41,42 For the expression of variant isoforms of CD44 this correlation is even more apparent.35,43 Certain variants are associated with metastasis formation.10,43 44 The upregulation of CD44s and CD44v on human endothelium upon activation and in solid tumors is therefore not a unique phenomenon. It is feasible that the upregulation of CD44 during and as a result of angiogenesis, contributes to an elevated level of CD44 signaling leading to a synergistic stimulation of EC proliferation, migration, and differentiation.
In a recent report by Bennet et al19 it has been described that CD44 isoforms containing exon v3 are responsible for the binding and presentation of heparin-binding growth factors. It is suggested that when v3 containing CD44 molecules are expressed by EC, these molecules may contribute to an inflammatory response by binding and presentation of these factors to leukocytes. The authors show, however, that activated cultured EC only express the CD44s isoform which does not contain any of the variably spliced exons. Our results demonstrated clearly that CD44 in EC is alternatively spliced giving rise to a number of different isoforms. Although we did not characterize these isoforms specifically we also showed that all exons except for exon v2 are present in activated EC at the mRNA level. This includes exon v3, which has an alternative splice acceptor site giving rise to exons v3I and v3II . Both exons are present in CD44 isoforms expressed by EC. Therefore, in our view endothelial CD44 can serve as a low-affinity receptor to present growth factors such as bFGF to leukocytes, or alternatively, to adjacent EC to promote angiogenesis.
We suggest in the present report that CD44 might be useful for targeting the tumor vasculature with immunotoxins. The major advantages of tumor treatment using this approach, as described by Thorpe et al,32 45 are that tumor EC are freely accessible whereas tumor cells in most cases are not and that the effect will be efficient after destroying a minority of EC. In addition, EC in tumors of different origin have a similar phenotype, so numerous types of cancer can be treated by a single reagent. Using this approach it is assumed that the selected target molecule is internalized (at least in part) by the target cells upon antibody binding and that the toxin can exert its ribosome-inactivating activity. The in vitro immunotoxin assay showed that the required internalization of toxin coupled to CD44 occurs in EC. This leads to killing percentages of 34% to 68%, which is efficient regarding the similar percentage of CD44 expressing EC in cultured HUVEC populations. Future studies will clarify the specificity of certain CD44 variants in regard to this concept. Alternatively, targeting immunotoxins to tumor endothelial CD44 may be used in isolated perfusion therapy.
In summary, CD44 is expressed on human EC as an activation marker associated with proliferation. The molecule is augmentedly expressed in the vasculature of tumors as a result of exposure to angiogenic factors. The role of CD44 in tumor angiogenesis is suggested by the enhanced binding to immobilized hyaluronan. Endothelial CD44 is a suitable target for immunotoxin treatment. Future research will have to clarify whether it is also a suitable target for clinical cancer therapy.
ACKNOWLEDGMENT
Dr Koolwijk (Leiden) is greatly acknowledged for isolation of foreskin microvascular endothelial cells. Dr N. Van Adrichem (Amersfoort) and Dr T. Boon (Utrecht) are greatly acknowledged for providing surgically removed tissue. Dr J. Lesley (San Diego, CA) is greatly acknowledged for providing HA-FITC and Dr G. Kansas (Iowa City, IA) for providing 515 anti-CD44 antibody.
Supported by the Dutch Research Foundation “De Drie Lichten” (Leiden, The Netherlands) and by the “Nijbakker Morra Stichting” (Ÿsselstein, The Netherlands).
Address reprint requests to A.W. Griffioen, PhD, Department of Internal Medicine, Tumor Angiogenesis Lab, University Hospital Maastricht, PO Box 5800, 6202 AZ Maastricht, The Netherlands.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal