Abstract
Ferritin (Ft) plays an important role in cellular iron metabolism. It can store substantial amounts of iron in a nontoxic soluble form. However, its ability to donate iron for cellular needs, in particular for hemoglobin (Hb) synthesis in human erythroid cells, is still controversial. We studied the role of intracellular Ft-iron in Hb synthesis and the involvement of lysosomal proteolysis in iron release from Ft. Ft-iron release and its subsequent incorporation into heme was investigated in normal human erythroid precursors developing in culture. Dual staining flow cytometry with antibody (Ab)-specific for Ft and for Hb showed a decrease in cellular Ft content in erythroid cells during their maturation. Cellular Ft-iron participation in heme synthesis was studied by labeling cells with 59Fe. Cells were incubated with 59Fe-labeled human diferric transferrin (Tf), then chased, and intracellular radioiron distribution between Ft and Hb was determined on subsequent days by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and/or Ft immunoprecipitation and heme extraction. On day 6, most of the 59Fe accumulated in Ft. Thereafter, a progressive decrease of radioiron in Ft and a corresponding increase of the label in Hb was observed. Inhibition of heme synthesis with succinylacetone caused radioiron to remain in Ft and prevented its redistribution. Addition of unlabeled diferric Tf to the culture medium did not prevent radioiron from appearing in Hb. Chloroquine repression of lysosomal function prevented radio-iron redistribution between Ft and Hb. Inhibition of proteolysis by chymostatin and/or leupeptin led to Ft-protein accumulation in the cells and also prevented radioiron transfer from Ft to Hb. The results of the present study suggest that intracellular Ft donates iron for heme synthesis and that proteolytic Ft degradation in a lysosmal-like compartment is necessary for iron release and its transfer to heme.
IRON IS AN ESSENTIAL element for all living cells. However, its tendency to catalyze free-radical formation makes free iron potentially cytotoxic. Furthermore, the amount of iron in cells is far above the solubility, and it is necessary to concentrate the excessive iron and to keep it soluble. Storage of excessive cytoplasmic iron in soluble and nontoxic form is performed in many cell types by ferritin (Ft).
Isoferritins (iso-Fts) are heteropolymers, containing different combinations of heavy (H) and light (L) type subunits of about 21 kD and 19 kD molecular mass, respectively; iso-Fts rich in H-type subunits have a lower pI (acid iso-Fts) than iso-Fts composed predominantly of L-type subunits (basic iso-Fts). The Ft protein shell surrounds a central iron core containing up to 4,500 iron atoms.1-3
Developing erythroid cells take up substantial amounts of iron, predominantly for heme synthesis. Iron that is in excess of immediate cell requirements is stored within Ft.4 Several studies relate to the possibility that Ft-iron participates in heme synthesis.5-13 Thus, isolated rat mitochondria were reported to use iron from added Ft for the ferrochelatase reaction.6 There are indications for a gradual decrease in cellular Ft content during erythroid cell maturation; that is, immature erythroblasts have been shown to contain more Ft than mature erythrocytes,7-11 suggesting that Ft in erythroid cells is degraded and donates its iron for heme synthesis. In murine erythroleukemic cells, some of the Ft-iron was found in hemoglobin (Hb) following induction of Hb synthesis,12 and radio-iron previously accumulated in bullfrog tadpole red cell Ft was found later in the adult Hb.13 However, attempts to demonstrate the participation of intracellular Ft-iron in heme synthesis with rabbit and rat reticulocytes were unsuccessful.14 15
The important role of Ft in erythroid cell iron metabolism is highlighted by the tight regulation of its synthesis by the cellular iron status as part of an intricate mechanism of posttranscriptional coordinated regulation of iron acquisition, storage, and use. The biosynthesis of Ft, as well as of erythroid δ-aminolevulonic acid synthase (e-δ-ALAS), the key enzyme in the heme synthesis pathway, are regulated on the molecular level through the translation of their mRNA. Their mRNA contain on the 5′ untranslated region a specific stem-loop structure, the iron responsive element (IRE), which reversibly binds iron regulatory protein (IRP). Low cellular iron levels activate IRP, causing it to bind to the IRE and inhibit translation. When iron levels increase, IRP is inactivated; it does not bind to the IREs allowing for continued translation. Transferrin receptor (TfR) expression is regulated through the stability of its mRNA. This mRNA contains five IRE structures similar to those of the Ft mRNA, however these IREs are located on the 3′ end of the mRNA. Activation of the IRP by low cellular iron levels causes it to bind to the IREs and renders the TfR mRNA inaccessible for RNAse, thus stabilizing it and allowing for continued translation. When iron stores increase, the IRP is inactivated, resulting mRNA degradation and consequently decreased TfR expression.16-19
In the present study, we followed the fate of intracellular Ft-iron and explored the potential of intracellular Ft to function as an iron donor for heme synthesis and investigated the mechanisms involved. For this purpose, we used the two-phase liquid culture procedure for growing normal human erythroid progenitors, which supports the proliferation, differentiation, and maturation of erythroid committed progenitors into orthochromatic normoblasts.20 21
Using immunofluorescence flow cytometry, we followed the temporal relationship between intracellular Ft content and the developmental stage of erythroid cells. The results demonstrated a decline in intracellular Ft content concomitant with erythroid maturation.
With radioactive iron, we followed the ability of erythroid cells to use Ft-iron for heme and Hb synthesis. The results showed that at the pre-Hb production stage, iron was taken up and accumulated mainly in Ft. Following maturation, it was gradually removed from Ft and appeared in Hb. Succinylacetone inhibition of heme synthesis prevented radioiron transfer from Ft to Hb. Inhibition of lysosomal functions by chloroquine or inhibition of intracellular proteases by chymostatin and/or leupeptin caused the radio-iron to remain in Ft and prevented its redistribution. These results suggest that at early stages of erythroid maturation, iron taken up via the Tf pathway, accumulates within the cells, mainly in Ft. Then, during the stage of intense Hb synthesis, intracellular Ft is gradually degraded, in a process that requires lysosomal proteolytic activity, and its iron is used for heme synthesis.
MATERIALS AND METHODS
Erythroid cell cultures.Blood was obtained from normal volunteers. The two-phase liquid culture was employed as previously described.20 21 Briefly, mononuclear cells were isolated from peripheral blood samples by Ficoll-Hypaque density gradient centrifugation and seeded in alpha-minimal essential medium (α-MEM) supplemented with 10% fetal calf serum (FCS; both from GIBCO, Grand Island, NY), 1 μg/mL cyclosporin A (Sandoz, Basel, Switzerland), and 10% conditioned medium from 5637 bladder carcinoma cell cultures, containing different hematopoietic growth factors not including eryhtropoietin (EPO). The cultures were incubated at 37°C, under an atmosphere of 5% CO2 in air with extra humidity. After a 7-day incubation in this phase-I culture, the nonadherent cells were harvested, washed, and recultured in fresh medium composed of α-MEM, 30% FCS, 1% deionized bovine serum albumin, 10−5 mol/L β-mercaptoethanol, 15 mmol/L glutamine, 10−6 mol/L dexamethasone, and 1 U/mL human recombinant EPO (Ortho Pharmaceutical Co, Raritan, NJ). This part of the culture is referred to as phase II. After 5 days of incubation, erythroblasts were purified by centrifugation on 45% Percol (Pharmacia, Uppsala, Sweden, density 1.0585 g/mL). The upper layer, containing mainly proerythroblasts and basophilic normoblasts, was collected, washed, and resuspended in the original medium for future incubation. Cell samples were analyzed usually between the sixth and twelfth days of phase II. Viability of the cells was determined by trypan blue exclusion and was higher than 95%. Maturation was confirmed by cell morphology, which was assessed microscopically on cytocentrifuge-prepared slides after May-Grunwald-Giemsa staining. The majority of erythroid cells, on day 6 of phase II, were basophilic normoblasts, on days 8 to 10, polychromatophilic normoblasts, and on day 12, orthochromatic normoblasts.
Preparation of cell lysates.Cells were harvested, washed, and lysed on ice in solubilization buffer containing 1% Triton X-100 (Pierce, Rechovot, Israel), aprotinin 10 μg/mL and leupeptin 10 μg/mL (both from Boehringer, Mannheim, Germany), benzamidine 10 μg/mL, N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) 3.7 μg/mL, N-tosyl-L-lysine chloromethyl ketone (TLCK) 3.7 μg/mL, pepstatine 1 μmol/L and phenylmethylsulphonyl fluoride (PMSF) 0.25 mmol/L and sodium azide 0.02% (all from Sigma-Israel, Cholon, Israel) in Tris-HCl 10 mmol/L, pH 7.4. The lysates were centrifuged at 10,000xg for 10 minutes, and the supernatants were collected and stored at −80°C until use.
Preparation of rabbit antihuman-Ft serum.Human Ft was isolated from human term placenta as previously described (subunit ratio of H-and L-subunits is about 1:3).22 The purity of the Ft was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) without heating; nonreducing conditions were used. When subunits were separated by SDS-PAGE, reducing conditions and heating were used. Antibodies (Ab) to this Ft were prepared in New Zealand rabbits (body weight 2.5 to 3.0 kg). They were injected with 1.5 to 2.0 mg of the human Ft solubilized in complete Freund's adjuvant. After 30 days, they were given a booster of 1.0 to 1.5 mg Ft in incomplete Freund's adjuvant. The rabbits were bled 7 days after the second injection. The titer of the antiserum obtained was tested by double immunodiffusion in agar. The serum was centrifuged at 10,000xg for 10 minutes and stored at −20°C.
Ft determination by enzyme-linked immunosorbent assay (ELISA).The concentration of total cellular Ft was measured by ELISA using antitotal-placental–Ft-Ab containing Ab against L- as well as H-Ft subunits. Placental Ft-Ab were affinity purified from the immune serum on agarose-immobilized total-placental-Ft, and coupled to β-galactosidase (obtained from Boehringer-Manheim) as previously described.23 Optimal working concentrations for the ELISA reagents were found by checkerboard titration.
Two-color flow cytometric analysis.Cultured cells were washed, fixed in 3% paraformaldehyde for 1 hour, and the membranes were permeabilized with saponin-containing buffer (phosphate-buffered saline [PBS] containing 0.1% saponin, 1% glycine, (both from Polysciences, Warrington, PA) and 2% human albumin (Kamada, Ltd, Kibutz Beth-Kama, Israel). The cells were then incubated with antihuman placental Ft rabbit serum for 1 hour, washed, and incubated simultaneously with fluorescein isothiocyanate (FITC)-labeled goat antirabbit IgG (Sigma-Makor, Rehovot, Israel) and phycoerythrin-labeled monoclonal Ab to human hemoglobin-A (Isolab, Akron, OH). After a 30-minute incubation, the cells were washed and submitted to flow cytometry. Nonimmune rabbit serum was used for control.
The cells were analyzed using a FACStarplus flow cytometer (Becton-Dickinson, Immunofluorometry Systems, Mountain View, CA). Cells were passed at a rate of 1,000 cells/second through a 70-mm nozzle, using saline as the sheath fluid. A 488-nm argon laser beam at 250 mW served as the light source for excitation. Green (FITC-derived) fluorescence was measured using a 530 ± 30 nm band-pass filter and red (phycoerythrin-derived) fluorescence using a 575 ± 26 nm band-pass filter. At least 104 cells were analyzed.
Preparation of 59Fe-labeled human diferric Tf (hdTf).Apo-Tf was labeled with 59Fe by incubating 100 μmol/L human apo-Tf (Kamada, Israel) with a small excess (210 μmol/L) of 59FeCl3 (du Pont, NEN, Dreieich, Germany) in 50 mmol/L Tris-HCl buffer, pH 7.4 for 2 hours at room temperature and then overnight at 4°C. The solution was subsequently passed through a Chelex-100 or Bio-Gel 6 DG (Bio-Rad, Hercules, CA) column to remove residual low molecular weight iron, and the purified radiolabeled Tf was sterilized by filtration.
Ft immunoprecipitation and 59Fe-Ft determination.For Ft immunoprecipitation, carrier human placental Ft was added to cell lysates containing 1% Triton x-100, followed by a saturating titer of antihuman placental Ft rabbit serum. The mixture was incubated for 1 hour at 37°C and then overnight at 4°C. The immunoprecipitate was then centrifuged, washed three times, and counted.
Labeling of cells with 59Fe.Cells were incubated with 1 μmol/L 59Fe-labeled hdTf (7.44 kBq or 0.2 μCi per mL) during the first 6 days in phase II. The cells were then harvested, washed three times with PBS, and reincubated in radioactive-free medium supplemented with 30% FCS and 1 μmol/L hdTf. On days 6, 8, and 10, cell aliquots were washed, lysed, and analyzed.
Heme extraction and 59Fe-heme determination.Heme was extracted as described by Thunell.24 A total of 0.5 mL of 1 N HCl was added to 0.2 mL of cell lysate and extracted twice with 0.5 mL ethylacetate:acetic acid (3:1 vol/vol) mixture. The organic extracts were pooled and their 59Fe-radioactivity was measured.
SDS-PAGE and autoradiography.Cell lysates were submitted to SDS-PAGE on 6% (T) gels under nonreducing conditions and without heating the samples. The gels were subsequently dried and exposed to phosphor-imaging and/or autoradiography.
RESULTS
Changes in cellular Ft content during erythroblast development.Ft content of cells at different stages of the culture was assessed by immunofluorescence flow cytometry. Cells were harvested, washed, fixed, and simultaneously stained with antihuman Ft rabbit serum followed by FITC-conjugated antirabbit IgG and with phycoerythrin-conjugated antihuman HbA monoclonal antibodies. The staining procedure was performed in saponin-containing buffer, permitting membrane permeabilization and penetration of the Ab into the cell.
The results (Fig 1) show that almost the entire Hb-positive (erythroid) cell population stained positive for Ft on day 6 of phase II, when the cells were mainly basophilic normoblasts (determined by morphology). A significant proportion of the Hb-positive population was Ft-negative on day 12, when the majority of the cells became orthochromatic normoblasts. Thus, a decline in intracellular Ft content during erythroid maturation was shown.
Flow cytometric analysis of ferritin content in cultured erythroid cells. Erythroid precursors were grown according to the two-phase liquid culture procedure. On different days of phase II (EPO-dependent phase, see Materials and Methods), cells were stained for ferritin with antihuman placental ferritin rabbit serum and antirabbit IgG labeled with FITC and for Hb with phycoerythrin-labeled antihuman HbA monoclonal Ab. Controls were stained with rabbit nonimmune serum. After 6 days in phase II, 93% of Hb-positive (erythroid) cells were also positive for ferritin (upper right quadrant) and only 7% of Hb-positive cells were ferritin-negative (upper left quadrant). After 12 days in phase II, 34% of the Hb-positive population became ferritin-negative. The plots shown are representative of three separate experiments.
Flow cytometric analysis of ferritin content in cultured erythroid cells. Erythroid precursors were grown according to the two-phase liquid culture procedure. On different days of phase II (EPO-dependent phase, see Materials and Methods), cells were stained for ferritin with antihuman placental ferritin rabbit serum and antirabbit IgG labeled with FITC and for Hb with phycoerythrin-labeled antihuman HbA monoclonal Ab. Controls were stained with rabbit nonimmune serum. After 6 days in phase II, 93% of Hb-positive (erythroid) cells were also positive for ferritin (upper right quadrant) and only 7% of Hb-positive cells were ferritin-negative (upper left quadrant). After 12 days in phase II, 34% of the Hb-positive population became ferritin-negative. The plots shown are representative of three separate experiments.
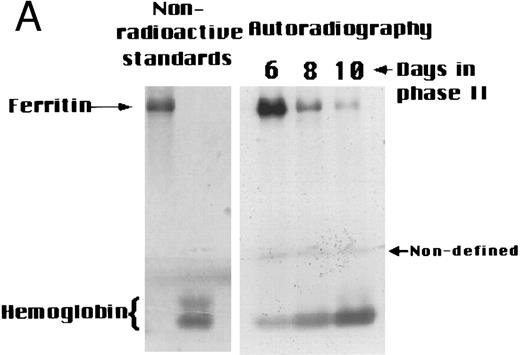
Changes in intracellular radioiron distribution during maturation of erythroid precursors.The above results raise the possibility that in erythroid precursors intracellular Ft undergoes gradual degradation during maturation and thereby provides its iron for heme synthesis and other cellular needs. To test this hypothesis, cultured cells were labeled with radioiron at a prehemoglobin stage, then chased with nonradioactive medium, and the distribution of their radioiron between Ft and Hb during maturation was followed. On days 6, 8, and 10, cell samples from equal culture volumes were taken, lysed, and applied to a 6% nonreducing SDS-PAGE gel followed by autoradiography and/or phosphor-imaging (Fig 2A). A gradual increase of 59Fe in Hb concurrent with a decrease of 59Fe in Ft was observed. Densitometric analysis of the images of three independent experiments with cells originating from three different donors demonstrate (Fig 2B) that on day 6, 83% ± 9% of the radiolabel was found in Ft and 11% ± 8% in Hb. On days 8 and 10, 52% ± 25% and 30% ± 22% were found in Ft and 37% ± 20% and 61% ± 19% in Hb, respectively, suggesting that the iron in Hb originated from Ft. Despite the relative large standard deviation, examination of the individual cultures from these donors showed a common trend: a decrease in 59Fe-Ft and a coordinated increase in 59Fe-Hb during maturation. A band of radioactivity representing an undefined iron binding protein, which migrated behind the Hb band and faster than the Ft band represented between 5% to 11% of the cellular radioactivity on the gel. This band did not change during erythroid maturation (Fig 2C).
Changes in intracellular radio-iron distribution during erythroid cell development. Three individual cultures were established from cells derived from different donors. Cells were labeled with 59Fe-human–diferric-transferrin during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed and reincubated in fresh nonradioactive medium. On days 6, 8, and 10, cell samples from equal culture volumes were taken, lysed, and applied to a 6% nonreducing SDS-PAGE gel. Human placental ferritin and human Hb (normal erythrocyte cell lysate) were used for markers. The markers were visible without staining. (A) Autoradiography of 59Fe-containing proteins of one representative culture. (B) Quantitative analysis by densitometry (mean ± SD of three cultures). (C) Quantitative analysis by densitometry of each individual culture.
Changes in intracellular radio-iron distribution during erythroid cell development. Three individual cultures were established from cells derived from different donors. Cells were labeled with 59Fe-human–diferric-transferrin during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed and reincubated in fresh nonradioactive medium. On days 6, 8, and 10, cell samples from equal culture volumes were taken, lysed, and applied to a 6% nonreducing SDS-PAGE gel. Human placental ferritin and human Hb (normal erythrocyte cell lysate) were used for markers. The markers were visible without staining. (A) Autoradiography of 59Fe-containing proteins of one representative culture. (B) Quantitative analysis by densitometry (mean ± SD of three cultures). (C) Quantitative analysis by densitometry of each individual culture.
Addition to the cell culture of 2.5 or 5.0 μmol/L hdTf did not affect the redistribution of 59Fe from Ft to Hb (results not shown).
Effect of inhibiting heme synthesis on intracellular radioiron distribution.To corroborate that Ft-iron is conveyed to Hb and to rule out the possibility that the decrease in intracellular 59Fe-Ft is caused by release of 59Fe-Ft from the cells, we analyzed cellular radioiron distribution following inhibition of heme synthesis. Cells were labeled with 59Fe for 6 days, and following washings as described above, incubated in fresh radioactive-free medium supplemented with serum, nonradioactive hdTf, and 0.5 mmol/L succinylacetone. The latter is an inhibitor of δ-aminolevulinic acid dehydratase, the second enzyme in the heme biosynthetic pathway.25 On different days, cells were harvested, lysed, and the lysates submitted to SDS-PAGE. The results (Fig 3A) confirmed that in cells without succinylacetone (control), there was redistribution of 59Fe from Ft to Hb from day 6 to day 10 of phase II. Treatment of the cells with succinylacetone prevented the accumulation of 59Fe in Hb and it remained in Ft. The results obtained with SDS-PAGE were corroborated by immunoprecipitation of the Ft and extraction of heme from the succinylacetone-treated cells and measuring their 59Fe radioactivity (Fig 3B). About 90% of the total cellular radioiron was recovered in Ft precipitates or extracted with heme, circa 10% was not.
Effect of inhibition of heme synthesis on intracellular radio-iron distribution during erythroid development. Cells were labeled with 59Fe-human–diferric transferrin during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed, and reincubated in fresh nonradioactive medium with or without 0.5 mmol/L succinylacetone. (A) Analysis by SDS-PAGE. Equal amounts of cell lysate radioactivity were applied to each lane of a 6% nonreducing SDS-PAGE gel followed by phosphor-imaging. The figure represents a typical result of four independent experiments from four different donors. (B) Analysis by immunoprecipitation and heme extraction. Ferritin was immunoprecipitated from cell lysates with antihuman placental ferritin rabbit serum. Heme was extracted with an ethylacetate-acetic acid (3:1 vol/vol) mixture. The results shown are from cultures of two different normal donors. Both experiments were performed in duplicate.
Effect of inhibition of heme synthesis on intracellular radio-iron distribution during erythroid development. Cells were labeled with 59Fe-human–diferric transferrin during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed, and reincubated in fresh nonradioactive medium with or without 0.5 mmol/L succinylacetone. (A) Analysis by SDS-PAGE. Equal amounts of cell lysate radioactivity were applied to each lane of a 6% nonreducing SDS-PAGE gel followed by phosphor-imaging. The figure represents a typical result of four independent experiments from four different donors. (B) Analysis by immunoprecipitation and heme extraction. Ferritin was immunoprecipitated from cell lysates with antihuman placental ferritin rabbit serum. Heme was extracted with an ethylacetate-acetic acid (3:1 vol/vol) mixture. The results shown are from cultures of two different normal donors. Both experiments were performed in duplicate.
Effect of inhibiting lysosomal function and protein degradation on intracellular radioiron distribution and Ft accumulation. To examine the involvement of lysosomes in the process of iron release from Ft and its transfer to heme, 59Fe-labeled cells were treated with chloroquine, a weak base known to inhibit lysosomal functions by raising pH in the lysosomal compartment.26 Chloroquine was used at concentrations of 5 to 20 μmol/L. At these concentrations, chloroquine showed little or no toxic effects and the cells survived and proliferated. Three days after addition of chloroquine, cells were harvested, lysed, and the radioactivity in heme and in Ft was determined after Ft immunoprecipitation and heme extraction. Other cultures grown under the same experimental conditions were analyzed by SDS-PAGE followed by phosphor-imaging. Iron transfer from Ft to heme was inversely related to the concentration of chloroquine: the higher the concentration of chloroquine the greater the fraction of intracellular 59Fe that remained in Ft and, thus, less was incorporated in heme (Fig 4A and B).
Effect of chloroquine on intracellular radioiron distribution. Cells were labeled with 59Fe-labeled human diferric transferrin during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed, and reincubated in fresh nonradioactive medium containing different concentrations of chloroquine. On day 9 of phase II, cells were harvested and lysed. (A) Results obtained by ferritin immunoprecipitation and heme extraction of three individual experiments obtained from cultures of three different normal donors. (B) Cell lysates were separated by SDS-PAGE followed by phosphor-imaging. Human placental ferritin and Hb were used as standards (not shown). Lane 1, 6 days in phase II, before treatment. Lanes 2 to 5, 9 days in phase II, chloroquine at 0, 5, 10, and 15 μmol/L, respectively.
Effect of chloroquine on intracellular radioiron distribution. Cells were labeled with 59Fe-labeled human diferric transferrin during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed, and reincubated in fresh nonradioactive medium containing different concentrations of chloroquine. On day 9 of phase II, cells were harvested and lysed. (A) Results obtained by ferritin immunoprecipitation and heme extraction of three individual experiments obtained from cultures of three different normal donors. (B) Cell lysates were separated by SDS-PAGE followed by phosphor-imaging. Human placental ferritin and Hb were used as standards (not shown). Lane 1, 6 days in phase II, before treatment. Lanes 2 to 5, 9 days in phase II, chloroquine at 0, 5, 10, and 15 μmol/L, respectively.
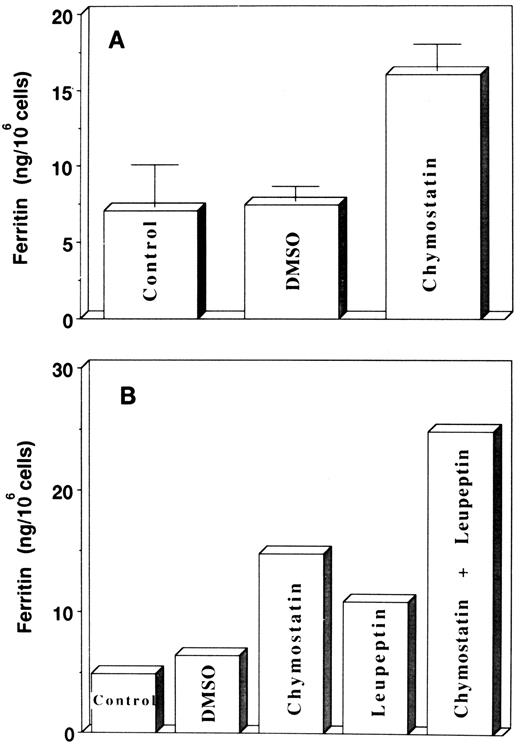
The inhibition by chloroquine of radioiron transfer from Ft to heme suggests that lysosomal-like organelles are involved in the release of iron from Ft and eventually its transfer to heme. The question remains: is iron released from intact Ft or is Ft degraded by lysosomal proteases and as a result iron is released and becomes available for heme synthesis? The protease inhibitors chymostatin and leupeptin were used to resolve this question. Chymostatin is a reversible serine and cysteine protease inhibitor, and leupeptin is a reversible inhibitor of trypsin-like and cysteine proteases. Chymostatin was dissolved in dimethylsulfoxide (DMSO) and added to cell cultures at a concentration of 200 μg/mL on day 7 or 8 of phase II. The following day, the same quantity of chymostatin was added again. DMSO was added to cultures in parallel flasks, which served as controls. On day 10 or 11, cells were harvested, lysed, and Ft content was determined by ELISA. The results (Fig 5A) showed that there was a 2.1-fold increase in cellular Ft content after treatment with chymostatin. In experiments presented in Fig 5B, the cells were treated with 10 μg/mL leupeptin, 200 μg/mL chymostatin, or both inhibitors together. Leupeptin alone caused a 2.2-fold increase in cellular Ft content, chymostatin led to a 2.3-fold increase, and both inhibitors together caused a 3.9-fold increase in cellular Ft content (Fig 5B). These results suggest that inhibition of lysosomal proteases leads to a decrease in Ft-protein degradation.
Effect of protease inhibitors on ferritin accumulation in cultured cells. (A) Effect of chymostatin. Chymostatin (200 μg/mL) was added at days 7 or 8 of phase II (EPO-dependent phase, see Materials and Methods), after 24 hours the same amount of chymostatin was added again. The cells were harvested at days 10 or 11 and the ferritin concentrations in the lysates were determined by ELISA. Results are expressed as mean ± SD of three individual experiments performed in duplicate. (B) The effect of chymostatin and leupeptin. Leupeptin (10 μg/mL) was added together with chymostatin (added as above).
Effect of protease inhibitors on ferritin accumulation in cultured cells. (A) Effect of chymostatin. Chymostatin (200 μg/mL) was added at days 7 or 8 of phase II (EPO-dependent phase, see Materials and Methods), after 24 hours the same amount of chymostatin was added again. The cells were harvested at days 10 or 11 and the ferritin concentrations in the lysates were determined by ELISA. Results are expressed as mean ± SD of three individual experiments performed in duplicate. (B) The effect of chymostatin and leupeptin. Leupeptin (10 μg/mL) was added together with chymostatin (added as above).
In another experiment, 59Fe-labeled cells were chased and then reincubated at day 6 of phase II in fresh, radioactive-free medium, supplemented with 1 μmol/L hdTf and the protease inhibitors chymostatin (200 μg/mL) and leupeptin (20 μg/mL). Samples of the cultures were harvested after 2 and 4 days and analyzed by SDS-PAGE. The results (Fig 6) demonstrate that in treated cells 59Fe remained in Ft and its transport to Hb was inhibited.
Effect of protease inhibitors on cellular radio-iron redistribution. Cells were labeled with 59Fe-labeled human diferric Tf during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed, and reincubated in fresh radioactive-free medium and treated with chymostastin (200 μg/mL) and leupeptin (20 μg/mL). At days 8 and 10, cells were harvested, lysed, and analyzed by SDS-PAGE followed by phosphor-imaging. The figure is representative of two independent experiments with cells obtained from two donors.
Effect of protease inhibitors on cellular radio-iron redistribution. Cells were labeled with 59Fe-labeled human diferric Tf during the first 6 days of phase II (EPO-dependent phase, see Materials and Methods), then washed, and reincubated in fresh radioactive-free medium and treated with chymostastin (200 μg/mL) and leupeptin (20 μg/mL). At days 8 and 10, cells were harvested, lysed, and analyzed by SDS-PAGE followed by phosphor-imaging. The figure is representative of two independent experiments with cells obtained from two donors.
DISCUSSION
Erythroid precursors have an extremely high iron requirement, especially during the period of Hb synthesis. Their developmental program involves, therefore, a drastic increase in transferrin receptor (TfR) expression,27,28 which allows accelerated uptake of iron. Excessive iron, especially before production of Hb, is stored in Ft. Another potential source of cellular iron is extracellular Ft,29 whose uptake by early erythroid cells is regulated and whose iron can be used for heme synthesis.27 The fate of the iron of intracellularly synthesized Ft during erythroid cell maturation, the potential of Ft to supply iron for Hb synthesis, and the mechanisms involved are still poorly understood.
To answer these questions, we followed changes in intracellular Ft content as a function of erythroid maturation. For this purpose, we developed and used an immunofluorescence flow cytometry method. Cells were dually stained with specific Ab for Ft and Hb. The procedure allowed us to avoid contaminating nonerythroid (Hb-negative) cells (eg, myeloid and lymphoid cells), which could bias the results. Analysis of cultures at different stages of developement, demonstrated a decline in intracellular Ft content concomitant with erythroid maturation. Our results are in agreement with previous studies in which erythroblasts from human bone marrow were used. In these studies Ft was measured by radioimmunoassay7,8 or assessed by microscopic immunocytochemical analysis.30
Ferritin, like other cellular proteins, has its own intracellular rate of turnover, during which it is likely to release its iron. This iron can join the cytoplasmic labile iron pool31 and thus can supply iron for Hb synthesis in developing erythroid cells.32 However, the question of the participation of Ft-iron in Hb synthesis in human erythroid cells remains unresolved. Human diferric Tf can be taken up via the TfR-Tf pathway during the entire period of intense Hb synthesis28,33,34 and thus, may provide all the iron needs of the cell. It was shown that iron taken up via the TfR-Tf pathway may pass directly to heme without using Ft as intermediate.35 In murine erythroleukemia cells, labeled with 59Fe and induced to synthesize Hb with DMSO, some of the 59Fe in the Hb originated from Ft.12 Similarly, radio-iron from amphibian larval red cell Ft was found in adult Hb.13 These results indicate that, potentially, Ft can donate iron to Hb. However, attempts to demonstrate the participation of cellular Ft-iron in heme synthesis in rabbit or rat reticulocytes have failed.14 15 Reticulocytes represent the penultimate stage of erythroid development, when the rate of iron uptake and heme synthesis are already deminished. In addition, in these experiments short observation periods were employed making detection of iron transfer from Ft to Hb extremely difficult.
In our studies, we used a two-phase liquid culture procedure,20 21 which allowed us to follow the fate of Ft-iron at early stages of developing erythroid cells. The cultured cells were labeled with radioiron at a pre-Hb stage (day 0 to 6 of phase II), and the distribution of their radioiron in Ft and heme was followed. The results showed that in early erythroid precursors (in day-6 cultures), iron was taken up and it accumulated mainly in Ft. Following maturation, this iron was redistributed; it was gradually removed from Ft and appeared in Hb (Fig 2). This redistribution of the radioiron strongly supports the possibility that the iron, which has accumulated in Ft before Hb production is released from this Ft at later stages and subsequently used for heme synthesis. Human diferric Tf did not affect the redistribution of 59Fe from Ft to Hb, indicating that Ft donates iron for heme synthesis also when the cells are supplemented with sufficient or even excessive iron.
To prove that the decrease in intracellular 59Fe-Ft was caused by the translocation of the 59Fe from Ft to heme rather than by leakage from the cells,36 cells were treated with succinylacetone, an inhibitor of δ-aminolevulinic acid dehydratase.25 Under these conditions, the radioiron was retained in Ft, thus indicating that Ft-iron in erythroid cells serves as a repository of iron for heme synthesis.
Mechanisms involved in the process of iron release from intracellular Ft are still an enigma. In vitro, this process is triggered by acidic pH and various reducing agents,37-42 and it is enhanced by free radicals,42-44 which alter the Ft protein shell and accelerate its degradation.38 However, such agents are either unphysiological or are not present in normal cells at sufficient concentrations necessary for iron release. In vitro studies suggested that iron release from Ft may be triggered by intermediates of the heme synthetic pathway. It was shown, in a cell free system, that δ-aminolevulinic acid was able to release iron directly from Ft.44
Our present study suggests that at least part of the process of iron release from Ft in normal, Hb synthesizing, cells takes place in an acid intracellular compartment and involves proteolytic activity. Thus, treatment of erythroid precursors with chloroquine inhibits iron redistribution between Ft and Hb in a dose-dependent manner, suggesting that increasing the compartmental pH leads to inhibition of the process. After treating the cells with the protease inhibitors, chymostatin and leupeptin, an increase in the Ft protein content was observed suggesting that lysosomal proteases are involved in Ft protein degradation. As a result, much of the iron remained in Ft and was not used for heme synthesis. These results are in agreement with previous studies in non-Hb synthesizing cells, which have shown that chymostatin leads to Ft accumulation in HepG2 hepatoma cells45 and inhibits the restoration of the deferoxamine (DFO) chelatable iron pool in cultured rat hepatocytes following iron depletion by DFO, presumably by inhibiting iron release from Ft.31 Leupeptin and the lysosomal inhibitor, methylamine, inhibited Ft degradation in uninduced K562 cells and decreases Ft-iron release and Ft-iron mobilization by DFO.46
The results of the present study suggest that at early stages of erythroid maturation, preceding intensive Hb synthesis, erythroid cells actively take up extracellular iron in Tf-bound form and accumulate it in Ft. Then, with the increase in the rate of Hb synthesis, iron is released from Ft and is used by the cells for heme synthesis. Iron release from Ft takes place in an acid compartment and requires protein shell degradation by proteases. It should be noted that these results do not rule out the possibility that iron can also pass directly from Tf to heme without using Ft as an intermediate, nor do they determine the relative contribution of Ft nor the Tf-TfR pathway to Hb synthesis at different stages of erythroid maturation.
ACKNOWLEDGMENT
This work contains part of the PhD thesis of B.V. We thank Aviva Sinberger and Gila Neyman for excellent performance of the FACS procedure and Dr E.G. Meyron-Holtz for plentiful discussions.
Supported in part by a grant from the Israel Cancer Association.
Address reprint requests to Abraham M. Konijn, PhD, Department of Human Nutrition and Metabolism, The Hebrew University, Faculty of Medicine, PO Box 12272, Jerusalem 91120, Israel.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal