Abstract
The putative chemokine receptor BLR1 has been identified as the first G-protein–coupled receptor involved in B-cell migration and in microenvironmental homing to B-cell follicles and to germinal centers. In healthy individuals, expression of BLR1 is restricted to all mature recirculating B cells and to a subpopulation of T-helper memory cells. In the present study, we analyzed the distribution of BLR1 on defined lymphocyte subsets during the progression of acquired immunodeficiency syndrome. It is shown that the proportion of T-helper memory cells coexpressing BLR1 continuously decreases during the infection, whereas a high proportion of γ/δ T cells expressing BLR1 can be found in peripheral blood. The latter subpopulation is restricted to lymphoid tissues in healthy individuals. Most interestingly, in 75% of all human immunodeficiency virus (HIV)+ individuals, peripheral blood B cells were identified as not expressing BLR1 and phenotypically resembling germinal center cells of lymphoid tissue. Using BLR1 as a marker molecule, this study identifies peripheral blood lymphocytes in HIV+ individuals that are usually restricted to lymphoid tissue in healthy individuals. Because HIV infection is active in lymphoid tissue even at the clinically latent stage, aberrant expression of the B-cell homing chemokine receptor BLR1 might be an early indicator for the onset of destruction of lymphoid tissue.
INFECTION WITH THE human immunodeficiency virus (HIV) is the initial event of a complex and multifactoral disease characterized by progressive dysfunction and final destruction of the immune system.1 Recent advancement in the understanding of both virus infection and virus replication led to a new understanding of HIV pathogenesis. Until recently, the lag period between infection with the virus and onset of the disease had been interpreted as the virus laying quiescent in infected individuals for years before it is activated. However, recent evidence suggests that up to 109 virions are produced and removed daily by the immune system, even at the latent phase of the disease.2,3 Because lymphoid organs such as lymph nodes can harbor high amounts of the virus even when they cannot be detected in peripheral blood,4-6 these organs are of particular importance in the study of the pathogenesis of acquired immunodeficiency syndrome (AIDS). Major progress in the understanding of the mechanisms underlying the initial interactions between virus and target cell has been recently achieved by the identification of new HIV receptors. It has been convincingly shown that the chemokine receptor CXCR4 (formerly termed fusin or LESTR) is used by lymphotropic strains of HIV-1 as a coreceptor needed for virus entry,7 whereas macrophage-tropic strains and primary isolates need the chemokine recepors CCR5 or CCR3 for productive infection of target cells.8-12 These results help to explain earlier observations that showed that expression of CD4 in nonhuman cell lines is not sufficient for productive infection with HIV-1, although CD4 mediates firm attachment of the virus to the surface of the cells.13 14
Chemokine receptors belong to the large family of heterotrimeric, G-protein–coupled receptors that are characterized by seven hydrophobic transmembrane spanning domains (reviewed in Murphy15 ). These receptors have been shown to play a major role in inflammatory and allergic processes by directing neutrophils, eosinophils, and macrophages to inflammed or infected areas of the body (reviewed in Baggiolini et al16 ). We have identified a novel member of this superfamily termed BLR117,18 and shown that BLR1 is the first lymphocyte-specific member of this gene family, because it is only expressed on mature recirculating B cells and a subpopulation of T-helper memory cells.19 Sequence alignment of chemokine receptors identified a subgroup within this receptor family currently consisting of BLR1, BLR2/EBI1, and CXCR4.15 Because the physiologic ligands for BLR1 and BLR2 have not yet been identified and as SDF-1 has only recently been recognized as a ligand for CXCR4,20,21 little is known about the function of these receptors. Using blr1 knock-out mice, we succeeded in identifying BLR1 as a major regulator for B-cell migration. Mice lacking this receptor had no or only a few morpholocically altered Peyer's patches, showed altered structure of the splenic white pulp, and had no functional germinal centers (GC) in the spleen and in Peyer's patches.22
Because GC, the area of isotype switching, and B-cell maturation have been identified as major reservoirs for HIV and are subjected to progressive histopathologic changes during infection with the virus,4,5 23-27 we analyzed the expression of BLR1 in HIV+ individuals. We show aberrant expression of the receptor on both B and T cells, even at the clinically latent stage, and provide evidence that expression of BLR1 on defined peripheral blood lymphocyte subsets might reflect activating and remodelling processes leading to the destruction of lymphoid tissues by HIV.
MATERIALS AND METHODS
Seventy-seven adult HIV+ patients at various stages of the disease were admitted to the Staedtische Krankenhaus Muenchen-Schwabing and randomly enrolled in this study. Patients were classified either according to the Walter Reed staging classification 28 or according to their absolute CD4 cell counts (>500/μL, between 200 and 500/μL, and <200/μL). The control group consisted of age-matched healthy individuals working at our laboratories.
The following antibodies were used in this study: anti-CD19, anti-CD4, anti-CD8, anti–αβ-T-cell receptor (TCR), anti–γ/δ-TCR, and anti-CD25 were purchased from Becton Dickinson (Heidelberg, Germany). Anti-CD45R0, anti-CD23, anti-CD38, and anti-CD10 were purchased from Dianova (Hamburg, Germany), and anti-CD3 (clone 26-II-8; rat IgG2b ) was kindly provided by Dr Rolf Schuh (Fresenius, Munich, Germany). The anti-BLR1 monoclonal antibody (MoAb) RF8B2 has been described elsewhere.19
Peripheral blood was anticoagulated with EDTA and erythrocytes were lysed with a NH4Cl solution. For triple fluorescence analysis, white blood cells were incubated with biotinylated anti-BLR1 MoAb (1 μg/mL for 20 minutes at 12°C). Cells were then stained simultaneously with streptavidin-cychrome (1:200; Pharmingen, San Diego, CA) and further stained by fluorescein isothiocyanate (FITC)- and/or phycoerythrin (PE)-conjugated primary antibodies at saturating amounts for 20 minutes at 12°C. After two final washings, lymphocytes were analyzed with a FACScan flow cytometer (Becton Dickinson).
Statistical analysis was performed using the Mann-Whitney test. In those experiments in which HIV+ individuals were allocated into subgroups, P values were further subjected to Bonferroni-Holm conditions (α = .05).
RESULTS
Expression and distribution of the G-protein–coupled receptor BLR1 on peripheral blood lymphocytes was analyzed using a panel of MoAbs and three-color flow cytometry. HIV-seropositive individuals were either allocated into 6 groups in accordance to the Walter Reed classification (WR1 through WR6) or into three groups reflecting to the total numbers of CD4+ cells per microliter (>500/μL, 200 to 500/μL, and <200/μL). Because the expression of BLR1 on T cells is restricted to a subpopulation of CD4+CD45RO+19 T-helper memory cells, we analyzed these subpopulations in HIV+ individuals. Interestingly, the proportion of T-helper cells with a memory phenotype (CD4+CD45RO+) significantly increased with decreasing lining total CD4 counts, whereas the proportion of CD4+CD45RO+ coexpressing BLR1 decreased (Fig 1). In healthy controls, 45% of CD4+ cells were associated with the CD45RO isoform, and 25% of this double-positive population coexpressed BLR1. In the group of HIV+ individuals with absolute CD4 counts greater than 500/μL, between 200 and 500/μL, and less than 200/μL, the percentage of CD4+ cells expressing CD45R0 increased to 57%, 68%, and 85%, respectively. In contrast, the percentage of CD4+CD45RO+ cells coexpressing BLR1 decreased to 17%, 13%, and 10%, respectively (Fig 1). These data suggest that the percentage of T-helper cells with a memory phenotype increases during the progression of the disease, whereas T-helper memory cells coexpressing BLR1 are progressively diminished.
Frequency of CD4+ CD45R0+ memory cells and of CD4+ CD45R0+ memory cells coexpressing BLR1 in healthy and HIV-infected subjects. Peripheral blood lymphocytes (PBL) were stained with CD4-FITC, CD45R0-PE, and BLR1-biotin/streptavidin-cychrome as described in the Materials and Methods. Gates were either set at CD4+ cells to determine the percentage of T-helper cells associated with the CD45R0 isoform (○) or at CD4+ CD45R0+ cells to determine the percentage of T-helper memory cells coexpressing BLR1 (•). Results are the mean ± SD. *P < .05, **P < .01, and ***P < .001, always versus HIV− control (Mann-Whitney test).
Frequency of CD4+ CD45R0+ memory cells and of CD4+ CD45R0+ memory cells coexpressing BLR1 in healthy and HIV-infected subjects. Peripheral blood lymphocytes (PBL) were stained with CD4-FITC, CD45R0-PE, and BLR1-biotin/streptavidin-cychrome as described in the Materials and Methods. Gates were either set at CD4+ cells to determine the percentage of T-helper cells associated with the CD45R0 isoform (○) or at CD4+ CD45R0+ cells to determine the percentage of T-helper memory cells coexpressing BLR1 (•). Results are the mean ± SD. *P < .05, **P < .01, and ***P < .001, always versus HIV− control (Mann-Whitney test).
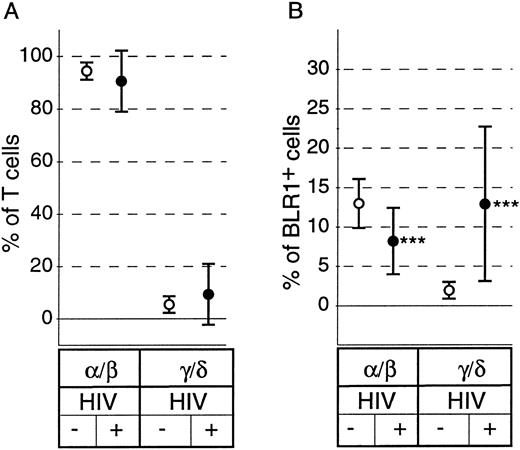
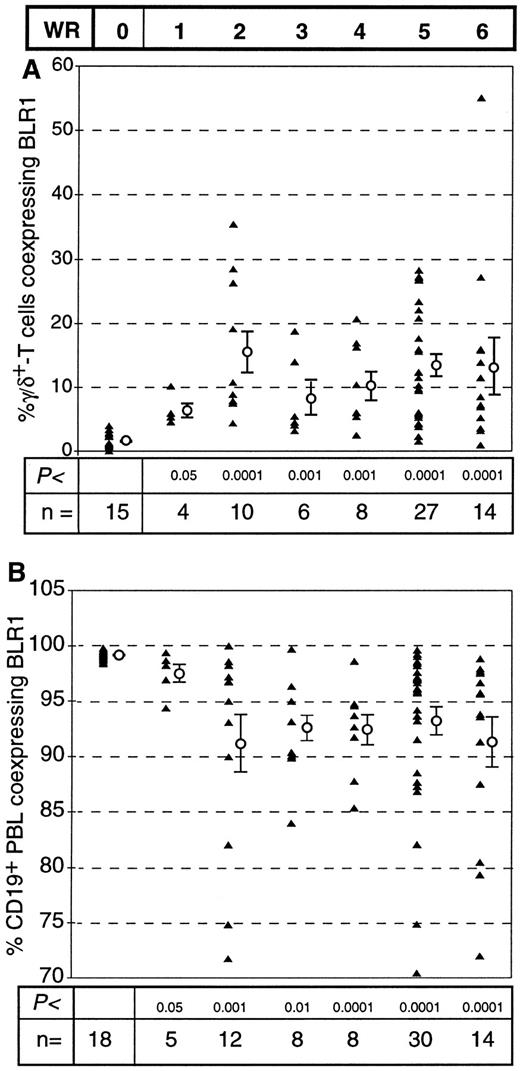
Although there have been conflicting reports on the relative percentage of peripheral blood γ/δ+ T cells, there is evidence that characteristic changes of the subset distribution within the γ/δ+ T cells occur in HIV+ individuals.29 As shown in Fig 2A, there was no significant difference in the percentage of peripheral blood α/β+ T cells or γ/δ+ T cells between healthy controls and HIV+ individuals (P = .34 and P = .36, respectively). However, striking differences in the distribution of BLR1+ cells within the α/β or γ/δ compartment were observed between controls and HIV+ individuals. The percentage of α/β+ T cells expressing BLR1 was reduced (13.1% v 8.1%, P < .0001; Fig 2B), whereas the percentage of γ/δ+ T cells expressing BLR1 was strongly increased in HIV patients compared with normal controls (1.8% v 12.7%, P < .0001; Fig 2B). Further analysis showed that the frequency of γ/δ+ T cells coexpressing BLR1 had already significantly increased in HIV+ patients without any sign of the disease (WR1) and that progression of the disease leads to increased levels of peripheral recirculating γ/δ+ BLR1+ T cells (Fig 3A). Interestingly, these observations are paralleled by the irregular expression of BLR1 on B cells, because BLR1− B cells are also detected in WR1 HIV+ individuals (Fig 3B). Their frequency increases during the progression of AIDS and approximately 75% of all HIV patients contain B cells not expressing BLR1 (Fig 3B). Further analysis of this subpopulation showed that their phenotype resembles those of germinal center cells: they are large cells (as determined by forward scatter intensities; Fig 4A) and the majority (75%; range, 50% to 99%; n = 8) express high levels of CD38 (Fig 4B). Interestingly, other markers frequently observed on activated B cells, such as CD10, CD23, or CD25, were not expressed at detectable levels (data not shown).
Distribution of α/β+ and γ/δ+ T cells and coexpression of BLR1 on these subpopulations on PBL derived from healthy (○; n = 15) or HIV-infected subjects (•; n = 69). PBL were stained with anti-BLR1-biotin/strepavidin-cychrome and were either incubated with anti-α/β+ TCR-FITC MoAb or with anti-γ/δ+ TCR-FITC MoAb. The percentage of T cells expressing α/β or γ/δ chains was calculated (A) and the percentage of BLR1+ cells in each subpopulation was determined (B). Results are the mean ± SD. ***P < .001 versus HIV− control.
Distribution of α/β+ and γ/δ+ T cells and coexpression of BLR1 on these subpopulations on PBL derived from healthy (○; n = 15) or HIV-infected subjects (•; n = 69). PBL were stained with anti-BLR1-biotin/strepavidin-cychrome and were either incubated with anti-α/β+ TCR-FITC MoAb or with anti-γ/δ+ TCR-FITC MoAb. The percentage of T cells expressing α/β or γ/δ chains was calculated (A) and the percentage of BLR1+ cells in each subpopulation was determined (B). Results are the mean ± SD. ***P < .001 versus HIV− control.
Different expression pattern of BLR1 on γ/δ+ T cells (A) and on B cells (B) during the progression of AIDS. The BLR1-phenotype of peripheral blood B cells was determined as described for Fig 1 using anti-BLR1 MoAb and either anti-γ/δ MoAb or anti-CD19 MoAb. HIV+ individuals were grouped according to the Walter Reed classification. Results of each experiment (▴) and the mean ± SEM for each group are shown (Mann-Whitney test with Bonferroni-Holm conditions, α = .05).
Different expression pattern of BLR1 on γ/δ+ T cells (A) and on B cells (B) during the progression of AIDS. The BLR1-phenotype of peripheral blood B cells was determined as described for Fig 1 using anti-BLR1 MoAb and either anti-γ/δ MoAb or anti-CD19 MoAb. HIV+ individuals were grouped according to the Walter Reed classification. Results of each experiment (▴) and the mean ± SEM for each group are shown (Mann-Whitney test with Bonferroni-Holm conditions, α = .05).
Characterization of BLR1− B cells. PBL were incubated with CD19-PE, BLR1-biotin/strepavidin-cychrome, and FITC-conjugated CD38 MoAb. CD19+ cells were gated and analyzed. If not indicated otherwise, the horizontal and vertical scales are log 10 antibody fluorescence intensities. Lymphocyte forward scatter intensity (FSC) reflects cell size. Representative experiments from an HIV+ (WR5) and an HIV− individual are shown.
Characterization of BLR1− B cells. PBL were incubated with CD19-PE, BLR1-biotin/strepavidin-cychrome, and FITC-conjugated CD38 MoAb. CD19+ cells were gated and analyzed. If not indicated otherwise, the horizontal and vertical scales are log 10 antibody fluorescence intensities. Lymphocyte forward scatter intensity (FSC) reflects cell size. Representative experiments from an HIV+ (WR5) and an HIV− individual are shown.
DISCUSSION
We identified the G-protein–coupled receptor BLR1 that is expressed on mature B cells and a subpopulation of T-helper memory cells as the first lymphocyte-specific member of this gene family.19 BLR1 shows significant relationship to receptors for interleukin-8 and other members of the chemokine receptor family.17,18 Although the ligand for BLR1 is currently unknown, it seems likely that this receptor belongs to the chemokine receptor family, because a characteristic sequence motive at the end of transmembrane domain III, DRYLAIVH, that is unique to members of this family is also present in BLR1 (reviewed in Murphy15 ). Chemokine receptors are known to act as regulators of leukocyte migration during inflammatory processes (reviewed in Baggiolini et al16 ). Using gene targeted mice carrying a mutation in the blr1 locus, we could identify BLR1 as the first chemokine receptor regulating B-cell homing to defined lymphoid organs.22 These animals showed a severe impairment in the development of Peyer's patches and the formation of splenic follicles was found to be aberrant. Although high numbers of germinal founder cells were present in this organ, no functional GC developed. Furthermore, upon adoptive transfer into wild-type mice, B cells isolated from blr1-mutant mice entered the T-cell–rich area but failed to migrate to the B-cell follicle.22 These data strongly suggest that expression of BLR1 is required for navigating GC founder cells through the T-cell zone into the B-cell follicle, where they then initiate the formation of GC. Interestingly, once GC cells undergo proliferation and isotype switching, BLR1 gets downregulated.19 These data indicate that BLR1 is essentially involved in the formation of GC. Interestingly, recent evidence shows that chemokine receptors not only direct leukocyte migration but also that some members such as CXCR4, CCR5, or CCR3 are essentially required to allow productive infection with the human immunodeficiency viruses HIV-1 and HIV-2.7-12
Both activation and aberrant function of B cells are known to occur during HIV infections and it seems that processes within GC are involved in the progression of the disease. In situ hybridization showed that high numbers of virus particles can be identified in germinal centers.30 Furthermore, persistent generalized lymphadenopathy, characterized by progressive fragmentation and degeneration of follicular dentritic cells leading to follicular depletion, represents an early abnormality in HIV patients.31 Early reports have described an increased responsiveness of peripheral B cells to mitogens, hypergammaglobulinemia with a predominant anti-HIV reaction, and spontaneous Ig secretion in vitro.32,33 The source of B-cell activation has been unclear, because in vitro stimulation with native viral proteins did not trigger lymphocyte proliferation. Moreover, removal of CD8+ lymphocytes from culture could not prevent spontaneous Ig secretion and it has been suggested that membrane-bound tumor necrosis factor-α may be involved in this process.34,35 These effects could be explained also by the escape of activated germinal center B cells to the peripheral blood pool. GC B cells are large cells in rapid cell cycle and are further characterized by the expression of high levels of CD38 and by the absence or low expression of BLR1.19 Alternatively, the B cells may undergo abnormal maturation in the GC, where they become persistently activated and enlarged.
In HIV-seropositive individuals, we identified a subpopulation of large CD19+ B cells with high levels of CD38 but not expressing BLR1 (Fig 4). This cell type is not observed in the peripheral blood of healthy individuals (Figs 3B and 4). It is conceivable that they may represent B blasts or centroblasts that escaped from the GC, eg, due to the destruction of the follicular dentritic cell network31 or due to a failure to undergo apoptosis caused by altered expression of cytokines or other cellular molecules (eg, bcl-2 and Apo). The appearance of BLR1− peripheral blood B cells represents an early abnormality in HIV patients, because it is already detectable in WR1 patients. Soon after the patients show any clinical symptoms (WR2 through WR6), the frequency of peripheral blood B cells not expressing BLR1 increases (Fig 3B).
The expression of different isoforms of CD45 has been postulated to distinguish naive from memory T cells. It is thought that expression of the high molecular weight isoform CD45RA characterizes naive T cells, whereas CD45R0, the low molecular weight isoform, characterizes antigen-experienced memory T cells.36 In this study, we have shown that the percentage of classically defined T-helper memory cells (CD4+ cells coexpressing CD45R0) increases with decreasing total CD4 counts. These findings are contrasted by the functional loss of immunologic memory during the progression of the AIDS reported earlier (reviewed in Helbert et al37 ). However, because the proportion of CD4+ CD45R0+ cells coexpressing BLR1 parallels both loss of immunologic memory and progression of the disease (Fig 2), it seems that BLR1 is a suitable marker to define functional competent T-helper memory cells in HIV+ individuals.
At present, the function of lymphocytes expressing γ/δ TCR and its alteration under pathologic conditions is poorly understood. These cells are frequently found in the spleen and, to a lesser extent, in the thymus, tonsils, and the lung.38,39 A transient increase in the number of circulating γ/δ+ T cells has been reported in malaria infected individuals,40 and data show changes within the γ/δ+ T-cell subset in HIV+ individuals.29 Interestingly, in healthy individuals, only 1% to 2% of peripheral blood γ/δ+ T cells coexpress BLR1, whereas about 23% of tonsillar γ/δ+ T cells express BLR1 (R.F., unpublished results). In the present study, we did not find significantly altered γ/δ+ T-cell numbers in the HIV+ group; however, there was a significant shift from α/β+ T cells to γ/δ+ T cells coexpressing the BLR1 molecule in HIV individuals (Fig 3). As shown above for B cells, it seems that, in the peripheral blood pool of HIV+ individuals, a γ/δ+ T-cell subpopulation can be identified that is usually restricted to lymphoid tissue. These findings are supported by others describing HIV infection as both active and progressive in the lymphoid organs during the clinically latent period of HIV infection.4-6 Because BLR1 belongs to a receptor family involved in lymphocyte migration, it will be of interest to elucidate whether deregulated expression of BLR1 participates in the pathogenesis of lymphomas frequently found in HIV patients.
In summary, this study shows that deregulated expression of the B homing chemokine receptor BLR1 is an early event during the pathogenesis of AIDS, most probably reflecting the stage of activation and destruction of lymphoid tissues even during the clinically latent period of HIV infection.
ACKNOWLEDGMENT
The authors thank Brigitte Scherz and Dr G. Bernhardt for critically reading this manuscript.
Address reprint requests to Reinhold Förster, Dr Med Vet, Max-Delbrück-Center for Molecular Medicine, Robert-Rössle-Str. 10, D-13122 Berlin-Buch, Germany.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal