Abstract
Constitutive expression of a rat CD44 variant isoform, rCD44v4-v7, on murine T cells accelerates immune responsiveness. Because prolonged immunodeficiency can be a major drawback in allogeneic bone marrow transplantation, we considered it of special interest to see whether repopulation of lethally irradiated syngeneic and allogeneic mice may be influenced by constitutive expression of the rCD44v4-v7 transgene. When lethally irradiated syngeneic and allogeneic mice were reconstituted with bone marrow cells (BMC) from rCD44v4-v7 transgenic (TG) or nontransgenic (NTG) mice, the former had a clear repopulation advantage: thymocytes expanded earlier after reconstitution and, as a consequence, higher numbers of lymphocytes were recovered from spleen and lymph nodes. Lymphocytes also displayed functional activity in advance to those from mice reconstituted with BMC from NTG mice. Most importantly, after the transfer of BMC from TG mice into an allogeneic host, the frequency of host-reactive T cells decreased rapidly. Apparently, this was due to accelerated induction of tolerance. Because these effects were counterregulated by an rCD44v6-specific antibody, it is likely that they could be attributed to the rCD44v4-v7 TG product. Thus, expression of a CD44 variant isoform at high levels facilitated reconstitution with allogeneic BMC by accelerated establishment of tolerance and the regaining of immunocompetence.
ALLOGENEIC BONE marrow transplantation may be the ultimate possibility of curative therapy in a variety of diseases, including malignancies of the hematopoietic system.1,2 One of the drawbacks after allogeneic bone marrow transplantation is the prolonged period of immunodeficiency,3,4 which may be based on immunosuppressive medications, graft-versus-host (GVH) disease, and intrinsic T-cell dysfunction.5-7 Considering the latter aspect, it is known that T-cell activation requires an accessory signal, which can be provided by a number of molecules expressed on the T-cell surface.8 Thus, manipulation of costimulatory molecules may provide a means of interfering with T-cell dysfunctions after allogeneic bone marrow transplantation.
CD44 comprises a family of adhesion molecules that vary by protein structure and glycosylation.9-17 The former is due to alternative splicing, whereby 10 additional exons can be inserted into the hematopoietic or standard isoform of CD44 (CD44s) either individually or in multiple combinations.18-20 There is evidence that the different CD44 isoforms fulfill distinct functions.21,22 The CD44 standard isoform, originally described as a lymphocyte homing receptor, is known to be involved in myelopoiesis and lymphopoiesis23as well as in bone marrow cell (BMC) homing and seeding.24-26 CD44 also has been described to function as an accessory molecule in T-cell activation.27-37 Although it is not known so far whether costimulatory activities can be mediated by CD44s, by CD44 variant isoforms (CD44v), or by both, there are several reports that show by upregulation in autoimmune diseases and allergic reactions38-40 as well as by antibody blockade that the variant exon CD44v6 should be involved.41,42 Furthermore, we have shown recently that rat CD44v4-v7 constitutively expressed on the surface of Thy1.2+ cells is functionally active in the mouse, particularly in modulating the response profile after antigenic stimulation.43 Therefore, we became interested in finding out whether this transgenic (TG) product might also offer an advantage during repopulation after BMC transplantation. Because the transgene product has been derived from rat CD44-cDNA and because a rat CD44v6-specific monoclonal antibody (MoAb)17 44 has been available, the model allowed us to differentiate between functions of CD44s and this particular CD44 variant isoform in BMC transplantation. It could be shown that rCD44v4-v7 facilitated the regaining of immunocompetence and exerted a clear-cut effect on the establishment of tolerance towards the host's major histocompatibility complex (MHC).
MATERIALS AND METHODS
Mice.C57BL/6 and BALB/c mice were obtained from Charles River (Sulzfeld, Germany). Severe combined immunodeficiency (SCID) mice were bred at the animal facilities of the German Cancer Research Center (Heidelberg, Germany). Rat CD44v4-v7-TG mice were generated by introducing the rat meta-1 expression construct (rCD44 containing exon v4-v7)17 into the genome of C57BL/6 × (C57BL/6xBALB/c)F1 mice. The rCD44v4-v7-cDNA was expressed under the control of the Thy1 promoter and the Igε heavy chain enhancer.45,46 Founder mice were crossed with C57BL/6 mice. Transgenic mice were identified by Southern blot analysis.47 Rat CD44v4-v7 transgenic mice were bred at the central animal facilities of the German Cancer Research Center. Animals were housed under specific pathogen-free conditions and were kept under conventional diet and water ad libitum. Mice were used for experiments at the age of 8 to 12 weeks.
Antibodies, purification, and flow cytometry.The following MoAbs were used: antimouse CD44s (IM-7, rIgG2b),48 antirat CD44v6 (1.1ASML, mIgG1),17,44 anti-Thy1 (YTS154.7.711, rIgG2b),49 anti-CD4 (GK1.5, rIgG2a),50 and anti-CD8 (YTS169.4.2.1, rIgG2b).49 The MoAbs anti–H-2Dd (K9-18, mIgG2b) and anti–H-2Db (K7-65, mIgG2a)51 were kindly provided by G. Hämmerling (Department of Immunogenetics, German Cancer Research Center). An isotype-matched control to anti-rCD44v6, 3-9 (anti-Ga-chelate, mIgG1)52 was used in some experiments. Culture supernatant-derived MoAbs were purified by passage over protein G Sepharose.53 In some instances, F(ab′)2 fragments were prepared. The eluted fractions were dialyzed, concentrated, and filter sterilized. Optimal working concentrations were determined by flow cytometry. Phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-labeled polyclonal antisera were used as second antibodies. B cells were stained directly with a PE-labeled antimouse μ.
For flow cytometry (fluorescence-activated cell sorting [FACS]), 5 × 105 cells were stained according to routine procedures. Fluorescence was determined with an EPICS XL (Coulter, Hialeah, FL).
Purified MoAbs 1.1ASML and 3-9 were used at a concentration of 10 μg/mL in in vitro experiments. In vivo, animals received twice per week an intravenous (IV) injection of 200 μg MoAb.
Reconstitution.Syngeneic C57BL/6 and allogeneic BALB/c mice were irradiated with 850 and 800 R, respectively; SCID mice were irradiated with 300 R. Animals received a single IV injection of 5 × 106 BMC 24 hours after irradiation. In some experiments, BMC were depleted of mature T and B cells by two rounds of panning on anti-Ig–coated and anti-Thy1 (YTS154.7.711)–coated Petri dishes according to the protocol by Wysocki and Sato.54 As shown by FACS analysis, neither IgM+ nor Thy1.2+ cells could be detected in the nonadherent population. Furthermore, from similar depletion studies using lymph node cells or spleen cells, we knew that the efficiency of depletion ranged between 95% and 98%. Where indicated, the reconstituted mice received twice per week an IV injection of 200 μg anti-rCD44v6 or a control IgG1 MoAb. Mice were killed after 7, 14, 21, 28, 42, and 56 days and central and peripheral lymphoid organs were excised. Cells were isolated by meshing through fine gauze. The percentage of host- and donor-derived lymphocytes and of T cells expressing the transgene product was evaluated by FACS staining.
To exclude that possible effects of the TG product on the reconstitution process may have been due to alteration in the migratory behavior, the short-term distribution of radiolabeled BMC was evaluated. BMC were depleted of B and T cells as described above. The nonadherent cells were collected and incubated with 1 mCi 51Cr for 90 minutes at 37°C. After washing, cells were resuspended at a concentration of 2.5 × 107 cells/mL phosphate-buffered saline containing 1 mg/mL control or anti-rCD44v6 (1.1ASML) F(ab′)2 fragments. Animals received 200 μL of cell suspension IV. The injection of F(ab′)2 fragments was repeated after 3 days. Animals were bled from the retroorbital sinus and were killed after 1, 8, 24, 48, 72, and 96 hours. Organs (bone marrow, spleen, thymus, liver, lung, kidney, and muscle) were excised and weighed. Radioactivity was determined in a γ-counter and the radioactivity per organ was calculated.
Lymphocyte proliferation.Functional maturity of thymocytes (TC), spleen cells (SC), and lymph node cells (LNC) was evaluated in proliferation assays using either trinitrophenyl-ovalbumin (TNP-OA) at a concentration of 50 μg/mL or irradiated (3,000 R) syngeneic or allogeneic lymphocytes (5 × 104/well). Cells were titrated in triplicates in 96-well U-shaped plates, starting with a concentration of 2 × 105 cells/well. Cultures were kept for 4 days adding 3H-thymidine (10 μCi/mL) during the last 16 hours of culture. Plates were harvested with an automatic harvester and 3H-thymidine incorporation was determined in a β-counter. Where indicated, T cells were enriched by panning on anti–Thy-1 — coated plates54 collecting the adherent fraction (purity was 98% according to FACS analysis). Cells were plated under limiting dilution (LD) condition, titrating 24 replicates from 200 to 25,600 cells/well and adding 5 × 104 irradiated (3,000 R) syngeneic or allogeneic spleen cells as stimulator cells. Frequencies were calculated according to the formula F0 (fraction of nonresponding cultures) = e−u, where u = c/w (number of cells [c] distributed in wells [w]).55
Statistical analysis.Significance of differences were calculated according to the Wilcoxon rank sum test.
RESULTS
Regaining immunocompetence is one of the major problems in allogeneic stem cell transplantation. Because we recently could show that constitutive expression of rCD44v4-v7 on Thy1.2+ cells modulated immune responsiveness,43 we became interested to see whether this effect may be observed on a systemic level during reconstitution of lethally irradiated mice.
As described,43 the transgene is expressed exclusively on Thy1+ T cells in thymus, lymph nodes, and spleen, but not on Thy1+ cells in the peritoneal cavity and the Peyer's patches. In the bone marrow (Table 1) a small population of medium-sized cells expressed the transgene. These cells are CD43+, partly SCA-1+, and, surprisingly, only partly Thy1+ and CD4+. Thus, the TG-expressing cells in the bone marrow are likely to be early progenitors including the B-cell lineage.
Expression of the rCD44v4-v7 TG Product on BMC
| Donor . | FS/logSS* . | % of Cells . | % Positive Cells . | % Double-Positive Cells CD44v6+ . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | SCA-1 . | Thy.1 . | CD4 . | CD8 . | B220 . | CD43 . | IgM . | IgD . | CD44s . | rCD44v6 . | SCA-1 . | Thy1 . | CD43 . |
| NTG | 206/8.3 | 7.8 | 6.2 | 6.5 | 7.3 | 4.2 | 2.3 | 2.0 | 3.7 | 3.0 | 74.1 | 0.0 | |||
| 328/9.0 | 24.3 | 13.6 | 10.6 | 9.9 | 3.8 | 18.6 | 28.3 | 7.1 | 7.8 | 99.8 | 0.0 | ||||
| 616/46.4 | 30.0 | 1.2 | 13.2 | 9.0 | 1.5 | 10.4 | 88.8 | 9.0 | 12.5 | 99.6 | 0.0 | ||||
| Total | 100.0 | 6.8 | 10.4 | 9.2 | 2.7 | 11.8 | 38.7 | 7.6 | 7.3 | 84.5 | 0.1 | ||||
| TG | 208/8.3 | 6.6 | 2.3 | 3.2 | 5.1 | 3.0 | 2.8 | 2.9 | 3.4 | 1.4 | 76.6 | 1.3 | 0.0 | 0.1 | 0.1 |
| 336/8.9 | 25.3 | 12.1 | 15.1 | 13.9 | 3.9 | 22.3 | 28.3 | 6.8 | 5.2 | 93.8 | 6.8 | 3.6 | 3.0 | 6.5 | |
| 616/46.4 | 30.5 | 0.5 | 10.4 | 9.9 | 1.5 | 10.3 | 89.3 | 8.3 | 9.4 | 99.1 | 6.9 | 0.4 | 2.1 | 6.9 | |
| Total | 100.0 | 5.4 | 12.2 | 11.8 | 2.2 | 12.7 | 35.8 | 7.3 | 8.6 | 85.3 | 6.1 | 1.8 | 2.3 | 6.0 | |
| Donor . | FS/logSS* . | % of Cells . | % Positive Cells . | % Double-Positive Cells CD44v6+ . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | SCA-1 . | Thy.1 . | CD4 . | CD8 . | B220 . | CD43 . | IgM . | IgD . | CD44s . | rCD44v6 . | SCA-1 . | Thy1 . | CD43 . |
| NTG | 206/8.3 | 7.8 | 6.2 | 6.5 | 7.3 | 4.2 | 2.3 | 2.0 | 3.7 | 3.0 | 74.1 | 0.0 | |||
| 328/9.0 | 24.3 | 13.6 | 10.6 | 9.9 | 3.8 | 18.6 | 28.3 | 7.1 | 7.8 | 99.8 | 0.0 | ||||
| 616/46.4 | 30.0 | 1.2 | 13.2 | 9.0 | 1.5 | 10.4 | 88.8 | 9.0 | 12.5 | 99.6 | 0.0 | ||||
| Total | 100.0 | 6.8 | 10.4 | 9.2 | 2.7 | 11.8 | 38.7 | 7.6 | 7.3 | 84.5 | 0.1 | ||||
| TG | 208/8.3 | 6.6 | 2.3 | 3.2 | 5.1 | 3.0 | 2.8 | 2.9 | 3.4 | 1.4 | 76.6 | 1.3 | 0.0 | 0.1 | 0.1 |
| 336/8.9 | 25.3 | 12.1 | 15.1 | 13.9 | 3.9 | 22.3 | 28.3 | 6.8 | 5.2 | 93.8 | 6.8 | 3.6 | 3.0 | 6.5 | |
| 616/46.4 | 30.5 | 0.5 | 10.4 | 9.9 | 1.5 | 10.3 | 89.3 | 8.3 | 9.4 | 99.1 | 6.9 | 0.4 | 2.1 | 6.9 | |
| Total | 100.0 | 5.4 | 12.2 | 11.8 | 2.2 | 12.7 | 35.8 | 7.3 | 8.6 | 85.3 | 6.1 | 1.8 | 2.3 | 6.0 | |
Cells were gated according to size (FS, forward scatter) and granulation (SS, sideward scatter).
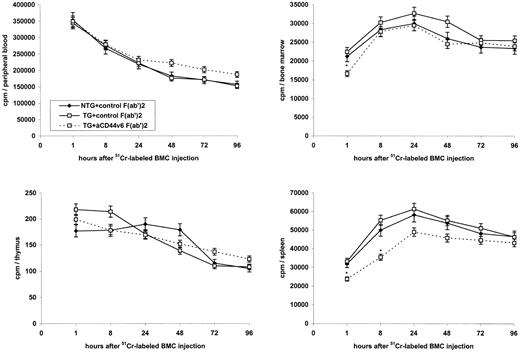
Repopulation of the thymus in lethally irradiated and reconstituted mice.Although only a minority of BMC expressed the TG product, we wanted to be reassured that possible effects on reconstitution were not biased by an altered homing behavior of BMC from TG as compared with nontransgenic (NTG) mice. Therefore, we evaluated the distribution of 51Cr-labeled BMC, which were depleted of IgM+ and Thy1+ cells, in lymphoid organs of the lethally irradiated, syngeneic host (C57BL/6; Fig 1). As could have been expected by expression of the TG product predominantly on thymocytes and peripheral T cells, no major differences were noted in homing of BMC from TG as compared with NTG mice into the bone marrow. Surprisingly, blocking studies showed that anti-rCD44v6 had a minor, but significant effect on homing into the bone marrow and the spleen, although only during the first 8 hours after injection. BMC from neither TG nor NTG mice homed into the thymus during the observation period of 4 days. The same overall distribution profile was seen in the lethally irradiated allogeneic host (data not shown).
Influence of rCD44v4-v7 on the distribution of BMC in lymphoid organs of the lethally irradiated host. Lethally irradiated (850 R) C57BL/6 mice received 5 × 106 51Cr-labeled BMC from NTG or rCD44v4-v7 TG C57BL/6 mice together with 200 μg control (3-9) or anti-rCD44v6 (1.1ASML) F(ab′)2 fragments, IV. Injections of F(ab′)2 fragments were repeated after 3 days. Animals were bled from the retroorbital sinus and were killed after 1, 8, 24, 48, 72, and 96 hours. Bone marrow, thymus, and spleen were excised and weighed. Radioactivity was determined in a γ-counter and the radioactivity per organ was calculated. Mean cpm ± SD are shown; significant differences (P ≤ .01) between cpm in mice receiving TG BMC and control F(ab′)2 or anti-rCD44v6 F(ab′)2 are indicated by an asterisk.
Influence of rCD44v4-v7 on the distribution of BMC in lymphoid organs of the lethally irradiated host. Lethally irradiated (850 R) C57BL/6 mice received 5 × 106 51Cr-labeled BMC from NTG or rCD44v4-v7 TG C57BL/6 mice together with 200 μg control (3-9) or anti-rCD44v6 (1.1ASML) F(ab′)2 fragments, IV. Injections of F(ab′)2 fragments were repeated after 3 days. Animals were bled from the retroorbital sinus and were killed after 1, 8, 24, 48, 72, and 96 hours. Bone marrow, thymus, and spleen were excised and weighed. Radioactivity was determined in a γ-counter and the radioactivity per organ was calculated. Mean cpm ± SD are shown; significant differences (P ≤ .01) between cpm in mice receiving TG BMC and control F(ab′)2 or anti-rCD44v6 F(ab′)2 are indicated by an asterisk.
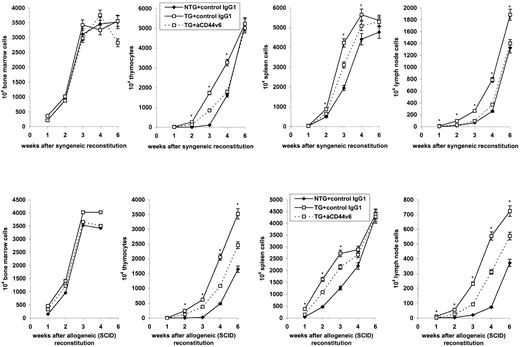
A clear difference between transferred BMC from TG versus NTG mice was observed during the following period of repopulation and/or expansion (Fig 2). In the syngeneic and the allogeneic host (data not shown) as well as in the allogenic SCID mouse, repopulation of the thymus as well as of the spleen and the lymph nodes was accelerated with BMC from TG mice. No such difference was observed in the bone marrow. Two additional observations supported the assumption that the accelerated repopulation could be assigned to expression of the transgene product. (1) The accelerated repopulation could be inhibited to some extent by anti-rCD44v6. (2) After the transfer of a 1:1 mixture of BMC from TG and NTG mice (Table 2), the number of recovered thymocytes resembled that observed with BMC from TG mice. FACS analysis confirmed that, at 10 and 14 days after reconstitution, the vast majority of thymocytes expressed the transgene product.
Expansion of BMC from NTG and rCD44v4-v7 TG donors in the syngeneic and the allogeneic host. Lethally irradiated (850 R) syngeneic C57BL/6 and irradiated (300 R) allogeneic BALB/c SCID mice were reconstituted with 5 × 106 BMC from NTG or from rCD44v4-v7 TG C57BL/6 mice. The reconstituted mice received either 200 μg control (3-9) or anti-rCD44v6 (1.1ASML) IgG1, IV. Injections of IgG1 were repeated twice per week. Mice were killed after 1 to 6 weeks. The numbers of cells (mean ± SD) in central and peripheral lymphoid organs of 3 mice/group are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk.
Expansion of BMC from NTG and rCD44v4-v7 TG donors in the syngeneic and the allogeneic host. Lethally irradiated (850 R) syngeneic C57BL/6 and irradiated (300 R) allogeneic BALB/c SCID mice were reconstituted with 5 × 106 BMC from NTG or from rCD44v4-v7 TG C57BL/6 mice. The reconstituted mice received either 200 μg control (3-9) or anti-rCD44v6 (1.1ASML) IgG1, IV. Injections of IgG1 were repeated twice per week. Mice were killed after 1 to 6 weeks. The numbers of cells (mean ± SD) in central and peripheral lymphoid organs of 3 mice/group are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk.
Recovery of Thymocytes After Reconstitution of the SCID Mouse With Mixtures of BMC From NTG and rCD44v4-v7 TG Mice
| BMC* Donor . | No. of Cells (×105)/Thymus† . | % rCD44v6+ Cells† . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | 7 d . | 10 d . | 14 d . | 21 d . | 7 d . | 10 d . | 14 d . | 21 d . |
| NTG | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.5 ± 0.3 | 4.4 ± 0.8 | ND | ND | ND | ND |
| TG + NTG | 0.1 ± 0.1 | 0.6 ± 1.3 | 14.3 ± 3.1 | 31.6 ± 2.9 | 2.5 ± 2.4 | 53.2 ± 6.3 | 69.8 ± 7.8 | 40.3 ± 7.9 |
| TG | 0.3 ± 0.5 | 0.7 ± 1.7 | 18.4 ± 2.5 | 37.8 ± 4.1 | 2.3 ± 1.6 | 58.6 ± 7.5 | 77.8 ± 8.9 | 78.9 ± 8.4 |
| BMC* Donor . | No. of Cells (×105)/Thymus† . | % rCD44v6+ Cells† . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | 7 d . | 10 d . | 14 d . | 21 d . | 7 d . | 10 d . | 14 d . | 21 d . |
| NTG | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.5 ± 0.3 | 4.4 ± 0.8 | ND | ND | ND | ND |
| TG + NTG | 0.1 ± 0.1 | 0.6 ± 1.3 | 14.3 ± 3.1 | 31.6 ± 2.9 | 2.5 ± 2.4 | 53.2 ± 6.3 | 69.8 ± 7.8 | 40.3 ± 7.9 |
| TG | 0.3 ± 0.5 | 0.7 ± 1.7 | 18.4 ± 2.5 | 37.8 ± 4.1 | 2.3 ± 1.6 | 58.6 ± 7.5 | 77.8 ± 8.9 | 78.9 ± 8.4 |
Abbreviation: ND, not detectable.
Mice received 5 × 106 BMC from NTG or TG mice or mixed at equal parts from NTG and TG mice, IV.
Mean ± SD of 5 mice per group.
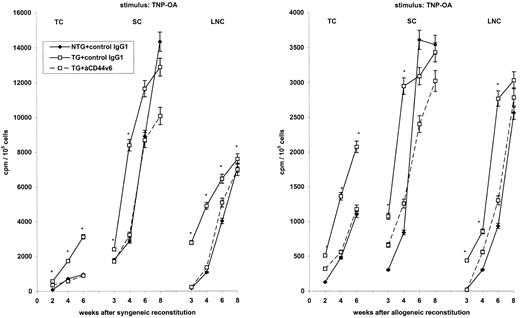
Regaining responsiveness.The finding that lymphocytes constitutively expressing rCD44v4-v7 showed an accelerated immune response after contact with nominal antigen43 could indeed be confirmed at the systemic level after the transfer of BMC from TG mice into the syngeneic and the allogeneic host (Fig 3). Responsiveness towards nominal antigen was noted earlier after the transfer of BMC from TG mice. In animals receiving BMC from NTG mice, comparable response levels were only reached 6 and 8 weeks after reconstitution in spleen and lymph nodes, respectively. Accelerated regain of responsiveness after the transfer of BMC from TG mice was abrogated by anti-rCD44v6 treatment. Beyond that, at 6 and 8 weeks after reconstitution, the proliferative activity of SC appeared to be inhibited. This is shown for the response towards TNP-OA in the syngeneic and the allogeneic host.
Influence of rCD44v4-v7 on the regain of responsiveness after syngeneic and allogeneic reconstitution. TC, SC, and LNC of lethally irradiated syngeneic C57BL/6 and allogeneic BALB/c mice that were reconstituted with BMC from NTG and rCD44v4-v7 TG C57BL/6 mice were collected 2 to 8 weeks after BMC transfer. Cells from 3 mice per group were pooled and were cultured in triplicates for 3 days in the presence of TNP-OA. Where indicated, lethally irradiated and reconstituted mice had received anti-rCD44v6 (200 μg twice per week). During the last 8 hours of culture, 3H-thymidine was added, cells were harvested, and incorporation of 3H-thymidine was determined in a β-counter. Background values were subtracted (cpm in the absence of an antigenic stimulus). Mean cpm ± SD of triplicate cultures are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk. The experiment was repeated three times showing similar results.
Influence of rCD44v4-v7 on the regain of responsiveness after syngeneic and allogeneic reconstitution. TC, SC, and LNC of lethally irradiated syngeneic C57BL/6 and allogeneic BALB/c mice that were reconstituted with BMC from NTG and rCD44v4-v7 TG C57BL/6 mice were collected 2 to 8 weeks after BMC transfer. Cells from 3 mice per group were pooled and were cultured in triplicates for 3 days in the presence of TNP-OA. Where indicated, lethally irradiated and reconstituted mice had received anti-rCD44v6 (200 μg twice per week). During the last 8 hours of culture, 3H-thymidine was added, cells were harvested, and incorporation of 3H-thymidine was determined in a β-counter. Background values were subtracted (cpm in the absence of an antigenic stimulus). Mean cpm ± SD of triplicate cultures are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk. The experiment was repeated three times showing similar results.
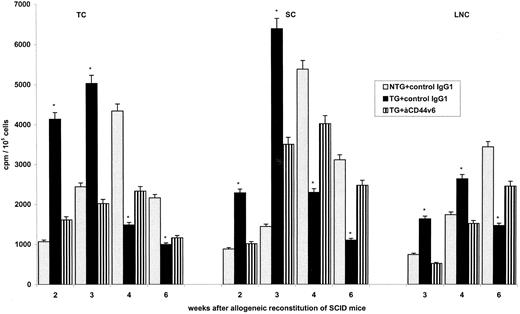
Establishment of tolerance.In view of the accelerated repopulation of the thymus, it was tempting to speculate that possibly tolerance towards the host may become more readily established after the transfer of BMC from TG than from NTG mice. This was first tested in the allogeneic SCID mouse (Fig 4). Particularly in the thymus, the onset of the antihost response was noted earlier after the transfer of BMC from TG as compared with BMC from NTG mice. This was not the case when mice receiving BMC from TG animals were treated with anti-rCD44v6, pointing towards the transgene product as the relevant molecule. More importantly, in the thymus and in peripheral lymphoid organs, antihost reactivity decreased more rapidly after the transfer of BMC from TG as compared with BMC from NTG mice. LD analysis confirmed the high frequency of host-reactive T cells at 2 weeks after transplantation as well as the striking decrease during the following weeks in SCID mice reconstituted with BMC from rCD44v4-v7 TG mice (Table 3).
Influence of rCD44v4-v7 on antihost reactivity after reconstitution of allogeneic SCID mice. Allogeneic SCID mice were irradiated with 300 R and were reconstituted with 5 × 106 BMC from NTG and rCD44v4-v7 TG C57BL/6 mice. Mice received, in addition, either a control IgG1 or anti-rCD44v6 (200 μg twice per week). TC, SC, and LNC were harvested after 2 to 6 weeks. Cells from 3 mice per group were pooled and were cultured in the presence of irradiated lymphocytes from BALB/c mice. After 3 days of culture and the addition of 3H-thymidine during the last 16 hours, cells were harvested and incorporation of 3H-thymidine was determined in a β-counter. Background values were subtracted (cpm in the absence of an antigenic stimulus). Mean cpm ± SD of triplicate cultures are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk. The experiment was repeated one time showing similar results.
Influence of rCD44v4-v7 on antihost reactivity after reconstitution of allogeneic SCID mice. Allogeneic SCID mice were irradiated with 300 R and were reconstituted with 5 × 106 BMC from NTG and rCD44v4-v7 TG C57BL/6 mice. Mice received, in addition, either a control IgG1 or anti-rCD44v6 (200 μg twice per week). TC, SC, and LNC were harvested after 2 to 6 weeks. Cells from 3 mice per group were pooled and were cultured in the presence of irradiated lymphocytes from BALB/c mice. After 3 days of culture and the addition of 3H-thymidine during the last 16 hours, cells were harvested and incorporation of 3H-thymidine was determined in a β-counter. Background values were subtracted (cpm in the absence of an antigenic stimulus). Mean cpm ± SD of triplicate cultures are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk. The experiment was repeated one time showing similar results.
Frequencies of Host-Reactive T Cells in the Allogeneic SCID Host After Transfer of BMC From rCD44v4-v7-TG and NTG Mice
| Organ . | Days After Reconstitution3-150 . | Host-Reactive T Cells: Frequency/106 Cells (frequency/organ) . | |
|---|---|---|---|
| . | . | NTG Donor . | TG donor . |
| TC | 21 | 123 (27) | 4,0003-151 (69,200) |
| 28 | 424 (2,027) | 523-151 (1,700) | |
| 42 | 39 (641) | 83-151 (281) | |
| SC | 21 | 2,941 (45,968) | 7223-151 (19,653) |
| 28 | 363 (7,968) | 933-151 (2,531) | |
| 42 | 106 (4,540) | 43-151 (176) | |
| LNC | 28 | 394 (291) | 623-151 (344) |
| 42 | 79 (295) | 93-151 (65) | |
| Organ . | Days After Reconstitution3-150 . | Host-Reactive T Cells: Frequency/106 Cells (frequency/organ) . | |
|---|---|---|---|
| . | . | NTG Donor . | TG donor . |
| TC | 21 | 123 (27) | 4,0003-151 (69,200) |
| 28 | 424 (2,027) | 523-151 (1,700) | |
| 42 | 39 (641) | 83-151 (281) | |
| SC | 21 | 2,941 (45,968) | 7223-151 (19,653) |
| 28 | 363 (7,968) | 933-151 (2,531) | |
| 42 | 106 (4,540) | 43-151 (176) | |
| LNC | 28 | 394 (291) | 623-151 (344) |
| 42 | 79 (295) | 93-151 (65) | |
5 × 106 BMC, IV.
P ≤ .01.
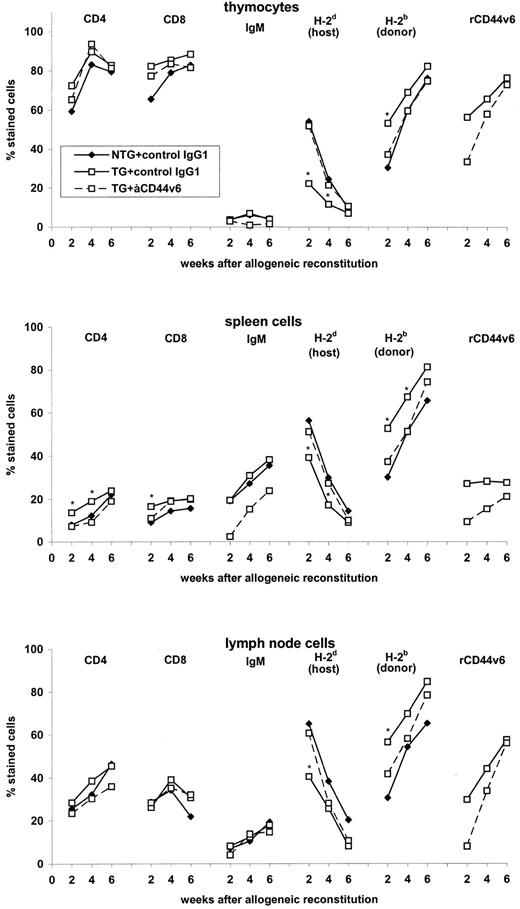
We next asked whether the same phenomenon may be observed in lethally irradiated allogeneic mice reconstituted with BMC from TG and NTG mice. To allow for quantification of donor- and host-reactive lymphocytes in this experimental setting, we evaluated in a pilot experiment the distribution of host-and donor-derived lymphocytes as well as the distribution of B cells and T-cell subsets in central and peripheral lymphoid organs (Fig 5). The distribution of T-cell subsets and B cells in thymus and lymph nodes of the reconstituted allogeneic host did not differ significantly between mice receiving BMC from TG or NTG mice and treatment with anti-rCD44v6 had no major impact. Only in the spleen was an increased percentage of CD4+ and CD8+ cells recovered early after reconstitution with BMC from TG mice. This may have been a consequence of the accelerated T-cell maturation in the thymus. Furthermore, whereas there was no difference in the percentage of IgM+ cells in the spleen of mice receiving NTG or TG BMC, in anti-rCD44v6–treated mice the percentage of IgM+ cells was reduced. Considering the distribution of host- and donor-derived lymphocytes, mice receiving BMC from TG and NTG animals differed only inasmuch as the percentage of donor cells increased more rapidly after the transfer of BMC from TG mice. At 6 weeks after reconstitution, the remaining differences were marginal.
Leukocyte distribution after reconstitution of allogeneic mice with BMC from rCD44v4-v7-TG and NTG mice. Lethally irradiated allogeneic BALB/c mice were reconstituted with 5 × 106 BMC from NTG and rCD44v4-v7 TG C57BL/6 mice. Mice received, in addition, either a control IgG1 or anti-rCD44v6 (200 μg twice per week). TC, SC, and LNC were harvested after 2 to 6 weeks. Expression of CD4, CD8, sIgM, H-2d (host), H-2b (donor), and the transgene product was analyzed by flow cytometry. The mean percentage of stained cells in 2 pools (TC and LNC from 3 mice, 2 weeks after reconstitution) or in 6 individually tested mice are shown. SD were in the range of 3% to 7%. Significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk.
Leukocyte distribution after reconstitution of allogeneic mice with BMC from rCD44v4-v7-TG and NTG mice. Lethally irradiated allogeneic BALB/c mice were reconstituted with 5 × 106 BMC from NTG and rCD44v4-v7 TG C57BL/6 mice. Mice received, in addition, either a control IgG1 or anti-rCD44v6 (200 μg twice per week). TC, SC, and LNC were harvested after 2 to 6 weeks. Expression of CD4, CD8, sIgM, H-2d (host), H-2b (donor), and the transgene product was analyzed by flow cytometry. The mean percentage of stained cells in 2 pools (TC and LNC from 3 mice, 2 weeks after reconstitution) or in 6 individually tested mice are shown. SD were in the range of 3% to 7%. Significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk.
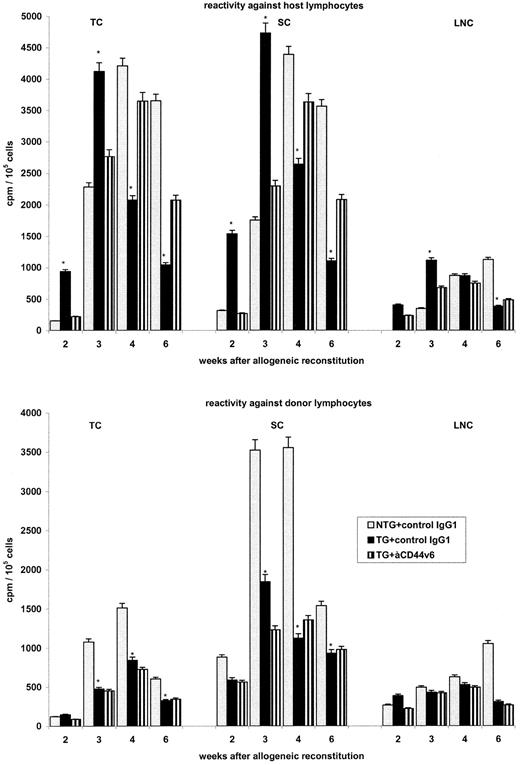
Although bulk reactivities against donor- or host-derived lymphocytes will be skewed by the concomitant presence of donor and host cells in the responder population, they can provide an indication as to the in vivo situation. As shown in Fig 6, the results supported the idea that BMC from TG mice may have an advantage in becoming tolerant towards the host's MHC. Antidonor reactivity was lower after the transfer of BMC from TG as compared with BMC from NTG mice. This could have been due to the accelerated expansion of lymphocytes from TG donors in the thymus. More interestingly, although antihost reactivity was high 2 weeks after reconstitution with BMC from TG mice, it had decreased significantly at 4 to 6 weeks after reconstitution, when the majority of lymphocytes were donor-derived. As could have been expected, anti-rCD44v6 had no influence on the reactivity of the host against the donor. The influence on host-reactive lymphocytes was similar to that seen in the SCID mouse reconstituted with BMC from allogeneic TG mice. In the presence of anti-rCD44v6, the onset of antihost reactivity was delayed but persisted for a prolonged period.
Influence of rCD44v4-v7 on antihost and antidonor reactivity after reconstitution of irradiated allogeneic BALB/c mice. Lethally irradiated BALB/c mice were reconstituted with 5 × 106 BMC from NTG and rCD44v4-v7 TG C57BL/6 mice. Mice received, in addition, either a control IgG1 or anti-rCD44v6 (200 μg twice per week). TC, SC, and LNC were harvested after 2 to 6 weeks. Cells from 3 mice per group were pooled and were cultured in the presence of irradiated lymphocytes from BALB/c (host) and C57BL/6 (donor) mice, respectively. After 3 days of culture and the addition of 3H-thymidine during the last 16 hours, cells were harvested and incorporation of 3H-thymidine was determined in a β-counter. Background values were subtracted (cpm in the absence of an antigenic stimulus). Mean cpm ± SD of triplicate cultures are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk. The experiment was repeated two times showing similar results.
Influence of rCD44v4-v7 on antihost and antidonor reactivity after reconstitution of irradiated allogeneic BALB/c mice. Lethally irradiated BALB/c mice were reconstituted with 5 × 106 BMC from NTG and rCD44v4-v7 TG C57BL/6 mice. Mice received, in addition, either a control IgG1 or anti-rCD44v6 (200 μg twice per week). TC, SC, and LNC were harvested after 2 to 6 weeks. Cells from 3 mice per group were pooled and were cultured in the presence of irradiated lymphocytes from BALB/c (host) and C57BL/6 (donor) mice, respectively. After 3 days of culture and the addition of 3H-thymidine during the last 16 hours, cells were harvested and incorporation of 3H-thymidine was determined in a β-counter. Background values were subtracted (cpm in the absence of an antigenic stimulus). Mean cpm ± SD of triplicate cultures are shown; significant differences between NTG and TG BMC (P ≤ .01) are indicated by an asterisk. The experiment was repeated two times showing similar results.
The efficiency of accelerated tolerance induction was further proven by analyzing the frequencies of host-reactive T cells after reconstitution of lethally irradiated mice with allogeneic BMC from TG and NTG animals (Table 4). As in the allogeneically reconstituted SCID mouse, frequencies of host-reactive lymphocytes decreased rapidly after reconstitution with BMC from TG mice. Although the question of how the TG product may have facilitated tolerance induction towards the host could not be answered definitively, there is evidence that supports a direct involvement of rCD44v4-v7 in the selection process. We tested in an experiment whether tolerance induction may be a consequence of contaminating T cells. This was not the case. When allogeneic mice were reconstituted with T- and B-cell–depleted BMC from TG mice, repopulation of the thymus and the periphery still proceeded in an accelerated fashion and the regaining of immunoreactivity was not influenced (data not shown). Instead, reconstitution differed only with respect to one aspect, ie, the frequency of host-reactive T cells early after reconstitution was reduced (Table 4). Thus, the rapid appearance of antihost reactivity may have depended on the few contaminating T cells in the inoculum, but this apparently was independent from the accelerated regaining of immunocompetence and tolerance induction. This assumption was also supported by the following experiment, in which lethally irradiated mice received a 9:1 mixture of BMC from NTG mice with T cells from TG mice. Repopulation was significantly impaired in mice receiving the mixture and they suffered from severe GVH reactions with rapid weight loss and greater than 30% lethality (data not shown). Importantly, the frequency of host-reactive T cells in peripheral lymphoid organs was by no means reduced after the transfer of the mixture of BMC from NTG and T cells from TG mice (Table 4). Hence, it can be concluded that accelerated responsiveness and rapidly decreasing host-reactivity after the transfer of BMC from TG mice relied on the emergence of newly maturing T cells.
Frequencies of Host-Reactive T Cells in the Lethally Irradiated, Allogeneic Host After Transfer of BMC From rCD44v4-v7-TG and NTG Mice
| . | . | Host-Reactive T Cells†: Frequency/106 Cells . | ||||
|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . |
| Organ . | Days After Reconstitution4-150 . | Transfer: BMC . | Transfer: BMC Without T and B . | Transfer: BMC Without T and B NTG Donor + TG T Cells . | ||
| . | . | NTG Donor . | TG Donor . | NTG Donor . | TG Donor . | . |
| BMC | 14 | 323 (564) | 333 [NS] (564) | 294 (510) | 323 [NS] (514) | 345 (48) |
| 21 | 1,084 (1,608) | 244‡ (364) | 1,350 (2,015) | 214‡ (332) | 1,280 (1,988) | |
| 28 | 448 (575) | 24‡ (319) | 454 (593) | 29‡ (39) | 440 (598) | |
| 42 | 17 (20) | 6‡ (7) | 16 (19) | 5‡ (6) | 19 (23) | |
| TC | 14 | 2 (8) | 15‡ (26) | 2 (7) | ND [NS] | ND |
| 21 | 302 (667) | 2,500‡ (4,082) | 240 (545) | 1,389‡ (2,130) | 320 (727) | |
| 28 | 743 (1,236) | 42‡ (61) | 400 (565) | 12‡ (18) | 370 (546) | |
| 42 | 20 (26) | 2‡ (3) | 15 (17) | 4‡ (5) | 29 (33) | |
| SC | 14 | 135 (365) | 345 [NS] (759) | 102 (304) | 22‡ (52) | 114 (374) |
| 21 | 1,087 (2,558) | 738 [NS] (1,175) | 1,250 (3,605) | 320‡ (451) | 1,043 (2,249) | |
| 28 | 565 (1,101) | 137‡ (203) | 555 (914) | 112‡ (156) | 355 (544) | |
| 42 | 99 (151) | 14‡ (18) | 64 (82) | 15‡ (20) | 53 (72) | |
| LNC | 14 | ND | 22‡ (40) | ND | 5 [NS] (10) | ND |
| 21 | 4,900 (1,225) | 5,000 [NS] (8,197) | 4,500 (10,638) | 2,000‡ (3,724) | 4,700 (13,277) | |
| 28 | 645 (1,187) | 122‡ (175) | 480 (714) | 143‡ (202) | 457 (774) | |
| 42 | 80 (123) | 10‡ (12) | 75 (114) | ND‡ | 78 (127) | |
| . | . | Host-Reactive T Cells†: Frequency/106 Cells . | ||||
|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . |
| Organ . | Days After Reconstitution4-150 . | Transfer: BMC . | Transfer: BMC Without T and B . | Transfer: BMC Without T and B NTG Donor + TG T Cells . | ||
| . | . | NTG Donor . | TG Donor . | NTG Donor . | TG Donor . | . |
| BMC | 14 | 323 (564) | 333 [NS] (564) | 294 (510) | 323 [NS] (514) | 345 (48) |
| 21 | 1,084 (1,608) | 244‡ (364) | 1,350 (2,015) | 214‡ (332) | 1,280 (1,988) | |
| 28 | 448 (575) | 24‡ (319) | 454 (593) | 29‡ (39) | 440 (598) | |
| 42 | 17 (20) | 6‡ (7) | 16 (19) | 5‡ (6) | 19 (23) | |
| TC | 14 | 2 (8) | 15‡ (26) | 2 (7) | ND [NS] | ND |
| 21 | 302 (667) | 2,500‡ (4,082) | 240 (545) | 1,389‡ (2,130) | 320 (727) | |
| 28 | 743 (1,236) | 42‡ (61) | 400 (565) | 12‡ (18) | 370 (546) | |
| 42 | 20 (26) | 2‡ (3) | 15 (17) | 4‡ (5) | 29 (33) | |
| SC | 14 | 135 (365) | 345 [NS] (759) | 102 (304) | 22‡ (52) | 114 (374) |
| 21 | 1,087 (2,558) | 738 [NS] (1,175) | 1,250 (3,605) | 320‡ (451) | 1,043 (2,249) | |
| 28 | 565 (1,101) | 137‡ (203) | 555 (914) | 112‡ (156) | 355 (544) | |
| 42 | 99 (151) | 14‡ (18) | 64 (82) | 15‡ (20) | 53 (72) | |
| LNC | 14 | ND | 22‡ (40) | ND | 5 [NS] (10) | ND |
| 21 | 4,900 (1,225) | 5,000 [NS] (8,197) | 4,500 (10,638) | 2,000‡ (3,724) | 4,700 (13,277) | |
| 28 | 645 (1,187) | 122‡ (175) | 480 (714) | 143‡ (202) | 457 (774) | |
| 42 | 80 (123) | 10‡ (12) | 75 (114) | ND‡ | 78 (127) | |
Abbreviations: NS, nonsignificant; ND, not detectable.
5 × 106 BMC, IV; where indicated, BMC were depleted of B and T cells as described in the Materials and Methods or BMC from NTG mice were depleted of B and T cells and substituted with mature T cells from rCD44v4-v7 TG mice (BMC:T cells = 9:1).
In parentheses is the frequency/106 cells corrected for the relative percentage of donor-derived lymphocytes.
P ≤ .01.
We interpret these findings in the sense that tolerance induction and the regaining of immunocompetence after the transfer of BMC from TG mice are linked phenomena that both depend on the maturation of the transplanted BMC in the host's thymus and are possibly a consequence of an accessory signal provided by rCD44v4-v7 during the process of positive selection.
Host antidonor reactivity after allogeneic reconstitution.Surprisingly, reactivity against donor lymphocytes, as determined in bulk cultures (Fig 6), as well as frequencies of donor-reactive lymphocytes also were lower after the transfer of BMC from TG mice (Table 5). This was also true when the minor differences in the percentage of host cells in mice reconstituted with BMC from TG or NTG animals was taken into account. One possible explanation for this finding could have been that BMC from TG mice contained potential veto cells for host lymphocytes. According to the outcome of the following two experiments (Table 5), this apparently was not the case. In one setting, BMC from TG mice were depleted of cells expressing the TG product. In the second setting, BMC from NTG mice were mixed with rCD44v4-v7+ BMC from TG mice. A veto mechanism by rCD44v4-v7+ BMC should have resulted in a higher percentage of donor-reactive T cells in the first experiment and in a lower one in the second. Instead, host antidonor reactivity was consistently lower after the transfer of BMC from TG mice, even when BMC were depleted of rCD44v6+ cells. On the other hand, antidonor reactivity was not significantly reduced when a mixture of BMC from NTG and rCD44v6+ BMC from TG mice was transferred.
Frequencies of Donor-Reactive T-Cells in the Lethally Irradiated, Allogeneic Host After Transfer of BMC From rCD44v4-v7-TG and NTG Mice
| Organ . | Days After Reconstitution5-150 . | Donor-Reactive T Cells†: Frequency/106 Cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Transfer: NTG BMC . | Transfer: TG BMC . | . | . | . | |||
| . | . | Unseparated . | Without T and B . | Without T and B + rCD44v6+ BMC . | Unseparated . | Without rCD44v6+ BMC . | . | . | . |
| BMC | 14 | ND | ND | ND [NS] | ND [NS] | ND [NS] | |||
| 21 | 93 (296) | 72 (212) | 120 [NS] (375) | 127 [NS] (358) | 56 [NS] (169) | ||||
| 28 | 424 (2,507) | 228 (1,562) | 500 [NS] (3,571) | 367 [NS] (1,782) | 255 [NS] (1,417) | ||||
| TC | 14 | 9 (26) | 7 (13) | 7 [NS] (16) | 10 [NS] (31) | 7 [NS] (14) | |||
| 21 | 37 (94) | 32 (97) | 34 [NS] (112) | 21 [NS] (121) | 13 [NS] (87) | ||||
| 28 | 37 (106) | 32 (91) | 15 [NS] (65) | 31 [NS] (265) | 17 [NS] (148) | ||||
| 42 | 63 (745) | 65 (619) | NT | 65-152 (85) | 4 [NS] (67) | ||||
| SC | 14 | ND | ND | ND [NS] | ND [NS] | ND [NS] | |||
| 21 | 149 (283) | 138 (317) | 164 [NS] (410) | 465-152 (158) | 50 [NS] (172) | ||||
| 28 | 1,852 (6,212) | 2,000 (6,667) | 833 [NS] (5,950) | 2865-152 (1,652) | 222 [NS] (1,586) | ||||
| 42 | 50 (345) | 81 (1,013) | 74 [NS] (1,088) | 85-152 (88) | 16 [NS] (134) | ||||
| LNC | 14 | ND | ND | ND [NS] | ND [NS] | ND [NS] | |||
| 21 | ND | ND | ND [NS] | ND [NS] | ND [NS] | ||||
| 28 | 167 (601) | 200 (500) | 111 [NS] (333) | 285-152 (116) | 31 [NS] (124) | ||||
| Organ . | Days After Reconstitution5-150 . | Donor-Reactive T Cells†: Frequency/106 Cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Transfer: NTG BMC . | Transfer: TG BMC . | . | . | . | |||
| . | . | Unseparated . | Without T and B . | Without T and B + rCD44v6+ BMC . | Unseparated . | Without rCD44v6+ BMC . | . | . | . |
| BMC | 14 | ND | ND | ND [NS] | ND [NS] | ND [NS] | |||
| 21 | 93 (296) | 72 (212) | 120 [NS] (375) | 127 [NS] (358) | 56 [NS] (169) | ||||
| 28 | 424 (2,507) | 228 (1,562) | 500 [NS] (3,571) | 367 [NS] (1,782) | 255 [NS] (1,417) | ||||
| TC | 14 | 9 (26) | 7 (13) | 7 [NS] (16) | 10 [NS] (31) | 7 [NS] (14) | |||
| 21 | 37 (94) | 32 (97) | 34 [NS] (112) | 21 [NS] (121) | 13 [NS] (87) | ||||
| 28 | 37 (106) | 32 (91) | 15 [NS] (65) | 31 [NS] (265) | 17 [NS] (148) | ||||
| 42 | 63 (745) | 65 (619) | NT | 65-152 (85) | 4 [NS] (67) | ||||
| SC | 14 | ND | ND | ND [NS] | ND [NS] | ND [NS] | |||
| 21 | 149 (283) | 138 (317) | 164 [NS] (410) | 465-152 (158) | 50 [NS] (172) | ||||
| 28 | 1,852 (6,212) | 2,000 (6,667) | 833 [NS] (5,950) | 2865-152 (1,652) | 222 [NS] (1,586) | ||||
| 42 | 50 (345) | 81 (1,013) | 74 [NS] (1,088) | 85-152 (88) | 16 [NS] (134) | ||||
| LNC | 14 | ND | ND | ND [NS] | ND [NS] | ND [NS] | |||
| 21 | ND | ND | ND [NS] | ND [NS] | ND [NS] | ||||
| 28 | 167 (601) | 200 (500) | 111 [NS] (333) | 285-152 (116) | 31 [NS] (124) | ||||
The compared populations (NTG BMC v TG BMC; NTG BMC without T and B cells v NTG BMC without T and B cells + CD44v6+ TG BMC; and TG BMC v TG BMC without CD44v6+ cells) are indicated by the horizontal brackets.
Abbreviations: NS, nonsignificant; ND, not detectable; NT, not tested.
5 × 106 BMC, IV; where indicated, BMC were depleted of B and T cells or of rCD44v6+ cells as described in the Materials and Methods or BMC from NTG mice were depleted of B and T cells and substituted with rCD44v6+ BMC from TG mice (NTG BMC:CD44v6+ TG BMC = 9:1).
In parentheses is the frequency/106 cells corrected for the relative percentage of host-derived lymphocytes.
P ≤ .01.
Thus, the transfer of BMC from TG mice promoted toleration of recovering host-derived T cells. Because CD44v6+ cells in the BM of TG mice did not display particular veto functions, toleration of the host towards the donor must have been acquired during the reconstitution process.
DISCUSSION
The hematopoietic isoform of CD44 is positively involved in the homing of BMC24-26,56 as well as in myelopoiesis and lymphopoiesis.23,57 Accordingly, syngeneic BMC engraftment will be delayed by anti-CD44s and allogeneic BMC engraftment may even be prevented by anti-CD44s (Zöller et al, manuscript submitted). On the other hand, there is evidence that CD44 variant isoforms modulate immune responsiveness (ie, a CD44v6-specific antibody had been described to interfere with T- and B-cell responses41 ) and that rCD44v4-v7 TG mice, which express the transgene on Thy1.2+ cells, are characterized by accelerated responsiveness.43 The latter finding prompted us to evaluate whether constitutive expression of a CD44 variant isoform could be favorable considering the regaining of immunocompetence and, if so, whether this may in a positive or negative manner influence GVH reactivities. The regaining of immunocompetence was indeed accelerated after the transfer of BMC from mice constitutively expressing rCD44v4-v7 on Thy1.2+ cells and GVH reactivities vanished already a few weeks after reconstitution.
BMC from rCD44v4-v7 TG mice homed into the bone marrow at a frequency comparable to those from NTG mice, but had a slight advantage in homing into the spleen, whereas BMC from neither TG nor NTG mice homed directly in the thymus. Nonetheless, repopulation of the thymus was the earliest parameter in which BMC from TG and NTG mice differed, ie, during the first weeks after transplantation, higher numbers of thymocytes were recovered after the transfer of BMC from TG mice. Because expression of the transgene product is mainly restricted to Thy1.2+ cells, a proliferation advantage during intrathymic maturation is more plausible than a facilitated homing into the thymus.
Repopulation of the thymus was accompanied by an earlier regaining of immune responsiveness in animals receiving BMC from TG as compared with animals receiving BMC from NTG mice. This was a first indication that expression of the TG product may facilitate T-cell maturation in the host's thymic environment. The observation that, during ontogeny, thymocytes are brightly CD44v6+58 supports the hypothesis that expression of CD44v6 may be of functional importance during the intrathymic selection processes. There are several possibilities whereby the TG product could have facilitated the expansion of thymocytes. It could have functioned as a cytokine receptor59-66 or as an accessory molecule27-32 34-37 actively involved in the process of positive selection. In the latter case, one would expect, eg, a significantly increased Ca2+ influx and elevated Ca2+ levels for a prolonged period of time after cross-linking of CD3 and CD44v6 on the thymocytes of TG mice. Because this is the case (N. Föger, unpublished data), we favor the latter hypothesis and are currently trying to elucidate the possible pathway of CD44v6-mediated signal transduction during T-cell selection in the thymus.
Early after the transfer of BMC from TG mice we noted a significant level of GVH reactivity. This was partly due to contaminating T cells, because the frequency of host-reactive T cells was reduced when T-cell–depleted BMC were transferred. Nonetheless, even after T-cell depletion, responsiveness was observed rapidly and GVH reactivity also decreased steeply between 2 and 3 weeks after reconstitution. According to the following observations, the rapid loss of antihost reactivity after the transfer of BMC from TG mice is likely based on the induction of central tolerance. (1) The same phenomenon was noted in the SCID and the allogeneic BALB/c mice, the former excluding that the decline in host-reactivity might have been shadowed by host-versus-graft reactions. (2) LD analysis showed a steep decrease in the frequency of host-reactive T cells in the thymus and the spleen between 3 and 4 weeks and in the lymph nodes between 4 and 6 weeks after transplantation of BMC from TG mice. (3) Tolerance induction was by no means accelerated in the allogeneic host receiving BMC from NTG mice together with mature T cells from TG mice. Instead, mice developed severe GVH reactions that were lethal in greater than 30% of the animals. Thus, it appears that both phenomena, the regaining of immunocompetence and the loss of host-reactivity, are linked to the wave of TG donor T cells maturing in the host's thymic environment. As mentioned above, we are currently elaborating on the function of CD44v6 expression during the process of positive selection. Furthermore, although likely, it still remains to be proven that positively selected, host-reactive thymocytes are subsequently subject of negative selection, possibly by activation induced cell death.67 68
Maturation of BMC from TG mice apparently was completed before the host's immune system had recovered. This was demonstrated by the low responsiveness against donor lymphocytes in bulk cultures. In the thymus, antidonor reactivity remained low throughout the observation period. In the spleen, although some reactivity was noted 3 and 4 weeks after transplantation of BMC from TG mice, antidonor reactivities were diminished as compared with animals receiving BMC from NTG mice. The reduced level of antidonor reactivity was partly due to the decreased numbers of host lymphocytes in animals reconstituted with BMC from TG mice. This could have been due to the prior occupancy of space by donor lymphocytes.69 More difficult to explain is the observation that, even after correction for the percentage of host-derived lymphocytes, the frequencies of donor-reactive T cells were lower after the transfer of BMC from TG than from NTG mice. One possible explanation could have been that immature host-derived T cells may have been confronted more frequently with donor lymphocytes after reconstitution with BMC from TG than from NTG mice. The findings that differences in the frequencies of donor-reactive T cells were more striking in the periphery and more pronounced 4 and 6 weeks (as compared with 2 and 3 weeks) after reconstitution would support the hypothesis of an activation-induced cell death of host-derived maturing T cells in a thymic environment presenting the allogeneic TG T cells in large excess. Alternatively, BMC from TG mice could have been more efficient in exerting veto functions.70,71 This possibility was excluded by the observation that neither depletion of rCD44v4-v7+ BMC nor their addition to BMC from NTG mice had any major effect on the appearance of donor-reactive host cells. Nonetheless, it cannot be excluded by the finding that newly emerging T cells efficiently vetoed donor-reactive host cells. Taken together, although we could exclude a veto function of rCD44v4-v7+ BMC, it remains to be explored whether the higher efficiency of TG donor cells was a consequence of their more rapid expansion or reflected an active involvement of rCD44v4-v7+ T cells in death signaling.68
Up to this point, our interest focused on T-cell recovery, toleration, and responsiveness, because the TG product is predominantly expressed on thymocytes and peripheral T cells. Nonetheless, three additional findings should be mentioned that were independent of the T-cell lineage. (1) To our surprise, the small population of BMC expressing the TG product was CD43+ and only partly Thy1+. There is no simple explanation for this expression pattern. It may have been a consequence of the integration site of the TG into the genome or it may have been directed by the ε enhancer or by both. (2) Transiently, a reduced number of B cells was recovered in the spleen of mice that received BMC from TG mice and were treated with anti-rCD44v6. (3) Particularly at 6 weeks after reconstitution, the proliferative response of SC from these animals was reduced. Both the second and third T-cell–unrelated phenomena may be linked to the first one. If BMC expressing the TG product belong (partly) to the B-cell lineage, which would be in line with the profile of surface markers, it could well be that the antibody inhibited directly or by steric hindrance a B-cell progenitor-stroma interaction that facilitates B-cell maturation. This could have led to reduced numbers of IgM+ cells in the spleen as well as to reduced proliferative activity, simply as a consequence of a delay in B-cell maturation.
Transplantation of allogeneic BMC or peripheral stem cells has several advantages.1,72-74 However, host-versus-graft and GVH reactivities75-78 as well as delayed regeneration and immunoincompetence can contribute to morbidity and mortality in the patients undergoing transplantation.79-82 Thus, functional maturation of T cells, besides of BMC homing and expansion of stem cells/progenitor cells, is of major concern in BMC transplantation. In this respect, allogeneic mice receiving a transplant of BMC from rCD44v4-v7 TG mice had a clear advantage. Although no major influence of the TG product on seeding of BMC was noted, expansion, functional maturation, and toleration of TG-donor–derived T cells was accelerated, whereby expedited repopulation, regaining of immunocompetence, and decreasing GVH reactivity are possibly consequences of a CD44v6-mediated second signal during the positive selection process.
Although the model of a rCD44v4-v7 transgene has been advantageous in differentiating the transgene product from endogenous murine CD44 variant isoforms, it also has its limitations due to its heterologous nature and the arbitrarily selected combination of constitutively expressed CD44 variant exons. Because establishment of TG mice covering the whole array of potentially important CD44 variant exon combinations is not feasible, we are currently verifying the function of CD44 variant isoforms in BMC transplantation by the transfer of retrovirally infected BMC.
ACKNOWLEDGMENT
Nicole Föhr and Karin Herrmann are thanked for invaluable help.
Supported by the Deutsche Forschungsgemeinschaft, Grant No. Zo40/5-1 (to M.Z.) and the FSP Transplantation, Heidelberg (to M.Z.).
Address reprint requests to Margot Zöller, MD, Department of Tumor Progression and Immune Defense, German Cancer Research Center, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal