Abstract
Because of the recommendation to avoid the concomitant administration of growth factors and chemotherapy, there is only limited information on colony-stimulating factor (CSF ) therapy in acute lymphoblastic leukemia (ALL) induction protocols, in which cytotoxic drugs are administered in divided doses over a prolonged period of time, thus requiring a simultaneous administration of growth factors and chemotherapy. We conducted a prospective, randomized, controlled study to determine the safety and efficacy of granulocyte colony-stimulating factor (G-CSF; filgrastim) as an adjunct to phase I of induction chemotherapy for adult ALL. Patients (n = 53) were randomized to receive no growth factor or G-CSF (5 μg/kg/d subcutaneously) starting on day 2 of chemotherapy consisting of daunorubicin (45 mg/m2) and vincristine (1.5 mg/m2) on days 1, 8, 15, and 22; L-asparaginase (2500 U/m2) on days 1 through 14; and prednisone (60 mg/m2) on days 1 through 28. A total of 25 patients in the G-CSF group and 26 patients in the control arm fulfilled the inclusion criteria of the study. G-CSF markedly ameliorated neutropenia because the median proportion of days with neutropenia less than 1,000/μL was 29% in the G-CSF group as compared with 84% in the control arm (P < .00005). The median time to reach absolute neutrophil counts (ANC) ≥ 1,000/μL was 16 days in G-CSF patients and 26 days in controls (P < .001). More importantly, G-CSF significantly reduced the incidence of febrile neutropenia (12% v 42% in controls, P < .05) and documented infections (40% v 77%, P < .05). No significant differences were found with regard to requirements for red blood cell transfusions and platelet concentrates. A total of 24 of 25 (96%) patients in the G-CSF group and 20 of 25 (80%) evaluable control patients had complete remission after phase I of induction therapy. We conclude that G-CSF can be safely administered as an adjunct to induction therapy of ALL and is clinically beneficial by ameliorating neutropenia and reducing infectious complications.
WITH MOST CURRENT induction protocols, complete remission (CR) can be achieved in more than 75% of adult patients with acute lymphoblastic leukemia (ALL).1-3 The causes of failure are refractoriness to chemotherapy and death from complications, which occurs in approximately 10% of patients.1,3 Death during induction treatment is mainly due to severe bacterial or fungal infections that are caused by the severe prolonged neutropenia as a result of disease and/or chemotherapy (CT).4
Granulocyte colony-stimulating factor (G-CSF ) is a hematopoietic growth factor that stimulates neutropoiesis and, as it has been shown by several controlled studies, has the potential to reduce incidence and severity of neutropenia associated infections after standard or myeloablative CT in a variety of malignancies.5-9 There are only a few studies in which G-CSF has been tested during induction therapy of ALL.10-14 The only randomized study in which G-CSF was tested in the induction protocol of the German cooperative multicenter ALL trial (GMALL) was performed by Ottmann et al.13 They administered G-CSF during phase II of the induction therapy at a time when most patients were already in CR. They showed that G-CSF treatment resulted in a reduction of neutropenia, a trend to fewer infections, and a more rapid completion of chemotherapy.
In a retrospective analysis, we have shown that the duration of neutropenia could be markedly shortened by G-CSF not only in phase II but also in the initial part (phase I) of the GMALL protocol.10 We report here the results of a prospective, randomized study of the effect of G-CSF in phase I of the GMALL protocol in untreated adult ALL patients.
PATIENTS AND METHODS
Patients.Patients were eligible for the study if they fulfilled the following criteria: diagnosis of ALL documented by morphology, cytochemistry, and immunphenotyping; French-American-British (FAB) type L1 or L2; age more than 14 years; Eastern Cooperative Oncology Group (ECOG) status ≤2; and written informed consent by the patient or the legal guardian. Exclusion criteria were B-ALL (L3), hybrid leukemia, contraindication to chemotherapy or G-CSF, ECOG status greater than 2, HIV positivity, and pregnancy. Cytogenetic and molecular analysis [translocation (4; 11), BCR/ABL rearrangement] were optional. Between April 1993 and January 1996, 6 participating centers enrolled a total of 53 patients, of whom 51 fullfilled the inclusion criteria of the study. The study was approved by the local ethical committee at each institution.
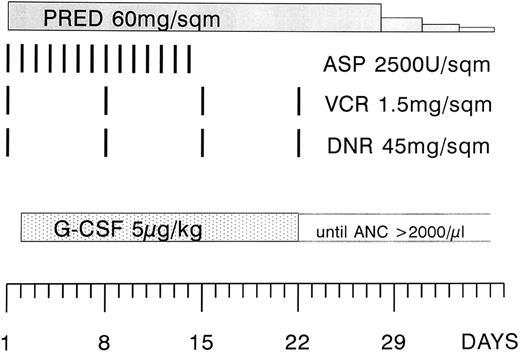
Treatment regimen.The patients were treated with a modified phase I of induction therapy according to the German Cooperative Multicenter ALL (GMALL) protocol (Fig 1).1 The modification consisted of a higher dose of daunorubicin (45 instead of 25 mg/m2 on days 1, 8, 15, and 22). The asparaginase preparation that was used in the study was Asparaginase Medac. The scheduled dose was 5,000 U/m2, but it was reduced to 2,500 U/m2 when it turned out that this preparation was more potent and also more toxic.15 16 The dose of daunorubicin and vincristine was adjusted to the bilirubin level (1.5 to 3.0 mg/dL 75% and 3.1 to 5.0 mg/dL 50% dose reduction, respectively). Patients with an initial leukocyte count of greater than 25,000/μL received a prephase therapy with vincristine and prednisone. All patients received infection prophylaxis consisting of trimethoprim 2 × 100 mg/d and fluconazole at 200 mg/d orally.
Modified phase I of the induction regimen according to the GMALL protocol 1984. The modification consisted of a higher dose of daunorubicin (45 instead of 25 mg/m2) and the administration of asparaginase during the first 2 weeks of the protocol, and at a lower dose (2,500 instead of 5,000 U/m2). The study period was 4 weeks, starting at the first day of G-CSF administration (day 2 through 29). PRED, prednisone; ASP, asparaginase; VCR, vincristine; DNR, daunorubicin; G-CSF, granulocyte colony-stimulating factor.
Modified phase I of the induction regimen according to the GMALL protocol 1984. The modification consisted of a higher dose of daunorubicin (45 instead of 25 mg/m2) and the administration of asparaginase during the first 2 weeks of the protocol, and at a lower dose (2,500 instead of 5,000 U/m2). The study period was 4 weeks, starting at the first day of G-CSF administration (day 2 through 29). PRED, prednisone; ASP, asparaginase; VCR, vincristine; DNR, daunorubicin; G-CSF, granulocyte colony-stimulating factor.
Recombinant metHu-G-CSF.Patients were put into random order centrally to receive either recombinant metHu-G-CSF (Neupogen R; Roche, Basel, Switzerland) or no growth factor. The protocol allowed G-CSF administration in the control group in case of a life-threatening infection in a severely neutropenic patient. In patients matched randomly to G-CSF, G-CSF was administered daily, subcutaneously (SC), at a dose of 5 μg/kg, starting 24 hours after the first daunorubicin administration and was continued at least until day 22. Thereafter, it was stopped when the absolute neutrophil count (ANC) exceeded 2,000/μL on 2 consecutive days.
Antibiotic treatment and supportive care.In case of fever, blood cultures were mandatory before starting antibiotic treatment. A careful examination of the lungs (x-ray, CT scan), skin, otolaryngeal region, and perianal region was performed to identify local causes of infection. Intravenous antibiotic (AB) treatment, antifungal, and antiviral treatment was administered according to local guidelines. There were no strictly defined rules as to the duration of AB treatment, but usually AB was stopped when the patient was afebrile for at least 3 days and the ANC was greater than 500/μL. Red cell transfusions were administered at a hemoglobin level less than 8 g/dL and platelet concentrates (single donor) at platelet counts less than 20,000/μL.
Treatment following the study period.Treatment was continued according to the GMALL protocol (phase II of the induction protocol) independent of the remission status after phase I.1 A total of 45 patients (23 G-CSF and 22 controls) proceeded to phase II. The reasons for not receiving phase II chemotherapy were death during phase I (1 G-CSF and 2 controls) or contraindications to further chemotherapy due to infections or other problems (1 G-CSF and 2 controls). Participating centers were free to use G-CSF in phase II of the induction. A total of 3 patients (2 G-CSF and 1 control) underwent allogeneic bone marrow transplantation (BMT) from siblings in first CR because of high-risk features of their disease (BCR/ABL positivity, failure to achieve CR after phase I). Two further patients (in the control arm) received an allogeneic graft beyond first remission.
Evaluation of study endpoints.The primary endpoints of the study were (1) the incidence of fever, febrile neutropenia, and the number of infectious episodes during the 4-week study period, ie, day 2 through 29 of the phase I of induction treatment (Fig 1); and (2) the duration of neutropenia. Infectious episodes were counted as septicemia, bacteremia, fever of unknown origin (FUO), pneumonia, and localized infections (skin and oral infection, etc). Septicemia was defined as fever greater than 38°C (axillary) and at least one blood culture positive for a pathogenetic organism. FUO was defined as fever greater than 38°C without positive blood cultures or localized infection. Bacteremia was defined as a positive blood culture and temperature ≤38°C. Pneumonia included patients with pulmonary infiltrates with or without positive blood cultures.
For the evaluation of the effect of G-CSF treatment on neutropenia, the following parameters were analyzed: the time of ANC recovery to ≥1,000/μL and ≥500/μL, the proportion of patients achieving complete reconstitution of neutropoiesis (ANC ≥2000/μL) at the end of the study period, and the proportion of days with neutropenia (ANC <1,000/μL and <500/μL) during the study period. Patients who died before their blood counts reached the designated recovery levels were censored at the day of death in the Kaplan-Meier curves for the cumulative probability of neutrophil recovery. The scheduled frequency of blood counts, including manual differential counts, was three times per week. Linear interpolation was used to estimate ANCs for days with no counts. For analysis of safety, the effects of the study drug on transfusion requirements, the incidence of nonhematological adverse events, and remission duration were assessed.
Statistical analysis.The incidence of fever, febrile neutropenia, and infectious episodes in the two treatment groups was compared with the χ2 test. This test was also used for comparison of remission rate and the proportion of patients with ANC ≥2,000/μL at the end of the study period. The U-test (Mann-Whitney) was applied to compare both groups with regard to the proportion of days with neutropenia less than 1,000/μL and less than 500/μL, respectively, and transfusion requirements during induction therapy. The time of neutrophil recovery, overall survival (OS), duration of continuous complete remission (CCR), and disease-free survival (DFS) were estimated using the Kaplan-Meier method and compared by the log-rank test. Survival was measured from the date of diagnosis until the date of death, and CCR from the date of achievement of CR to the date of relapse, respectively. DFS was the time of achievement of CR until either relapse or death of any cause. Patients undergoing allogeneic BMT were censored at the time of transplantation.
RESULTS
Patient characteristics at study entry.A total of 53 patients were put into random order. A total of 2 patients (both matched randomly to G-CSF ) not fulfilling the inclusion criteria, because the diagnosis of ALL could not be confirmed, were excluded. Thus, 25 patients in the G-CSF group and 26 patients in the control group were evaluated. The patients in the two treatment groups were well matched with regard to age, performance status, white blood cell count (WBC) at diagnosis, and immunophenotype (Table 1). Because of the limited number of patients, sex ratio (P = .125), BCR/ABL rearrangement (P = .159), and translocation (4; 11) (P = .268) were less well balanced, but not significantly different between both arms (χ2 test). A total of 9 G-CSF and 7 control patients received a prephase therapy with vincristine and prednisone because of a high initial leukocyte count (>25,000/μL).
Patient Characteristics
| . | Controls (n = 26) . | G-CSF (n = 25) . |
|---|---|---|
| Male/female | 17/9 | 10/15 |
| Age (yr) | ||
| Mean | 41.6 | 40.3 |
| Median | 41.7 | 36.4 |
| Range | 16-79 | 17-75 |
| ECOG status | ||
| 0-1 | 24 | 22 |
| 2 | 2 | 3 |
| WBC at diagnosis (×103/μL) | ||
| Mean | 22.8 | 31.0 |
| Median | 9.6 | 13.7 |
| Range | 1.9-124 | 1.1-171 |
| Immunophenotype | ||
| Early B-precursor | 5 | 5 |
| C-ALL | 12 | 11 |
| Pre-B | 5 | 2 |
| T-ALL | 4 | 7 |
| BCR/ABL rearrangement | 7/25 (28%) | 2/24 (8%) |
| Translocation (4;11) | 0/17 (0%) | 3/19 (16%) |
| . | Controls (n = 26) . | G-CSF (n = 25) . |
|---|---|---|
| Male/female | 17/9 | 10/15 |
| Age (yr) | ||
| Mean | 41.6 | 40.3 |
| Median | 41.7 | 36.4 |
| Range | 16-79 | 17-75 |
| ECOG status | ||
| 0-1 | 24 | 22 |
| 2 | 2 | 3 |
| WBC at diagnosis (×103/μL) | ||
| Mean | 22.8 | 31.0 |
| Median | 9.6 | 13.7 |
| Range | 1.9-124 | 1.1-171 |
| Immunophenotype | ||
| Early B-precursor | 5 | 5 |
| C-ALL | 12 | 11 |
| Pre-B | 5 | 2 |
| T-ALL | 4 | 7 |
| BCR/ABL rearrangement | 7/25 (28%) | 2/24 (8%) |
| Translocation (4;11) | 0/17 (0%) | 3/19 (16%) |
BCR/ABL rearrangement was determined by molecular analysis and translocation (4;11) by cytogenetic analysis. There was no statistically significant (P > 0.05) difference between both arms with regard to any of the patient characteristics given.
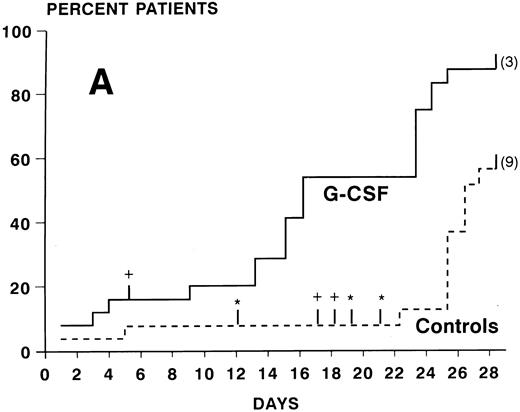
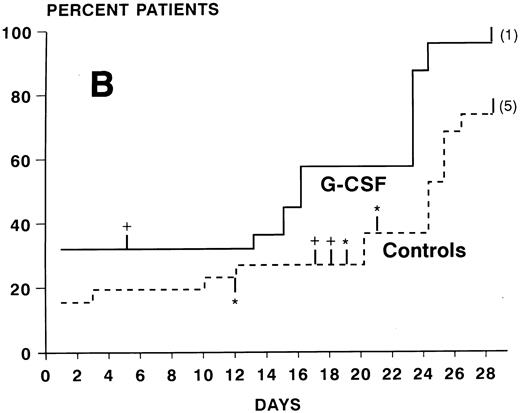
ANC during treatment.At study entry (day 2), ANC were slightly higher in the G-CSF group compared with the control arm. In the control group, there was a continuous slow decrease of ANC with median ANC levels less than 500/μL from day 7 to 23 and a slow recovery thereafter (Fig 2). In the patients receiving G-CSF, there was a substantial increase of ANC during the first week. The median ANC decreased to less than 500 at day 12 but only for 4 days. Subsequently, a steep increase in ANC values occured. The median times to recovery to ANC ≥1000 and ≥500/μL were significantly shorter in the G-CSF group as compared with the controls (ANC ≥1,000: 16 v 26 days, P < .0005, log-rank test; ANC ≥500: 16 v 24 days, P < .005) (Fig 3A and B). Whereas the ANC counts of 3 patients in the control group never decreased to less than 500/μL during induction chemotherapy, neutropenia less than 500 was prevented in 6 patients receiving G-CSF (Fig 3B). The median proportions of days with neutropenia below 1,000/μL or 500/μL were significantly lower in the G-CSF group as compared with the control group (29% v 84%, P < .00005; and 18% v 54%, P < .05, respectively; U-test). At the end of the study period, 20 of 25 G-CSF patients had complete neutrophil recovery (ANC counts ≥2,000/μl) as compared with 8 of 26 controls (P < .005, χ2 test).
ANC (median values and quartiles) in G-CSF patients (n = 25) and controls (n = 26).
ANC (median values and quartiles) in G-CSF patients (n = 25) and controls (n = 26).
Kaplan-Meier cumulative probability of neutrophil recovery ≥1,000/μL (A) and ≥500/μL (B) in G-CSF patients and controls. Tick marks indicate patients who have been censored because of death (+), because of G-CSF administration in the control group in case of a life-threatening infection in a severely neutropenic patient (*), or because of failure of recovery at day 28 (the numbers of these patients are indicated in parenthesis).
Kaplan-Meier cumulative probability of neutrophil recovery ≥1,000/μL (A) and ≥500/μL (B) in G-CSF patients and controls. Tick marks indicate patients who have been censored because of death (+), because of G-CSF administration in the control group in case of a life-threatening infection in a severely neutropenic patient (*), or because of failure of recovery at day 28 (the numbers of these patients are indicated in parenthesis).
Incidence of infectious complications.A total of 21 of 25 G-CSF patients remained afebrile during the study period, whereas almost half of the patients in the control arm (11/26) had fever greater than 38°C (Table 2). The percentage of patients with febrile neutropenia and with infections was significantly lower in the G-CSF group (12% and 40% v 42% and 77%, respectively, in controls; χ2 test). The reduction of infections was mainly due to fewer cases of septicemia, FUO, and pneumonia. The following microorganisms were cultured from the blood of controls and G-CSF patients, respectively: staphylococci (4/2), pseudomonas (2/0), streptococci (2/0), acinetobacter (1/1), Escherichia coli (1/0), enterobacter (0/1), and klebsiella (1/0). There was one documented pulmonary aspergillosis (in the control group) during the study period, which was fatal. A total of 3 patients of the control group received G-CSF (as provided in the protocol) at day 12, 19, and 21 because the treating physicians regarded these infections as life-threatening.
Frequency and Type of Infectious Episodes During Study Period
| . | Controls (n = 26) . | G-CSF . | P Value* . |
|---|---|---|---|
| . | . | (n = 25) . | . |
| No. of patients with fever | 11 (42%) | 4 (16%) | .079 |
| No. of patients with febrile neutropenia | 11 (42%) | 3 (12%) | .035 |
| No. of patients with documented infections | 20 (77%) | 10 (40%) | .017 |
| Septicemia | 8 | 2 | |
| Bacteremia | 3 | 2 | |
| FUO | 4 | 1 | |
| Pneumonia | 3 | 1 | |
| Oral infection | 5 | 3 | |
| Skin infection | 1 | 1 | |
| Herpes | 5 | 4 | |
| Otitis | 1 | 0 | |
| No. of episodes | 30 | 14 |
| . | Controls (n = 26) . | G-CSF . | P Value* . |
|---|---|---|---|
| . | . | (n = 25) . | . |
| No. of patients with fever | 11 (42%) | 4 (16%) | .079 |
| No. of patients with febrile neutropenia | 11 (42%) | 3 (12%) | .035 |
| No. of patients with documented infections | 20 (77%) | 10 (40%) | .017 |
| Septicemia | 8 | 2 | |
| Bacteremia | 3 | 2 | |
| FUO | 4 | 1 | |
| Pneumonia | 3 | 1 | |
| Oral infection | 5 | 3 | |
| Skin infection | 1 | 1 | |
| Herpes | 5 | 4 | |
| Otitis | 1 | 0 | |
| No. of episodes | 30 | 14 |
Fever is axillary temperature greater than 38°C; febrile neutropenia is fever and ANC less than 1,000/μL.
χ2 test.
Adverse events.The hematological toxicity on erythropoiesis and thrombopoiesis was similar in both treatment arms. The number of red blood cell transfusions and platelet concentrates was comparable in both groups (Table 3). The time to unsupported platelet counts of 100,000/μL was 21 days in the G-CSF group and 22 days in the control arm (data not shown). The main nonhematological toxicities were hyperbilirubinemia and hypofibrogenemia, which were similar in the 2 groups (Table 4). Hepatotoxicity required reduction of the daunorubicin dose in both groups (mean reduction 8% in the G-CSF arm v 7% in the control arm). There were 2 infectious deaths (invasive pulmonary aspergillosis at day 18 and klebsiella septicemia at day 17) in the control group and 1 death from pulmonary bleeding (without evidence of infection as proved by autopsy) at day 4 in the G-CSF arm.
Transfusion Requirements During Study Period
| . | Controls (n = 26) . | G-CSF (n = 25) . | P Value3-150 . |
|---|---|---|---|
| RBC concentrates | .62 | ||
| Mean | 5.9 | 5.6 | |
| Median | 6.0 | 5.5 | |
| Range | 0-12 | 0-15 | |
| Platelet concentrates | .72 | ||
| Mean | 1.7 | 1.8 | |
| Median | 0 | 1 | |
| Range | 0-10 | 0-11 |
| . | Controls (n = 26) . | G-CSF (n = 25) . | P Value3-150 . |
|---|---|---|---|
| RBC concentrates | .62 | ||
| Mean | 5.9 | 5.6 | |
| Median | 6.0 | 5.5 | |
| Range | 0-12 | 0-15 | |
| Platelet concentrates | .72 | ||
| Mean | 1.7 | 1.8 | |
| Median | 0 | 1 | |
| Range | 0-10 | 0-11 |
U-test (Mann-Whitney).
Adverse Events Recorded During Induction Therapy With (n = 25) and Without (n = 26) G-CSF
| Events . | Controls (all) . | G-CSF . | Controls (WHO > 2) . | G-CSF (WHO > 2) . |
|---|---|---|---|---|
| . | . | (all) . | . | . |
| Hyperbilirubinemia | 11 | 15 | 4 | 1 |
| Hypofibrinogenemia | 11 | 11 | 3 | 0 |
| Diarrhea | 5 | 1 | 1 | 1 |
| Constipation | 2 | 1 | 1 | 0 |
| Nausea/vomiting | 0 | 2 | 0 | 0 |
| Psychiatric symptoms | 0 | 1 | 0 | 1 |
| Peripheral neuropathy | 0 | 2 | 0 | 0 |
| Hyperglycemia | 2 | 1 | 1 | 0 |
| Gut perforation | 1 | 1 | 1 | 1 |
| Pneumothorax | 0 | 1 | 0 | 1 |
| Rash | 1 | 0 | 1 | 0 |
| Death | 2 | 1 | 2 | 1 |
| Events . | Controls (all) . | G-CSF . | Controls (WHO > 2) . | G-CSF (WHO > 2) . |
|---|---|---|---|---|
| . | . | (all) . | . | . |
| Hyperbilirubinemia | 11 | 15 | 4 | 1 |
| Hypofibrinogenemia | 11 | 11 | 3 | 0 |
| Diarrhea | 5 | 1 | 1 | 1 |
| Constipation | 2 | 1 | 1 | 0 |
| Nausea/vomiting | 0 | 2 | 0 | 0 |
| Psychiatric symptoms | 0 | 1 | 0 | 1 |
| Peripheral neuropathy | 0 | 2 | 0 | 0 |
| Hyperglycemia | 2 | 1 | 1 | 0 |
| Gut perforation | 1 | 1 | 1 | 1 |
| Pneumothorax | 0 | 1 | 0 | 1 |
| Rash | 1 | 0 | 1 | 0 |
| Death | 2 | 1 | 2 | 1 |
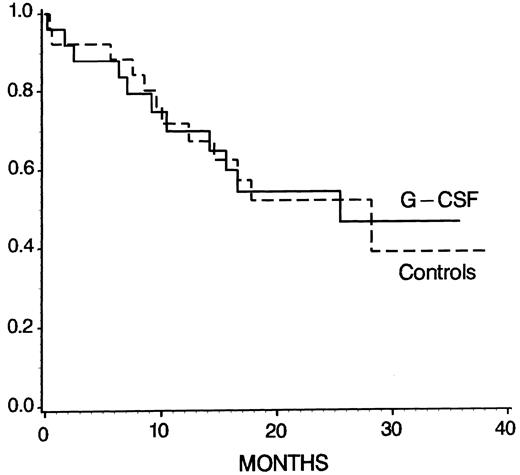
Overall outcome.The rate of complete remissions (CR) after the study period (phase I) was 24 of 25 (96%) in the G-CSF group and 20 of 25 (80%) in the controls, which was not significantly different (P = .192, χ2 test). In 1 patient of the control group, the remission status after the study period could not be determined because the patient was transferred to the surgical department because of gut perforation and refused further examination and treatment. In the control arm, 2 patients died and 3 were refractory after phase I but entered CR after phase II of the protocol. In the G-CSF group, 1 patient died but all others achieved CR after phase I. Figure 4 shows that the overall survival was not different among the treatment groups (at 28 months 47% ± 12% in G-CSF patients and 39% ± 14% in controls, P = .96; log-rank test). At 24 months, the probabilities of DFS and CCR were 55% ± 12% and 60% ± 12% in the G-CSF arm, and 46% ± 12% and 49% ± 13% in the control group, respectively, but again there were no statistically significant differences (DFS, P = .67; CCR, P = .49; log-rank test).
Kaplan-Meier survival probability for G-CSF patients (n = 25) and controls (n = 26) (P = .96, log-rank test). Patients undergoing allogeneic BMT were censored at the time of transplantation.
Kaplan-Meier survival probability for G-CSF patients (n = 25) and controls (n = 26) (P = .96, log-rank test). Patients undergoing allogeneic BMT were censored at the time of transplantation.
DISCUSSION
In contrast to other chemotherapy regimens in which cytostatic drugs are administered within a few days, the GMALL protocol, as well as some other ALL protocols, proposes the administration of drugs with hematotoxicity at weekly intervals for a prolonged period of time.1 G-CSF therapy in patients with acute myeloid leukemia (AML) and other malignancies is usually started after the end of CT. There may have been concern to use G-CSF in the GMALL protocol because of the recommendation to avoid the concomitant administration of growth factors and chemotherapy.17 However, our data indicate that, under the conditions of our study, ie, G-CSF administration starting at day 2 and continuing at least until day 22 of CT, G-CSF ameliorated neutropenia without any adverse effects on thrombopoiesis or erythropoiesis.
The results of this prospective randomized trial clearly show that G-CSF administered as an adjunct to induction therapy in patients with ALL not only shortens significantly the duration of neutropenia, but more importantly, reduces significantly the incidence of infections. In particular, the number of severe infections such as pneumonia and septicemia were much less frequent in patients receiving G-CSF. There were no infectious deaths in the G-CSF group but 2 deaths from infection in the control arm. It should be noted that 3 patients of the control group with life-threatening infections received G-CSF (according to the provision made in the protocol) and that this may have possibly prevented the fatal outcome of these infections.
In post-CT G-CSF studies, G-CSF shortens the time of severe neutropenia by approximately 1 week,7,18 but infectious complications occur mainly within the first 2 to 3 weeks from start of treatment when there is no effect of G-CSF on neutropoiesis. Under the condition of our study, the reduction of days with severe neutropenia was much more pronounced. Most of the patients had a biphasic neutrophil response that may be the result of G-CSF effects on both the postmitotic and mitotic pool of the myeloid compartment. Particularly important is the increase of neutrophils during the initial period of treatment. Subsequently, G-CSF was not able to completely prevent neutropenia in the majority of patients but significantly accelerated reconstitution of neutropoiesis as compared with controls. Thus, the additional effect of G-CSF in the early phase of treatment resulted in a marked reduction of days with neutropenia and may be one of the reasons for the fact that the clinical benefit of G-CSF to prevent chemotherapy associated neutropenic infections in our study was more impressive than in some post-CT G-CSF studies.18-20
The incidence of infections in the control group of our study was higher than it has been reported with the original protocol.1 In particular, septicemia was observed in almost 30% of patients not receiving G-CSF, as compared with 13% in the study by Hoelzer et al.1 The reason for this is not completely clear but it may be in part due to the higher dosage of daunorubicin used in this study (45 mg/m2) as compared with the original report (25 mg/m2). Despite this difference, the number of deaths during induction was approximately 10% similar in both studies.
One particularly important finding of this study is the fact that paradoxical myelosuppression due to the simultaneous administration of a growth factor and chemotherapy did not occur in this setting. There was also no evidence of a lineage steal on the other cell lineages by G-CSF because the number of platelet and red blood cell concentrates required was similar in both groups. In fact, paradoxically enhanced myelosuppression has been observed in patients receiving G-CSF and 5-FU concurrently.21 Moreover, an unexpectedly great incidence of severe thrombocytopenia occurred in breast cancer patients in whom G-CSF was administered, with the last dose administered 48 hours before the start of chemotherapy.22 GM-CSF coadministered with chemotherapy in a high-risk protocol for childhood ALL had neither a positive nor a negative effect on neutropoiesis.23 These findings indicate that the type and dose of cytostatic drugs used, as well as their schedule of administration, may be critical for the effect of a concurrent growth factor application and that each individual chemotherapy protocol has to be tested with regard to the feasibility of CSF administration.
There is some evidence in the literature suggesting that the actual dose of daunorubicin administered in induction plays a crucial role for the long-term outcome of adult ALL. In one retrospective analysis it has been found that patients receiving more or less than 175 mg/m2 of daunorubicin during induction had a median DFS of 44 and 12 months, respectively.24 Hoping to improve the results of induction treatment, we decided to escalate the dose of daunorubicin to 45 mg/m2 as compared with the original GMALL protocol. Despite this higher daunorubicin dose, hematological toxicity was mild in G-CSF–treated patients. However, surprisingly, hepatotoxicity was marked and observed in both treatment groups. In fact, hyperbilirubinemia occurred in more than half of patients that required similar dose reductions of daunorubicin in both arms. The reason for this finding remains unknown, but it may relate to the asparaginase preparation administered in this study that has been recently shown to be more effective and possibly more toxic than the asparaginase used in the original GMALL protocol.15,16 On the other hand, we cannot exclude that the escalation of the daunorubicin dose may have played a role because intensification of induction therapy for ALL was also associated with increased hepatic, gastrointestinal, and neurologic toxicity in another study.25 Therefore, despite ameliorating hematotoxicity by G-CSF, further dose escalation during this part of the protocol seems to be hampered by nonhematological toxicity.
The remission rate after the first phase of the induction protocol was slightly higher in the G-CSF arm as compared with the control group, but this difference was statistically not significant. Moreover, there was no significant difference in survival, DFS, and remission duration. Although these data may be of some interest, it must be stressed that the size and observation period of this study were not sufficient to draw major conclusions with regard to treatment outcome. Both groups were well-matched with respect to prognostic parameters like age, WBC count at diagnosis, and immunophenotype. Molecular and cytogenetic parameters were less well-balanced, but not significantly different. There were more BCR-ABL–positive patients in the control group, and more (4; 11) translocations in the G-CSF group that have also been shown to represent an unfavorable parameter.26
The fact that leukemic blasts can express G-CSF receptors in a subgroup of ALL patients and can be stimulated by G-CSF in vitro27,28 raises the concern about the potential stimulation of malignant cells by this growth factor. The limited data of this study do not indicate any increased relapse risk in G-CSF–treated patients. Moreover, remission duration was not negatively affected by G-CSF in a larger randomized trial that included patients with ALL and AML.7 The safety of CSFs in patients with acute leukemia is also supported by several AML trials, indicating that, despite the marked AML cell-stimulating potential of G-CSF and GM-CSF in vitro, in vivo propagation of AML did not occur if these growth factors were administered as an adjunct to chemotherapy.29
In conclusion, our data suggest that G-CSF is safe and effective, that it reduces neutropenia, and that it thereby reduces infectious morbidity in phase I of induction treatment of ALL if it is administered concurrently with chemotherapy. Although this was not an objective of this open label study, a potential reduction in the number of hospital days due to accelerated neutropoietic reconstitution by G-CSF may also translate into a socioeconomic benefit.
Presented in part at the American Society of Hematology Meeting, Nashville, TN, December 2-6, 1994
Address reprint requests to Klaus Geissler, MD, Abteilung für Hämatologie und Hämostaseologie, Klinik für Innere Medizin I, AKH Wien, Währinger Gürtel 18-20, A-1090 Vienna, Austria.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal