Abstract
The Flt3 receptor is expressed in primitive hematopoietic cells and its ligand exerts proliferative effects on these cells in vitro in synergy with other cytokines. To expand on the functional properties of Flt3 ligand (FL) in vivo we treated nonhuman primates with FL and tested its ability to mobilize stem/progenitor cells when given alone or in combination with granulocyte colony-stimulating factor (G-CSF ) treatment. FL alone (200 μg/kg/day) mobilizes progenitors with slow kinetics and with a peak effect at the end of 2 weeks of treatment. The spectrum of mobilized progenitors includes myeloid, lymphoid, megakaryocytic, and osteoclastogenic but a low proportion of burst-forming unit (BFU)e. Bone marrow (BM) studies before and during the treatment suggested that proliferative effects in BM may have preceded effects on peripheral blood mobilization. To assess the synergy of FL with G-CSF in mobilization of progenitors we used two schemes: one in which G-CSF was used for the last 5 days of a 12-day treatment with FL; the other in which both cytokines were given concurrently for 5 days only (FL, 200 μg/kg; G-CSF, 100 μg/kg). Both schemes yielded much higher progenitor mobilization levels (peak levels of colony-forming cells [CFSs] 41,000 to 95,000/mL blood) than observed with either FL (CFC 4,600 to 7,300/mL) or G-CSF (8,405 ± 3,024/mL) used alone at the same doses. Furthermore, there was a progressive and significant expansion of progenitors in vitro during 2 weeks in suspension cultures of mononuclear cells or of CD34+ cells only in the animal with the combined treatment. Likewise, substantial mobilization of osteoclastogenic progenitors was documented only with the combined treatment. Given the functional properties of FL, its synergistic mobilization with G-CSF, and its anticipated good tolerance (because of the absence of an effect on mast cell activation), a clinical use is projected for this cytokine in peripheral blood transplantation settings, as well as in experiments with ex vivo gene transfer.
CLONING OF GENES encoding hematopoietic cytokines and their receptors on hematopoietic cells has provided important insights into their own function and the biology of stem cells in general and has facilitated their therapeutic use. One class of hematopoietic growth factor receptors with a central role in early stages of hematopoiesis is class III,1,2 consisting of receptors with intrinsic kinase activity and ligand-activated tyrosine phosphorylation. Members of this receptor family include the c-kit,c-fms, platelet-derived growth factor (PDGF ), and Flt3/Flk2 receptor. The soluble forms of the ligands for these receptors exert proliferative effects in vitro on hematopoietic progenitors, each with a distinct spectrum of functional activity. The functional features of the newest ligand/member of this family, the Flt3 ligand (FL), have been recently described in several publications and compared with the effects of the other related ligands.3-13 FL acting alone enhances the survival of primitive hematopoietic cells.14,15 In higher concentrations it has a synergistic activity for augmentation of colonies of most primitive cells3-8,10-13 and in addition promotes limited self-renewal of more mature progenitors in vitro.8,13,15 The restricted expression of the Flt3 receptor on immature but not mature hematopoietic cells (with the exception of some B lymphoid cells and monocytes)14,16 is in keeping with its functional influence on early hematopoietic cells. FL, in contrast to colony- stimulating factor (CSF )1 and kit ligand (KL), has no species-specific restricted action,3 and despite its functional and structural similarities with KL, it does not affect mast cells and melanocytes.17,18 There is limited information about the in vivo role of FL in steady state hematopoiesis and its significance in long-term repopulation. However, Flt3/Flk2 null mice show normal basal hematopoiesis but have a defect predominantly in development of B-cell lineage, and Flt3/Flk2−/− stem cells have a decreased ability to compete with wild-type stem cells in competitive repopulation experiments.19 In addition, recent data indicate that the in vivo administration of FL induces a significant accumulation of functionally mature dendritic cells in multiple organs in mice.20
To expand on the functional properties of FL in vivo, we treated primates with daily subcutaneous injections of FL and monitored its effects on blood counts and on the frequency and spectrum of progenitor cell mobilization. FL appears to mobilize a wide but distinct spectrum of progenitors with slow, progressive kinetics peaking about 2 weeks after treatment, in contrast to kinetics with granulocyte colony-stimulating factor (G-CSF ) treatment. Despite the slow, gradual mobilization kinetics with FL alone, an unexpectedly strong synergy was uncovered when FL was combined with G-CSF in a short 5-day treatment. Because of this synergy and FL's lack of effect on mast cell activation, this cytokine may have a clinical potential in mobilization of cells for transplantation purposes.
MATERIALS AND METHODS
Animals
Primates used in this study (Papio cynocephalus anubis and Macaca nemestrina of either sex) were housed at the accredited facilities of the University of Washington Regional Primate Research Center. Our studies were approved by the Institutional Review Board and by the Animal Care and Use Committee at the University of Washington. All animals were provided with food and water ad libitum throughout the study. Blood sampling and BM sampling were done under anesthesia with ketamine hydrochloride, as previously described.21 Hematopoietic cytokines were given subcutaneously in the doses and schedules described next.
Cytokines and Treatment Schemes
Flt3 ligand (FL), both the yeast and the Chinese hamster ovary (CHO)-derived material were kindly provided by Immunex (Seattle, WA). Human recombinant G-CSF was purchased from Amgen (Thousand Oaks, CA). Two animals received yeast-derived FL as a single treatment for 13 days, 200 μg/kg/day, subcutaneously. This dose was chosen to simulate optimum doses of another ligand to a type III kinase receptor, stem cell factor (SCF), in terms of mobilization efficacy.22 CHO-derived FL in the same doses and schedule was given to two other animals. Single treatments with G-CSF for 5 days using a daily subcutaneous injection of 100 μg/kg/day have been used in a total of eight animals. Three animals were treated with FL and G-CSF combination; one received CHO-FL for 12 days and G-CSF from days 7 through 12. Two other animals received both cytokines (CHO-FL, 200 μg/kg/day and G-CSF 100 μg/kg/day) for 5 days.
Clonogenic Progenitor Assays
Peripheral blood was collected from primates 1 or 2 days before the treatment, daily during treatment, and on several occasions after the discontinuation of treatment. Mononuclear cells were isolated after centrifugation with Accuprep (Accurate Chemical, Westbury, NY). Interface cells were collected, washed, and cultured in methylcellulose medium (1.2% methylcellulose, Fisher Scientific, Fair Lawn, NJ) containing the following components: 50% fetal bovine serum (FBS; Summit Biotechnology, Ft Collins, CO), 1% bovine serum albumin (Intergen, Purchase, NY), 0.1 mmol/L 2-mercaptoethanol (Eastman Kodak, Rochester, NY), and Iscove's Modified Dulbecco's medium (HyClone, Logan, UT). The following cytokines were added: erythropoietin, 2 U/mL (Genetics Institute, Cambridge, MA); kit ligand (SCF ), 50 ng/mL (Amgen); granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL, Genetics Institute); and Gibbon interleukin-3 (IL-3), 50 U/mL (provided by K. Kaushansky, University of Washington, Seattle, WA). The above conditions were found to be optimal for the development of granulocytic and erythroid colonies. All cultures were set up in triplicate with an inoculum from 0.5 to 5 × 105/mL. The plates were incubated at 37°C with 5% CO2 at high humidity for 2 to 4 weeks. Erythroid burst-forming unit (BFU-E) and granulocyte macrophage colony-forming units (GM-CFU) were counted under a dissecting microscope in plates of live cells between days 12 and 14 using morphological criteria for their classification. Macroscopic, compact colonies (mixed or pure) that were greater than 0.5 mm were also counted separately in live plates at 24 to 26 days postplating and were categorized as high proliferative potential colony-forming cells (HPP-CFC). IL-7 (Biodesign, Kennebunk, ME) was added at 50 ng/mL and was used in combination with FL or KL (50 ng/mL) for assaying lymphoid colonies. These colonies had a tight characteristic appearance in live plates and were enumerated at days 12 through 14. CFU-megakaryocytes (CFU-Meg) were evaluated in plasma clot medium containing 10% bovine embryo extract, 10% human AB serum, 1% bovine serum albumin, 0.1 mmol/L 2-mercaptoethanol, 10% bovine citrated plasma, thrombopoietin at 5 ng/mL, and KL and IL-3 at concentrations indicated previously.21 Plasma clots were labeled with anti-CD41 antibody for identification of CFU-Meg–derived colonies, as described.23 Osteoclastogenic progenitors were evaluated in an agar culture assay using recombinant human macrophage colony-stimulating factor (rhM-CSF; 40 μg/mL) and osteoclast colony-stimulating factor (O-CSF) conditioned medium from SJ3 cells.24 Plates were incubated for 14 days and stained with TRAPase for colony enumeration. Aggregates of greater than eight osteoclasts were considered as osteoclastic colonies.
Suspension Cultures
Suspension cultures were performed on two occasions: one of the animals treated with G-CSF alone and one of the animals treated with G-CSF plus FL for 5 days. A total of 1 × 106 mononuclear cells were plated in 10 mL of IMDM with 20% FBS, KL 10 ng/mL, IL-6 1 ng/mL, and IL-1α 10 ng/mL. In addition, CD34+ cells were enriched from peripheral blood mononuclear cells after treatment with biotinylated anti-CD34 antibody (CellPro, Bothell, WA) and incubation with superparamagnetic streptavidin microbeads using a VarioMACS Column (Miltenyi Biotech, Auburn, CA). Purified cells were 70% positive for CD34. Enriched CD34+ cells were used for suspension cultures with the same media. Cell proliferation was assessed by enumeration of cells at 1 and 2 weeks postculture and expansion of progenitors in the suspension cultures was evaluated by replating the cells in methylcellulose after 1 and 2 weeks of suspension culture. Smears were prepared from cells in the suspension culture at weekly intervals for morphological observations. In addition, peripheral blood mononuclear cells (PBMCs) were cultured in suspension. From 1 to 5 × 106 PBMC cells were plated in wells in α-MEM with 10% FBS and no cytokines under conditions allowing the formation of osteoclastic cells, as previously described.25
RESULTS
Mobilization of Progenitors by FL
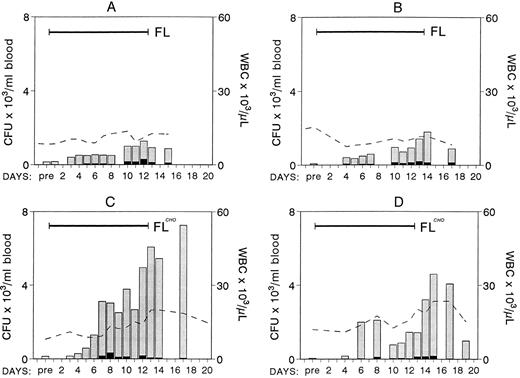
Four nonhuman primates were treated with 200 μg/kg of FL subcutaneously, two animals with the yeast-derived material, and two with the CHO-derived material. Blood counts were monitored daily during treatment and several days after treatment. Mononuclear cells from peripheral blood (PB) were also inoculated for clonogenic cell assays. The results from the four animals are shown in Fig 1. There were no significant changes in the white blood cell (WBC) count in the first two animals treated with yeast-derived FL, whereas a progressive increase to approximately two and a half times normal was observed in the animals with the CHO-derived FL (Fig 1). Hematocrits and platelets did not vary significantly (data not shown). Evaluation of clonogenic progenitors in PB showed significant differences in the output of mobilized progenitors between these two groups of animals. Peak levels of progenitors mobilized in the first two animals were 1,277 and 1,793/mL blood (8- to 25-fold enrichment from baseline), whereas there were 4,599 and 7,254/mL (51- to 98-fold enrichment) in the two animals given CHO-FL. Given the significantly longer half life of CHO-derived FL compared with yeast-derived FL (S. Lyman, unpublished data), these results may not have been totally unexpected. Although the amplitude of progenitor mobilization was significantly different between these two groups, the kinetics of mobilization was not. In all cases mobilization appears to peak very late, at the end of the 2-week treatment, but differences began to appear after 5 or 6 days of treatment. The majority of the progenitors mobilized per mL of blood were myeloid with very few erythroid progenitors (Fig 1 and Table 1). In addition to myeloid progenitors, there was a significant increase in small, compact, unicentric colonies consisting of small lymphoblast-like cells (morphology was evaluated in smears prepared from picked colonies) grown under the influence of IL-7 and kit ligand or FL. These colonies were at peak levels on day 14 (664/mL; 11-fold from baseline), whereas other types of colonies (Table 1) peaked at days 16 to 18. In two animals (animal A and C, Fig 1) BM was aspirated from two independent sites 1 day before treatment and at day 11 of treatment. Total nucleated cells per milliliter of marrow appear to be increased compared with pretreatment values (mean cell number in four aspirates [two per animal] was 76 × 106 ± 25.6/mL BM aspirate pretreatment versus 188 × 106 ± 25.5/mL posttreatment). The content of progenitors (CFC) was also significantly increased in these samples (pretreatment CFC, 307,496 ± 116,150/mL; posttreatment CFC, 624,069 ± 140,344/mL of BM aspirate). Even if one allows for 50% blood contamination posttreatment, this cannot account for the obtained differences.
Changes in WBCs (× 103; - - - - ) and in the number of clonogenic progenitors (per milliliter of blood) with FL treatment (200 μg/kg/day × 12 days; ▪ for BFU-E, for nonerythroid). Animals A and B were treated with the yeast-derived material and animals C and D with the CHO-derived material. Note the progressive mobilization of progenitors at the end of treatment, especially in animals C and D.
Changes in WBCs (× 103; - - - - ) and in the number of clonogenic progenitors (per milliliter of blood) with FL treatment (200 μg/kg/day × 12 days; ▪ for BFU-E, for nonerythroid). Animals A and B were treated with the yeast-derived material and animals C and D with the CHO-derived material. Note the progressive mobilization of progenitors at the end of treatment, especially in animals C and D.
Clonogenic Progenitors Differential in Peripheral Blood of Animals Treated With FL, G-CSF, or G-CSF Plus FL
| . | Clonogenic Progenitors* . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | BFU-E . | GM-CFU . | HPP-CFC . | CFU-Mk . | CFU-L . | O-CFC† . | . | . | . | . | . | . | ||||||
| . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | . | . | . | . | . | . |
| FL (2)‡ | 1.3 ± 0.5 | 169.4 ± 1.9 | 60.3 ± 17 | 5,595 ± 1,472 | 0.54 ± 0.54 | 46.5 ± 46.5 | 1.8 | 182 | 7.1 ± 3.1 | 664 ± 320 | ||||||||
| G-CSF (2) | 1.75 ± 0.75 | 467 ± 203 | 33 ± 18 | 8,770 ± 4,847 | 1.10 ± 0.4 | 388 ± 106 | 2.6 ± 2.0 | 742 ± 503 | 6.0 ± 2.0 | 2,199 ± 695 | 0 | 0 | ||||||
| FL + G-CSF (3) | 24.4 ± 17 | 7,689 ± 3,751 | 111 ± 22 | 40,910 ± 16,014 | 5.4 ± 0.6 | 4,213 ± 1,391 | 12 ± 9.0 | 2,122 ± 576 | 18.0 ± 11.0 | 3,737 ± 1,296 | 80 ± 70 | 26,690 ± 19,961 | ||||||
| . | Clonogenic Progenitors* . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | BFU-E . | GM-CFU . | HPP-CFC . | CFU-Mk . | CFU-L . | O-CFC† . | . | . | . | . | . | . | ||||||
| . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | No./105 . | No./mL . | . | . | . | . | . | . |
| FL (2)‡ | 1.3 ± 0.5 | 169.4 ± 1.9 | 60.3 ± 17 | 5,595 ± 1,472 | 0.54 ± 0.54 | 46.5 ± 46.5 | 1.8 | 182 | 7.1 ± 3.1 | 664 ± 320 | ||||||||
| G-CSF (2) | 1.75 ± 0.75 | 467 ± 203 | 33 ± 18 | 8,770 ± 4,847 | 1.10 ± 0.4 | 388 ± 106 | 2.6 ± 2.0 | 742 ± 503 | 6.0 ± 2.0 | 2,199 ± 695 | 0 | 0 | ||||||
| FL + G-CSF (3) | 24.4 ± 17 | 7,689 ± 3,751 | 111 ± 22 | 40,910 ± 16,014 | 5.4 ± 0.6 | 4,213 ± 1,391 | 12 ± 9.0 | 2,122 ± 576 | 18.0 ± 11.0 | 3,737 ± 1,296 | 80 ± 70 | 26,690 ± 19,961 | ||||||
Abbreviations: BFU-E, erythroid burst-forming unit; GM-CFU, granulocyte macrophage colony-forming unit; HPP-CFC, high proliferative potential-colony-forming cell; CFU-Mk, megakaryocyte colony-forming unit; CFU-L, lymphocyte colony-forming unit; O-CFC, osteoclastic colony-forming cell; FL, Flt3 ligand; G-CSF, granulocyte colony-stimulating factor.
At peak level.
Osteoclastic colonies were those over 8 cells each and were cultured with supernate from SJ3 cell line, as described.24 These progenitors are not included in calculations presented in Figs 1-3.
Number in parenthesis indicates numbers of animals in which the detailed progenitor differential was studied.
Mobilization by G-CSF
A total of eight animals were treated with G-CSF alone for 5 days at 100 μg/kg/day over the last 3 years in our institution. Mean value for WBC counts at their peak in these animals were at 93,400 ± 41,600 and were observed 4 days after initial injection. Peak progenitor levels were at 8,405 ± 3,024 mL of blood on day 7. (Partial data from six of the animals have been published before.22 ) These levels of WBCs and CFC/mL are very similar to data published from three additional animals after mobilization with the same dose of G-CSF.26 Detailed studies on the different spectrum of progenitors mobilized were done in two G-CSF–treated animals and are shown in Table 1.
Synergy of FL and G-CSF in Mobilization of Progenitors
Treatment with FL days 1 to 12 and G-CSF days 7 to 12.Because FL appears to mobilize with slow kinetics peaking at the end of 2 weeks, we reasoned that superimposition of G-CSF treatment at the end of FL treatment would provide the best boost in progenitor mobilization. Thus, G-CSF at 100 μg/kg was overlapped from days 7 to 12, the last 5 days of FL treatment. Results are shown in Fig 2C. No significant mobilization was seen until about 3 days into the G-CSF treatment, and a broad peak in progenitor mobilization was evident during the following 4 days. Total WBC counts reached a peak level of 122,200 (∼12.5-fold increase from baseline) at day 12 and the peak progenitor level (CFU-GM and BFUe) was 40,894 ± 2,762.8 per milliliter of blood at day 10.
Changes in WBCs (- - - - -) and in circulating clonogenic progenitors (per milliliter of blood) in animals treated with CHO-FL and G-CSF combinations. (Solid bars on left top of each panel correspond to duration of treatment.) Animal C was treated with CHO-FL for 12 days and with G-CSF from day 7 to 12. Panel D shows data from eight animals treated with G-CSF for 5 days (for values representing < 3 animals have no standard deviation [SD]). Note the amptitude of mobilization in animals A, B, and C compared with single treatments with FL (Fig 1; please note difference in scale) or G-CSF (D).
Changes in WBCs (- - - - -) and in circulating clonogenic progenitors (per milliliter of blood) in animals treated with CHO-FL and G-CSF combinations. (Solid bars on left top of each panel correspond to duration of treatment.) Animal C was treated with CHO-FL for 12 days and with G-CSF from day 7 to 12. Panel D shows data from eight animals treated with G-CSF for 5 days (for values representing < 3 animals have no standard deviation [SD]). Note the amptitude of mobilization in animals A, B, and C compared with single treatments with FL (Fig 1; please note difference in scale) or G-CSF (D).
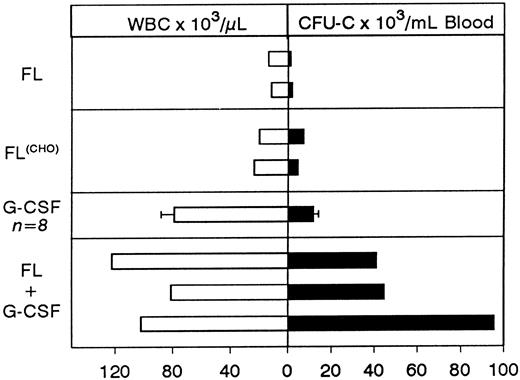
Concurrent treatment of FL and G-CSF for 5 days.Two macaques of similar age as in the above combined treatment were treated for 5 days with both G-CSF and FL at 100 μg/kg and 200 μg/kg, respectively, and the results are shown in Fig 2. A significant enhancement of progenitor mobilization was seen with these treatments, ie, progenitors peaked at 44,699/mL in one animal and 95,420/mL in the other. WBCs were at 81,200/mL and 102,000/mL respectively (ie, 2B and 2A, respectively). The responses from the three animals with FL plus G-CSF were much higher than those seen with either FL or G-CSF alone (Figs 1-3), supporting a syngergistic effect on mobilization. For example, with FL alone, the peak progenitor value was 7,254 and 4,598 per milliliter of blood, whereas peak levels in G-CSF animals were 8,405 ± 3,024 per milliliter of blood (Figs 2D and 3). The different types of progenitor cells mobilized with the single cytokine treatments (G or FL) are shown in Table 1 in comparison with data from the combined (G + FL) treatments. HPP-CFC were 388 ± 106 per milliliter of blood in the G-CSF treatment, and there were 4,213 ± 1,391 in the combined FL plus G-CSF at peak levels. Likewise, megakaryocytic progenitors (CFU-Meg) and osteoclastogenic progenitors were significantly higher with the combination treatment (Table 1). In one G-CSF–treated animal and one FL plus G-CSF–treated animal (animal A in Fig 2) we assessed the proportion of CD34+ cells (by fluorescence-activated cell sorting (FACS), after labeling with biotinylated 12.8 antibody) among peripheral blood mononuclear cells at day 6 of mobilization. In the G-CSF animal the proportion of CD34+ cells was 1.53%, whereas in the FL + G-CSF animal it was 2.29%. Considering the recovery of mononuclear cells per milliliter of blood (3.9 × 106/mL for the G-CSF animal and 25.2 × 106/mL for the G plus FL animal) the total output of CD34+ cells per milliliter of blood was 5.77 × 105/mL for the G-CSF plus FL animal and 0.597 × 105/mL for the G-CSF alone.
Comparison of peak levels of WBCs and of progenitors mobilized with the different treatment schemes. Each bar represents peak levels from a single animal with the exception of G-CSF treatment where the average ± SD of eight animals is shown.
Comparison of peak levels of WBCs and of progenitors mobilized with the different treatment schemes. Each bar represents peak levels from a single animal with the exception of G-CSF treatment where the average ± SD of eight animals is shown.
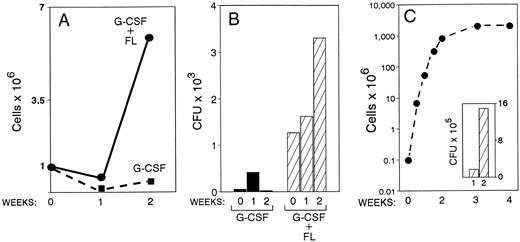
Furthermore, from the previous two animals, suspension cultures from PB mononuclear cells were initiated. A significant proliferative effect was observed with the mononuclear cells originating from the combined treatment animal compared with the one with G-CSF alone (Fig 4A). For example, under the culture conditions employed (KL, IL-6, IL-1α), 5.9 × 106 cells were generated by week 2 in the G plus FL treatment compared with 0.4 × 106 in the G-CSF alone with an initial inoculum of 106 mononuclear cells in both cultures. Furthermore, the cumulative number of all types of progenitors in the same cultures was far greater in the G plus FL animal compared with G-CSF alone (Fig 4B). A small increase of total progenitors by week 1, but a progressive expansion by week 2 was seen only in cultures of mononuclear cells derived from the combined-treatment animal. Morphological observations from smears prepared from the suspension cultures showed the presence of cells maturing along several lineages; ie, megakaryocytic, represented by large polyploid cells with megakaryocytic features and myeloid, by the presence of neutrophils, eosinophils, monocyte/macrophages, and osteoclasts with characteristic morphology and tartrate-resistant nonspecific esterase positivity. In addition, several cells with distinct morphological features (especially cytoplasmic projections) compatible with dendritic cells were noted. Unfortunately these types of cells were not quantitated in these preparations. Also, in methylcellulose cultures several putative dendritic cell colonies were noted and consisted of medium- to large-size cells with long and short cytoplasmic projections.
Blood drawn from one animal treated with G-CSF alone (100 μg/kg × 5 days) and from one animal treated with G-CSF plus FL for 5 days was drawn at day 6 of treatment. Suspension cultures of mononuclear cells (A and B) and of purified CD34 cells (C) from these mononuclear cells were set up, and cellular proliferation and expansion of progenitors were monitored for 2 weeks. Note the higher proliferative response (A) and accumulation of progenitors (B) in the G-CSF plus FL animal compared with G-CSF alone. In C the proliferative response for 4 weeks of CD34+ cells (∼70% CD34+) enriched from PBMC of the G-CSF plus FL animal and the expansion of CFC for 1 and 2 weeks in culture is shown. Similar data from G-CSF–only treated animal could not be generated, as the enrichment of CD34+ cells was poor and their total number was low.
Blood drawn from one animal treated with G-CSF alone (100 μg/kg × 5 days) and from one animal treated with G-CSF plus FL for 5 days was drawn at day 6 of treatment. Suspension cultures of mononuclear cells (A and B) and of purified CD34 cells (C) from these mononuclear cells were set up, and cellular proliferation and expansion of progenitors were monitored for 2 weeks. Note the higher proliferative response (A) and accumulation of progenitors (B) in the G-CSF plus FL animal compared with G-CSF alone. In C the proliferative response for 4 weeks of CD34+ cells (∼70% CD34+) enriched from PBMC of the G-CSF plus FL animal and the expansion of CFC for 1 and 2 weeks in culture is shown. Similar data from G-CSF–only treated animal could not be generated, as the enrichment of CD34+ cells was poor and their total number was low.
When suspension cultures of PB mononuclear cells were performed without any cytokines, initially several aggregates of lymphoid-like cells (based on morphological observations of picked and stained aggregates) were seen followed by the formation of islands of osteoclasts with characteristic morphological and cytochemical features, consistent with previous data.25 Osteoclast-like foci were not seen in the G-CSF animal, whereas there were 98 per 106 cells in the G-CSF plus FL animal. Both cultures were inoculated with the same number of PBMC cells (1 × 106 and 2.5 × 106 cells). (Studies were performed by Dr B. Torok-Storb at Fred Hutchinson Cancer Research Center.) In addition to suspension cultures, osteoclastogenic progenitor-derived colonies, assessed in an agar culture system,24 were numerous in G plus FL samples (Table 1), but no such colonies were seen in cultures inoculated with the same number of mononuclear cells (0.5 to 1 × 105/mL) from the G-CSF animal.
Apart from mononuclear cell cultures, CD34+ cells enriched from PB mononuclear cells from the above two animals on day 6 of treatment were also placed into suspension cultures. Figure 4C shows the exponential expansion of total cells and of progenitors from the animal treated with FL plus G-CSF. CD34 enrichment of cells from the animal with G-CSF alone was very poor (<30% CD34+ cells) and with a low cell yield, so it could not be used for comparison.
BM was aspirated 14 days after discontinuation of G-CSF or G-CSF plus FL treatment. Nucleated cells and clonogenic progenitors recovered per milliliter of BM were determined. The cellularity of the G-CSF animal was estimated at 205 × 106 ± 55/mL of marrow and CFC were 717 × 103/mL (268 × 103 ± 31.7 BFU-E, 440.2 × 103 ± 43.9/mL GM-CFU, and 8.2 × 103 ± 5.8 megakaryocyte colony-forming unit [CFU-Mk]). Both values are above average for a normal animal (see pretreatment levels) and may indicate a rebound increase after G-CSF treatment, as observed previously.27 28 BM cellularity from the G-CSF plus FL–treated animal was at 106 × 106 ± 76/mL BM, and progenitor content was 479 × 103/mL BM (172 × 103 ± 21.6 BFU-E, 285 × 103 ± 41.8/mL GM-CFU, and 21.8 × 103 ± 3.6 CFU-Mk). These values appear to be within the range of normal BM sampling suggesting that either rebound may not occur after the combined treatment, in contrast to G-CSF treatment, or that, if occurring, it may have been missed at the time of our sampling.
DISCUSSION
Mobilization by Hematopoietic Cytokines
G-CSF appears to be the most effective single agent with wide clinical application for mobilization of progenitors for transplantation purposes.29 Animal studies suggest that the G-CSF-induced mobilization is largely the result of a redistribution of progenitors from BM to the periphery with little change in the total progenitor content.30 Furthermore, given the protective effect of G-CSF on apoptosis of myeloid cells,31 prolongation of survival of circulating progenitors may also be a contributing factor. Proliferative expansion of progenitors in circulation is an unlikely contributor, as most mobilized progenitors are not in cycle.32-34 The molecular mechanism(s) by which progenitors redistribute themselves from BM to the blood after G-CSF treatment are poorly understood. Although several changes in cytoadhesion molecules and c-kit expression have been reported on mobilized progenitors,35-42 it has not been established whether these are primary effects or whether they are causally related. For example, one could propose that the primary effect of high doses of G-CSF is to keep cells out of active cycling (G0 /G1 ). Such cells could express less α4 integrins on their surface, which may not be functionally adequate. As a consequence, mobilization of relevant progenitors follows, providing an appealing mechanism for G-CSF–induced mobilization. However, there are conflicting data in the literature about the functional competency of integrins on mobilized cells.36,37,41,42 Furthermore, it is not clear to what extent a modulation of the same or different cytoadhesion molecules is at play when other cytokines are used for mobilization (ie, IL-1,43 IL-8,44 KL,45 and FL) In addition, with respect to G-CSF, the inability to maintain peak levels of mobilized progenitors despite continuation of G-CSF treatment22 and the fate of mobilized progenitors after discontinuation of treatment are other unexplained or unexplored events. Nevertheless, given a certain dose and duration of treatment with G-CSF, the kinetics of peak mobilization by G-CSF are fairly predictable.46,47 Because of this feature and its wide tolerance, G-CSF has been embraced as the cytokine of choice in progenitor mobilization. Several reports have compared G-CSF–induced mobilization with that of other cytokines. Treatments with IL-3 or KL appear to induce, in contrast to G-CSF alone, increased proliferation of progenitors in the BM and to mobilize progenitors with slower kinetics and different efficiencies.22,45,48-50 At the other end of the spectrum, the chemokines, IL-1 and IL-8, produce their mobilizing effects within minutes or hours of intravenous administration.43 44 Mobilization by these inflammatory cytokines is apparently unrelated to any proliferative effects and probably operates through different molecular pathways. Thus, extreme differences in mobilization kinetics among diverse cytokines/chemokines would support the view that several mechanisms are responsible for mobilizing stem/progenitor cells from BM.
In the present study we have explored the effects of yet another hematopoietic growth factor, FL, in stem/progenitor mobilization in primates. FL alone appears to have a delayed peak of progenitor mobilization at the end of 2 weeks of treatment. A similar dose of KL in primates induces a peak in mobilization at the end of the first week with no increment thereafter, whereas G-CSF mobilization peaks at 5 to 7 days22 similar to human studies.46,47 The CHO-derived FL, because of its longer half-life, had a significantly higher effect than the yeast-derived material used for treatments (Fig 1). Assessment of the spectrum of progenitor cells mobilized showed a negligible mobilization in BFU-E with most of the progenitors mobilized being of myeloid type (Table 1). Also, a significant mobilization of lymphoid type progenitors with an expanded progeny under the influence of IL-7 and FL was observed as well as mobilization of putative dendritic cells. Thus, in addition to kinetic differences, qualitative differences in KL-treated primates, inducing a significant BFU-E mobilization were noted22 (also from unpublished data from our laboratory). Collectively, the data suggest that the proliferative effects of these two cytokines on specific classes of hematopoietic progenitors in vitro (ie, special influence on erythroid progenitors by KL,51 or effects on lymphoid progenitors by FL only3 4 ) may be relevant to their in vivo effects including progenitor mobilization. It is tempting to speculate that the early proliferative effects on BM, which are induced by KL or FL but not by G-CSF, and which precede mobilization peaks in the periphery, may be responsible for delayed mobilization kinetics.
Cytokine Synergy in Mobilization
Because of the widespread use of mobilized blood for transplantation purposes, the need for multiple aphereses collections, and the individual variability in mobilization yields, especially in patients, new modalities aiming to enhance the yield of each apheresis are highly desirable. For this purpose, combinations of cytokines, for example G-CSF with GM-CSF, G-CSF with IL-3, or G-CSF with KL, have been explored.52-56 When G-CSF is combined with IL-3, optimal results were found only when it was used in a sequential fashion after IL-3 treatment.55 With KL the concurrent treatment of both cytokines gave optimal results both in murine54 and primate studies22 but with a more delayed peak in mobilization than G-CSF alone. Our present data, showing that a combination of FL plus G-CSF for 5 days has a synergistic effect in mobilization compared with single cytokine treatments, is in keeping with prior experience using cytokine combinations. Not only were myeloid progenitors mobilized, but an increase in lymphoid, osteoclastogenic, and CFU-Meg progenitor mobilization (Table 1) was noted with the combination. It is of interest that in our treatments the prolonged administration of FL did not appear to have any advantage compared with the short concurrent FL plus G-CSF treatment. What is the basis of FL plus G-CSF synergy? As the primary mobilization event(s) in the single cytokine treatments are as yet unclear, we can only provide speculative mechanisms for the synergy. An early proliferative effect of FL on BM could certainly expand the target cell pool on which G-CSF can exert its effect, thus enhancing mobilization. If the FL-mediated proliferation in BM peaks early, as observed in murine studies,57 it may not be surprising that the long FL treatment (12 days v 5 days) did not have an additional advantage in the combination treatment. Alternatively, one could propose that concurrent administration of pharmacological amounts of both cytokines brings about changes in adhesive interactions of hematopoietic progenitor cells with their BM microenvironment, unlike the ones induced by either cytokine alone. Whatever the possibility(s), it seems more likely that both cytokines act on the same target cell pool, rather than each on a different target cell population. If the latter were the case, an augmented early peak in mobilization would not have been expected when FL plus G-CSF were combined for 5 days only.
Given the specific functional features of FL and especially the lack of an effect on the differentiation and activation of mast cells,17,18 it is anticipated that this cytokine will be better tolerated in humans than KL.52 Because of this and the FL's ability to enhance a short G-CSF's mobilization course similar in duration to that applied clinically, it holds promise in clinical practice for boosting mobilization and reducing the number of aphereses needed. Implicit in this assumption is that the better mobilization of progenitors shown in our studies is also accompanied by mobilization of long-term repopulating cells, as has been clearly found in transplantation experiments in which cells mobilized with other cytokines, such as IL-845 or KL,26 were used. It would appear that the proportion of long-term repopulating cells mobilized is largely dependent both on the type of cytokine(s) used and the duration of cytokine treatment.29,58,59 Whether the high yield in 34+/38− subsets59 or LTC-IC56 observed in some of these combinations will ultimately improve transplantation outcome is unclear as yet. Therefore, additional studies to evaluate changes induced by FL or combination treatments in the content and distribution of long-term repopulating cells (by transplantation assays) are warranted. Furthermore, in the case of FL including combinations, the impact of FL-induced mobilization and expansion of functional dendritic cell populations20 on immunologic aspects of allogeneic transplantation needs to be carefully assessed.
ACKNOWLEDGMENT
We are grateful to Debra Glanister and Ray Colby for their help with the primate studies, to Lynn Ferguson for her help with the TRAPase staining of the osteoclastic cells, and to Sherri Brenner for her skillful secretarial assistance. We are also indebted to Dr B. Torok-Storb for providing part of the data with osteoclast suspension cultures.
Supported by NIH Grants No. HL46557, AI35191, HL54881, and RR00166.
Address reprint requests to Thalia Papayannopoulou, MD, Division of Hematology, University of Washington, Seattle, WA 98195.

![Fig. 2. Changes in WBCs (- - - - -) and in circulating clonogenic progenitors (per milliliter of blood) in animals treated with CHO-FL and G-CSF combinations. (Solid bars on left top of each panel correspond to duration of treatment.) Animal C was treated with CHO-FL for 12 days and with G-CSF from day 7 to 12. Panel D shows data from eight animals treated with G-CSF for 5 days (for values representing < 3 animals have no standard deviation [SD]). Note the amptitude of mobilization in animals A, B, and C compared with single treatments with FL (Fig 1; please note difference in scale) or G-CSF (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.620/4/m_bl_0027f2.jpeg?Expires=1769099647&Signature=4pf9axctCZv024Lcf~ea-RljA00doEdcM1CVK8kGC0t6TK7Dfd36yNvdeHruiUddK05yxqFW9Xw-3K~ZO6FeFnACVWxXXkzOCHLFnfqhXyRKJ9B4EjEnUQLj9oG8DJRO6SWloNPR8YKvB0T~~YwpnhBE3ZDLFfqFxj-ONR0khKRvvb4sSa9RdpYHLHiG8vgqikTo1tkBmWQC-gM1V44yqjlu~1VWT6sr2zYaZQjB~tskV1Zukc4L6djog4bLvO-VXx8Jfn~Lxlpf3czkVMlg4ev0uLBhm3TdnjbdaTIcSJoN9wFpt4Eh2qNVcPqydRA8awbwJFEfeV~cCQpY1hiEQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal