Abstract
The possibility that serum ferritin is a secreted protein and an acute phase reactant regulated by inflammatory hormones and iron was examined in a hepatic cell line that secretes plasma proteins. Differentiated rat hepatoma cells released albumin and ferritin into the medium, as determined by rocket immunoelectrophoresis and isolation of ferritin by standard procedures plus immunoaffinity chromatography, following labeling with radioactive amino acid. Administration of interleukin-1–β (IL-1) or tumor necrosis factor-α (TNF) doubled the amounts of ferritin released into the medium over 24 and 48 hours. Together, the cytokines had more than an additive effect. Albumin secretion was diminished by IL-1, but not TNF. Iron, administered as an iron dextran complex or as a 1:1 chelate with nitrilotriacetate (Fe-NTA), also enhanced ferritin release, but had no effect on albumin. Intracellular ferritin concentrations did not change significantly with cytokine treatment, but increased in response to iron. With or without treatments, release of ferritin and albumin from cells into the medium was inhibited by brefeldin A, an inhibitor of Golgi function. The effect of each of the cytokines and of iron on ferritin and albumin was also blocked by dichlorofuranosylbenzimidazole (DRB), an inhibitor of transcription. The stimulatory effect of Fe-NTA on ferritin secretion was diminished by TNF, and this was partially counteracted by IL-1, indicating additional regulatory complexity. These results show for the first time that hepatic cells secrete ferritin, that this ferritin secretion is regulated by iron and inflammatory cytokines, and that the mechanisms of regulation differ from those for intracellular ferritin. The results would explain why serum ferritin increases in inflammation or when iron flux is enhanced.
FERRITINS HAVE LONG been known as the main site for intracellular storage of excess iron in mammalian tissues.1-4 Wide ranging structural and molecular biological studies of this protein have established that most ferritins are composed of 24 subunits of at least two types (L and H) in the form of a symmetrical shell within which up to 4,500 atoms of iron are deposited. Ferritin synthesis is increased by iron, and this response is primarily translational,5,6 involving regulatory proteins IRP1 and IRP2 binding to a stem loop structure (iron response element; IRE) in the 5′ untranslated region of the mRNA.4 8-13
Most excess iron is in ferritin of liver, spleen, and bone marrow and comprises the largest iron pool outside of hemoglobin, the main iron pool.14 Lower concentrations of ferritin are probably found in the cells of all tissues, and traces are found extracellularly in blood and other body fluids, termed serum ferritin. In the history of ferritin, serum ferritin is a relative newcomer and its nature is poorly defined. Discovered in the early 1970s by immunoassays that use antibodies against liver and spleen ferritin,15 its existence has become widely known because of its ability to predict the abundance of body iron stores.16-18 In the absence of certain diseases, concentrations of serum ferritin correlate positively with concentrations of intracellular ferritin iron, suggesting some sort of equilibration between intra and extracellular ferritins. However, serum ferritin concentrations relate not just to iron status, but increased acutely in several physiologic conditions. These include iron treatment of iron deficiency, cancer, and inflammation or infection. The significance and mechanism of these elevations in serum ferritin have been obscure, and overall, it has been unclear whether it results from leakage of intracellular ferritin due to cell damage, and/or whether there is deliberate secretion of ferritin from specific cells and organs.
Studies of Worwood and others,15,19 many years ago found that some serum ferritin contains carbohydrate, as determined by binding to concanavalin A and the carbohydrate staining of a 23 k subunit (larger than the H or L subunits of intracellular ferritin20 ). Evidence from ongoing studies in our laboratory21 concurs that the subunits of serum ferritin are larger. Moreover, their amino acid sequence appears to be distinct from those of intracellular L and H ferritins, and they most likely are the product of different genes. The presence of carbohydrate suggested that serum ferritin is secreted (intracellular ferritin contains no carbohydrate). Bolstering the secretion concept were additional observations from our and another laboratory22-25 that mRNA hybridizing with cDNA for intracellular ferritin is with polyribosomes bound to the endoplasmic reticulum (ER), as would be expected for a secreted protein. Also, the proportion of ferritin message associated with the ER increased in inflammation,23 when serum ferritin increases.
Konijn et al26,27 were the first to establish that the liver responds to inflammation by increasing the rate of synthesis of intracellular ferritin by the liver, a result confirmed by us.23 This was supported by studies of Rogers et al28,29 with HepG2 cells, treated with IL-1 or IL-6 in which intracellular H and L ferritin synthesis was enhanced. The latter studies (and some of ours23 ) also indicated that regulation by IL-1 was at the level of translation. Thus, the rate of synthesis of intracellular ferritin appears to be stimulated both by inflammatory cytokines and by iron, both acting primarily at the level of translation.
Levels of serum ferritin also increase in response to these two kinds of stimulae, and it seemed possible to us that some of the increase in ferritin biosynthesis observed in liver cell extracts might reflect an increase in the rate of synthesis of ferritin destined for the serum. Indeed, we observed that inflammation did not increase the concentration of ferritin (or ferritin iron) in liver cells,24 suggesting that the increased ferritin synthesized might at least in part have been secreted. Although we had observed an increase in the proportion of ferritin L and H mRNA associated with the ER-bound polyribosomes after induction of turpentine inflammation in rats23,24 (as would be expected if ferritin were increasingly secreted during inflammation), no one had previously shown that ferritin is actually secreted by hepatic cells. In fact, Cairo et al24,25 were unable to show sequestration of H or L ferritin subunits in ER vesicles during in vitro translation in the presence of canine microsomes. Also, H and L ferritin mRNAs contain no sequence coding for a traditional signal peptide. Based on ongoing studies,21 we believe these apparent contradictions are the result of serum and intracellular ferritins being encoded by homologous, but different mRNAs; that both kinds of proteins interact (but interact differently) with antibodies against intracellular ferritin; and that serum ferritin mRNAs can hybridize with specific probes for intracellular ferritin L and H, depending upon the conditions used. The studies reported here establish for the first time that a form of ferritin is actually secreted by hepatic cells and that, contrary to what occurs with intracellular ferritin in hepatic cells, production and secretion of this ferritin is under transcriptional regulation by inflammatory hormones and iron.
MATERIALS AND METHODS
Cell culturing and treatments. Rat hepatoma cells (H4-II-E-C3) obtained from the American Type Culture Collection (Rockville, MD) were grown for 3 to 4 days on Dulbecco's Modified Eagle's Medium (Sigma Chemical Co, St Louis, MO), either with 20% horse serum and 5% fetal calf serum (for the leucine incorporation studies), or with 5% iron-supplemented bobby calf serum and 15% horse serum. This was followed by 24 to 72 hours in the same medium or in serum-free, protein-free hybridoma medium (S2772, from Sigma). During the last 48 hours, murine IL-1-β (1 to 20 ng/mL), murine tumor necrosis factor (TNF)-α (0.5 to 10 ng/mL) (both cytokines from Sigma), and/or iron were added to some of the cultures. The iron added was either in the form of the 1:1 molar complex of Fe(III)-nitrilotriacetate (Fe-NTA) or in the form of an iron-dextran complex (Imferon, Merrell National Laboratories, Cincinnati, OH). At the end of each experiment, the medium was aspirated off for analysis of albumin and ferritin in the medium, and for LDH activity.30 Cells were washed, released by 2 minutes incubation with 0.1% trypsin, transferred to centrifuge tubes with sterile isotonic saline, and collected by centrifugation. They were then either frozen at −85°C or homogenized directly, for determination of protein content and cell number. The cell line was found to have a protein content of 0.7 ± 0.2 mg per 106 cells (mean ± standard deviation [SD] for five determinations), which is close to the 0.8 mg value for adult rat liver cells.31 Nonfrozen cell homogenates (in 20 mmol/L K phosphate, pH 7.0, containing 0.02% NaN3), were assayed for ferritin after heating to 70°C for 10 minutes and centrifuging off coagulated proteins. Cell number was determined by counting orcein-stained nuclei in a hemocytometer, as previously described.32 Protein was determined by the Bradford assay using the reagent and protocol of BioRad (Richmond, CA). In one study, L-4,5-3H-leucine (specific activity 58 Ci/mmol; Amersham, Arlington Heights, IL) was added to cells during culturing, at a rate of 2 μCi per flask, to determine whether ferritin synthesized by the cells was released into the medium. Radioactive samples were counted in a Tri-Carb Liquid Scintillation Analyser (Model 2200 CA, Packard, Canberra, Australia), after addition of scintillation fluid (Fluorodyne; National Diagnostics, Manville, NJ). In some cases, brefeldin A (from Sigma) was added to the cultures at the same time as IL-1, TNF, or iron, at doses of 1 to 4 μg/mL to inhibit Golgi function.33 5,6-Dichloro-ribofuranosylbenzimidazole (DRB; from Sigma) was added to some flasks (4 ng/mL) to inhibit transcription.34
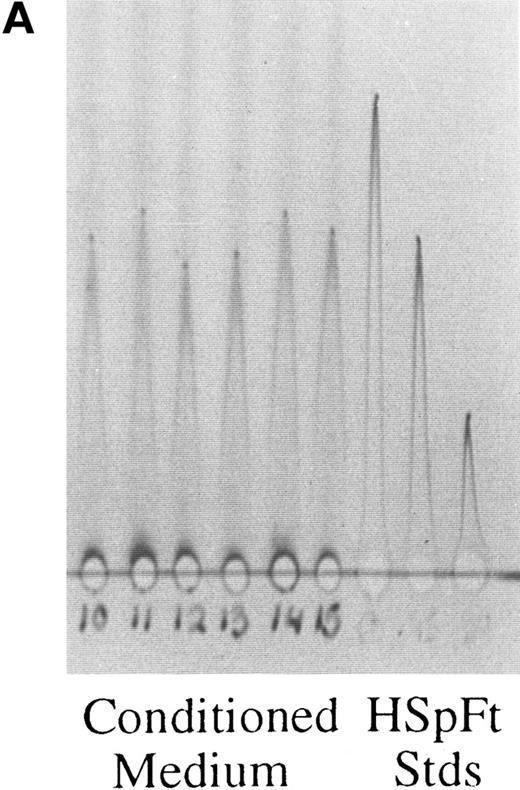
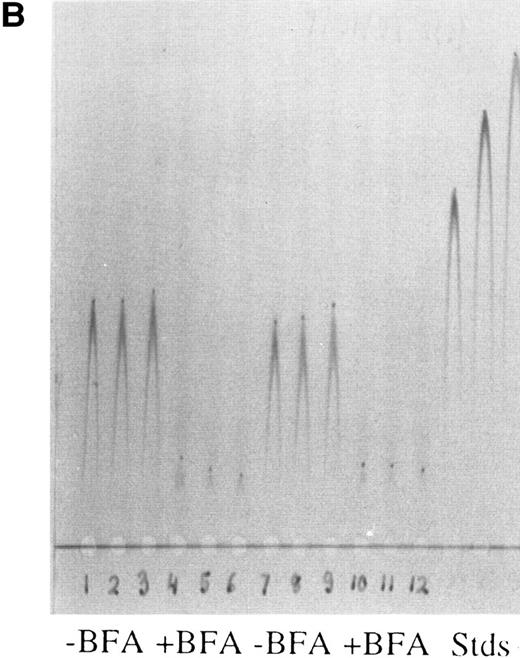
Rocket immunoelectrophoresis of ferritin (A) and albumin (B) in hepatoma cell medium. (A) Rockets obtained with concentrated samples of conditioned medium from hepatoma cells in comparison with standards (Stds) of horse spleen ferritin (HSpFt) (3 slots on far right). Samples (6 lanes on the left) are from individual flasks of cells grown for 48 hours in protein-free medium (see Materials and Methods). Standards contained 10, 6, and 2 μg/mL horse spleen ferritin protein (from left to right). (B) Samples of medium from cells that were and were not treated with the Golgi function inhibitor, brefeldin A (+ BFA; 4 μg/mL), in comparison with rat albumin standards (Stds; 3 lanes on far right). Lanes 1 to 3 and 7 to 9 from left were from untreated cells, lanes 4 to 6 and 10 to 12 were from BFA treated cells, as indicated.
Rocket immunoelectrophoresis of ferritin (A) and albumin (B) in hepatoma cell medium. (A) Rockets obtained with concentrated samples of conditioned medium from hepatoma cells in comparison with standards (Stds) of horse spleen ferritin (HSpFt) (3 slots on far right). Samples (6 lanes on the left) are from individual flasks of cells grown for 48 hours in protein-free medium (see Materials and Methods). Standards contained 10, 6, and 2 μg/mL horse spleen ferritin protein (from left to right). (B) Samples of medium from cells that were and were not treated with the Golgi function inhibitor, brefeldin A (+ BFA; 4 μg/mL), in comparison with rat albumin standards (Stds; 3 lanes on far right). Lanes 1 to 3 and 7 to 9 from left were from untreated cells, lanes 4 to 6 and 10 to 12 were from BFA treated cells, as indicated.
Isolation of ferritin from the medium. Ferritin was isolated from the medium by a modification of the procedure of Cragg et al20 for serum ferritin, as described elsewhere.21 Medium was brought to pH 4.8 with 0.1 mmol/L Na acetate (pH 4.8), and 0.1 N acetic acid and heated to 70°C for 10 minutes, then cooled on ice. After centrifugation, the precipitate was discarded, and the heating and centrifugation procedure repeated. The final supernatant, brought back to pH 6.8, was treated with granular ammonium sulfate to 50% saturation overnight at 4°C, and the ferritin-containing precipitate, dissolved in a minimal amount of K phosphate buffer (10 mmol/L, pH 7.0, containing 0.02% NaN3), was applied to immunoaffinity chromatography, using a gel prepared with the gamma globulin fraction of antiserum raised in a rabbit against horse spleen ferritin (the injected ferritin only had H and L subunits, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS PAGE]). After incubation of the extract with the affinity gel for 1 hour or more, at room temperature, nonbinding proteins were eluted by extensive washing of the gel in a column, until the 280 nm absorbance of effluent was less than 0.20. The ferritin was then eluted with 3 mmol/L K thiocyanate in the same buffer. The protein eluting was pooled and dialyzed into the nonthiocyanate containing phosphate buffer (with 0.02% NaN3). Protein determinations were by the Bradford procedure, using the protocol and reagent from BioRad and bovine serum albumin as the standard.
Immunoassays for ferritin and albumin. Immunoelectrophoresis was performed with a Bio-Phoresis system (BioRad), cooled to 4°C or 9°C. Antibodies were added to 1% agarose (Standard Low mT ; Bio-Rad) in 27 mmol/L Tricine Buffer IV, pH 8.6 (Bio-Rad) at about 55°C, just before pouring the plates. Samples (5 to 8 μL) were placed in wells on 10 × 10 or 10 × 15 cm, 1.5 mm thick agar plates immediately before application of current (100 V for a minimum of 4 hours, optimally overnight) at 9°C or 150 V for a minimum of 2 hours at 4°C). Samples and standards were usually interspersed, and several replicates were always analyzed. Finished plates were soaked in deionized water for 15 to 30 minutes and then pressed under several layers of filter paper and a weighted glass plate for 30 minutes. Glass plates were then soaked in 0.9% NaCl for 15 to 30 minutes and pressed as before. Pressed gels were dried completely with a hair dryer or in a gel drying apparatus, and were then immersed in Brilliant Blue R Staining solution (Sigma) for a minimum of 15 minutes. Gels were destained with two to three changes of 10% acetic acid until a clear background was obtained, then dried as before. Areas under each rocket were calculated from height × width at half height. Concentrations of antigen in the samples were determined from standard curves made with the area values for the three or more standards run on every plate. Samples were assayed in duplicate or triplicate. In the case of albumin, cell medium was assayed directly using commercial goat antirat albumin antiserum (Cappel, Westchester, PA) and rat albumin (Sigma) as the standard. In the case of ferritin, conditioned media and cell homogenates were heated to 70°C at pH 4.8 and (only in the case of the media) concentrated 20-fold (by Centricon-30; Amicon, Beverly, MA) before analysis. Ferritin antiserum was against horse spleen ferritin containing only L and H subunits and was raised in our laboratory in rabbits. The range of sensitivity of the rocket assays was from 0.2 to 20 μg/mL. Standards were horse spleen ferritin and therefore quantities are in terms of horse spleen ferritin units. Examples of typical assay plates are shown in Fig 1A and B for ferritin and albumin in the conditioned medium, respectively. It is evident that there was a linear relationship between the area of the rocket and dose of standard applied (Fig 1A, three rockets on right), and that samples of medium containing varying amounts of ferritin and albumin could be quantitated.
Synthesis of Ferritin by Rat Hepatoma Cells in Culture
| . | Cell Medium . | Hepatoma Cells . |
|---|---|---|
| Total protein | ||
| Amount (mg) | 2,390 | 51 |
| Total 3H (cpm × 10−6) | 3.49 | 21.2 |
| Specific activity (cpm/mg) | 1,460 | 419,000 |
| Total ferritin isolated | ||
| Amount (μg) | 546 | 199 |
| Total 3H (cpm) | 1,821 | 387 |
| Specific activity (cpm/mg) | 3,336 | 1,946 |
| . | Cell Medium . | Hepatoma Cells . |
|---|---|---|
| Total protein | ||
| Amount (mg) | 2,390 | 51 |
| Total 3H (cpm × 10−6) | 3.49 | 21.2 |
| Specific activity (cpm/mg) | 1,460 | 419,000 |
| Total ferritin isolated | ||
| Amount (μg) | 546 | 199 |
| Total 3H (cpm) | 1,821 | 387 |
| Specific activity (cpm/mg) | 3,336 | 1,946 |
Ten flasks of cells were grown a total of 7 days in serum containing medium. Tracer 3H-leucine was added to the last change in medium, and the medium and cells were harvested 72 hours later. Ferritin was isolated from medium and cells, and the total radioactivity incorporated into the ferritin and total protein was measured.
RESULTS
Synthesis and release of ferritin by rat hepatoma cells. To determine whether ferritin was synthesized and released by rat hepatoma cells, we followed the incorporation of 3H-leucine into intracellular ferritin and that in the medium. Medium and washed cells from 10 culture flasks that had been incubated for 72 hours with the radioactive amino acid, and represented about 5 × 108 cells, were pooled, and the ferritin present in each was isolated by the procedure used for serum ferritin (see Materials and Methods). The last step in this procedure is immunoaffinity chromatography with antibody against intracellular ferritin. The ferritin bound to the antibody was quantitated and analyzed for radioactivity.
Table 1 shows that significant amounts of ferritin were isolated from both the cells and the conditioned medium. In fact, there was considerably more ferritin in the medium than in the cells. Both portions of ferritin were radioactively labeled, indicating they had been synthesized by the hepatoma cells. The medium itself (with 20% horse serum and 5% fetal calf serum) contained negligible amounts of ferritin, as determined by our immunoassay (see Materials and Methods). Thus, virtually all of the ferritin recovered had been synthesized by the hepatoma cells.
Effects of TNF and IL-1 on Release of Ferritin and Albumin From Hepatoma Cells
| . | Dose (ng/mL) . | Ferritin Released . | Albumin Released . | LDH (% of control) . | Cell Ferritin (μg/mg cell protein) . | ||
|---|---|---|---|---|---|---|---|
| . | . | (μg/mg cell prot.) . | (% of control) . | (μg/mg cell protein) . | (% of control) . | . | . |
| Controls (12) | 0 | 1.8 ± 0.3 | 100 ± 10 | 22 ± 1 | 100 ± 3 | 100 ± 23 | 10.1 ± 2.3 (6) |
| IL-1 (12) | 5.0, 10.0 | 4.8 ± 1.1* | 280 ± 75* | 19 ± 1* | 88 ± 3* | 121 ± 31 | 9.2 ± 0.6 (4) |
| TNF (12) | 2.5, 5.0 | 5.7 ± 2.0* | 329 ± 111* | 21 ± 1 | 97 ± 4 | 125 ± 10 | 11.2 ± 0.3 (4) |
| IL-1 + TNF (16) | All combinations | 9.5 ± 1.9*† | 526 ± 160*† | 19 ± 1* | 89 ± 5* | 138 ± 12* | 10.6 ± 4.5 (4) |
| . | Dose (ng/mL) . | Ferritin Released . | Albumin Released . | LDH (% of control) . | Cell Ferritin (μg/mg cell protein) . | ||
|---|---|---|---|---|---|---|---|
| . | . | (μg/mg cell prot.) . | (% of control) . | (μg/mg cell protein) . | (% of control) . | . | . |
| Controls (12) | 0 | 1.8 ± 0.3 | 100 ± 10 | 22 ± 1 | 100 ± 3 | 100 ± 23 | 10.1 ± 2.3 (6) |
| IL-1 (12) | 5.0, 10.0 | 4.8 ± 1.1* | 280 ± 75* | 19 ± 1* | 88 ± 3* | 121 ± 31 | 9.2 ± 0.6 (4) |
| TNF (12) | 2.5, 5.0 | 5.7 ± 2.0* | 329 ± 111* | 21 ± 1 | 97 ± 4 | 125 ± 10 | 11.2 ± 0.3 (4) |
| IL-1 + TNF (16) | All combinations | 9.5 ± 1.9*† | 526 ± 160*† | 19 ± 1* | 89 ± 5* | 138 ± 12* | 10.6 ± 4.5 (4) |
Rat hepatoma cells (H4-II-E-C3) were grown in horse and bobby calf serum containing Dulbecco's modified Eagle's medium for 3 days, then adapted to protein free (serum free) hybridoma medium for several hours. Individual 10 mL flasks of cells were then given hybridoma medium with or without various doses of lymphokines, as indicated, and harvested 48 hours later. Both medium and cells were assayed for ferritin and albumin by rocket immunoelectrophoresis. LDH was assayed to assess potential damage to cells. Data for the two most optimal doses of each lymphokine administered (and all combinations thereof) did not show a significant difference and were thus combined. Cells were harvested and assayed for ferritin and total protein. Values are means ± SD per mg cell protein for the number of flasks in parentheses. Statistical analysis was by t-test.
P < .01 or <.001 for difference from controls.
P < .01 or <.001 for difference from IL-1 or TNF alone.
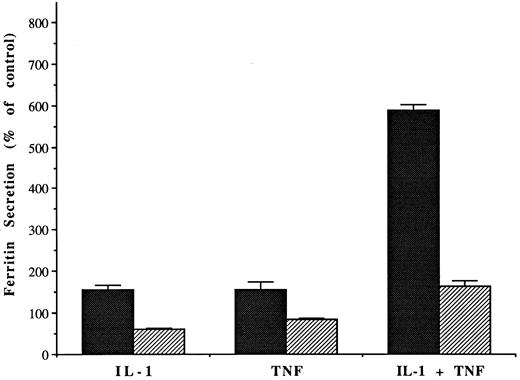
Effect of brefeldin A (1 μg/mL) on the release of ferritin from hepatoma cells, after stimulation with IL-1 or TNF (10 and 5 ng/mL, respectively). Bars show mean amounts of protein detected in the medium per mg cell protein, ± SD for 6 to 12 flasks per group, after 48 hours incubation, as a percent of mean values for the control (untreated) cells. Dark bars are without and lighter bars with brefeldin A. All differences with brefeldin treatment were significant.
Effect of brefeldin A (1 μg/mL) on the release of ferritin from hepatoma cells, after stimulation with IL-1 or TNF (10 and 5 ng/mL, respectively). Bars show mean amounts of protein detected in the medium per mg cell protein, ± SD for 6 to 12 flasks per group, after 48 hours incubation, as a percent of mean values for the control (untreated) cells. Dark bars are without and lighter bars with brefeldin A. All differences with brefeldin treatment were significant.
Immunoassays for ferritin and albumin were developed and confirmed that ferritin was being released from the hepatoma cells even in the absence of potential stimulae (Fig 1A), and that the cells were behaving like liver cells by releasing albumin into the medium (Fig 1B). We also confirmed that the albumin was secreted via the usual Golgi-dependent mechanism, as treatment of the cells with brefeldin A (BFA) greatly reduced the amounts of albumin released into the medium (Fig 1B); (BFA causes a fusion of the Golgi apparatus with the ER, preventing normal exocytosis and protein secretion33 ).
Effects of inflammatory cytokines on ferritin release and secretion. Serum ferritin concentrations are elevated in inflammatory conditions, and we wondered whether serum ferritin might be an acute phase reactant responsive to certain cytokines. We, therefore, treated our cell line with IL-1–β and TNF-α, alone and in combination, and quantitated the effects on release of ferritin and albumin into the medium. The lactate dehydrogenase (LDH) activity of the medium was monitored as an indicator of potential cell damage.
The data from several studies in which cells were treated with 5 to 10 ng/ml IL-1–β and/or 2.5 to 5 ng/mL TNF-α are summarized in Table 2. Lower doses were less effective; much higher doses induced cell damage, as shown by an increased release of LDH, particularly in the case of TNF (data not shown). All of the data are given as values per mg of cell protein, to correct for any differences in cell number among replicates and with treatments. There were no obvious effects of the cytokines on cell number, as determined by the amount of cell protein recovered per flask (data not shown).
IL-1 and TNF each increased ferritin release about 2.5-fold (Table 2). The administration of IL-1 and TNF together had a much greater effect than either cytokine alone, increasing the concentrations of ferritin in the medium more than five-fold. Overall, the effects of the two cytokines were additive, although in two of the four experiments, the effects were clearly synergistic. As the individual cytokines were each given at doses that had a maximal individual effect, one can conclude that the mechanisms used by these hormones to enhance ferritin release are not identical and that the mechanism of regulation used by one may enhance that of the other. Inflammation induces the release of both of these cytokines in vivo, so the effect of both together should be more representative of what can occur in vivo.
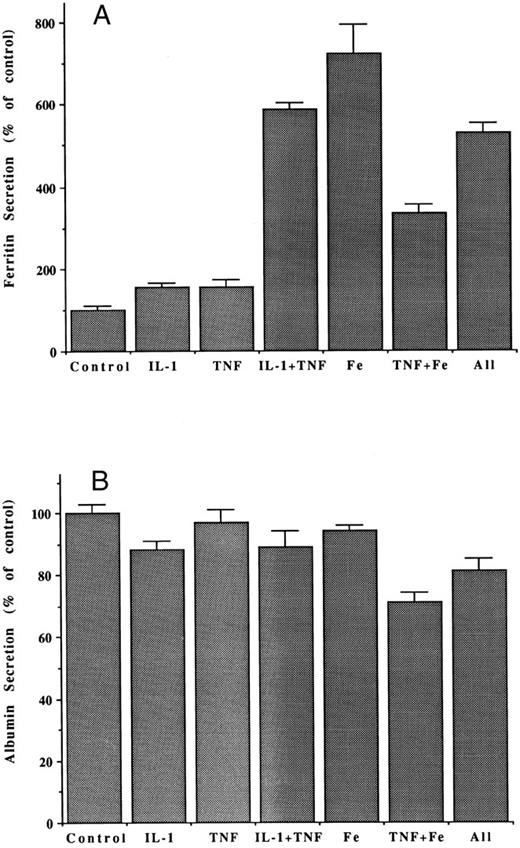
In vivo, during inflammation, it is also observed that the release of albumin from the liver is diminished. This phenomenon was also apparent in our hepatic cell culture system treated with cytokines (Table 2) and appeared to be due to IL-1. In contrast to what occurred with ferritin or albumin release, and consistent with in vivo findings,24 there were no significant effects of the cytokines on levels of intracellular ferritin.
To verify that the effects of IL-1 and TNF were on ferritin secretion, we again used BFA. BFA markedly inhibited the effects of IL-1 and TNF on release of ferritin into the medium (Fig 2). Secretion of albumin was also inhibited (data not shown). In one single experiment, secretion of still another plasma protein (ceruloplasmin) was also monitored; BFA almost completely blocked release (data not shown). Thus, increased secretion rather than leakage accounted for the increased release of ferritin from hepatoma cells in response to stimulation by the cytokines.
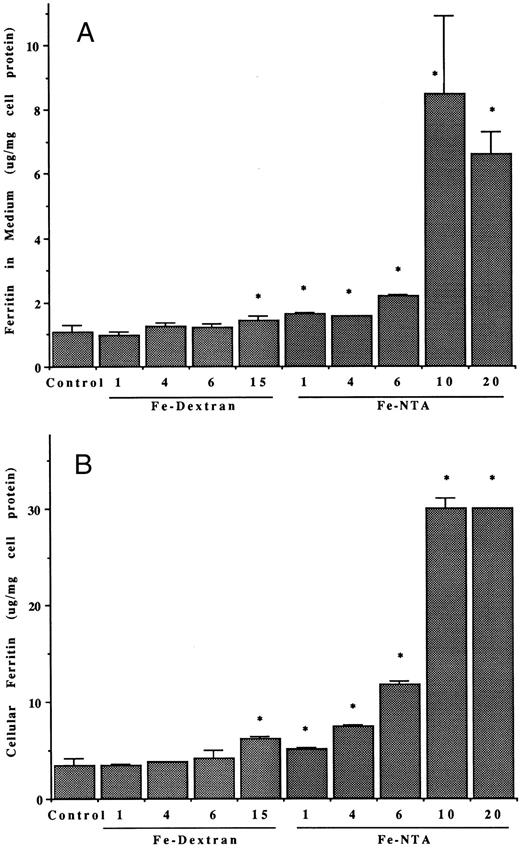
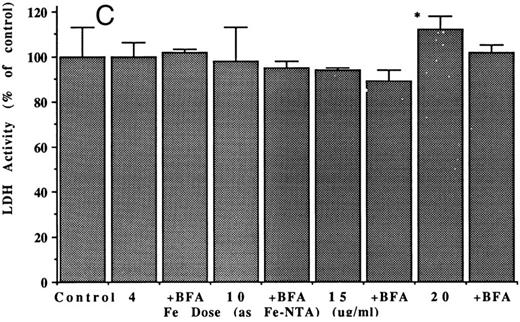
Effects of iron on ferritin release and secretion. Treating the hepatoma cells with iron also greatly enhanced release of serum ferritin. The effects of various doses of the iron, given either in the form of a 1:1 molar complex or Fe(III) with nitrilotriacetate (Fe-NTA) or as a dextran complex (Fe-dextran; Imferon), are shown in Fig 3. Both forms of iron stimulated release of ferritin into the medium (Fig 3A), although the Fe-NTA was more effective, at a given dose (it is noteworthy that iron dextran was able to deliver iron even in the absence of RE cells, although the latter are thought to be involved in release of iron from this complex in vivo). The highest doses of Fe-NTA tested had very dramatic effects on ferritin release, causing more than an eightfold increase in the ferritin content of the medium. As expected, iron also increased the concentration of intracellular ferritin (Fig 3B). The patterns of response of intracellular ferritin concentrations to the different types and doses of iron were similar to those for the ferritin released into the medium. At the very highest dose of Fe-NTA administered (20 μg Fe/mL), there was a small, but significant, increase of LDH activity in the medium (Fig 3C). Therefore, a dose of 10 μg Fe/mL was considered optimal for stimulating ferritin release without causing cell damage. As with cytokine stimulation, the effect of iron on ferritin in the medium was due to its secretion from the hepatoma cells, as BFA markedly inhibited the process (Table 3).
Effects of varying doses and types of iron on released and intracellular ferritins of hepatoma cells. (A and B) Ferritin concentrations in the medium (A) and in the hepatoma cells (B) after 48 hours, with and without (control) treatments with different doses of iron (during that period) in the form of Fe-dextran (Imferon; 1, 4, 6, and 15 μg Fe/mL) or the 1:1 complex of Fe(III) with nitrilotriacetate (Fe-NTA; 1, 4, 6, 10, and 20 μg Fe/mL). Data are means ± SD for 3 to 6 determinations (flasks), as microgram of protein per milligram of cell protein. Asterisk (*) indicates increases that are statistically significant (P < .01) by t-test, in comparison with the control. (C) LDH activity in the medium from cells untreated (control) or treated with 4, 10, 15, or 20 μg/mL doses of iron (Fe-NTA), ± brefeldin A (4 μg/mL), for 48 hours. After the control, under successive bars (from left to right) are the results at a given iron dose (for example, 4 μg Fe/mL) followed by the same, but also treated with BFA. The only significant change in LDH was with the highest dose of Fe-NTA (asterisk; P < .01).
Effects of varying doses and types of iron on released and intracellular ferritins of hepatoma cells. (A and B) Ferritin concentrations in the medium (A) and in the hepatoma cells (B) after 48 hours, with and without (control) treatments with different doses of iron (during that period) in the form of Fe-dextran (Imferon; 1, 4, 6, and 15 μg Fe/mL) or the 1:1 complex of Fe(III) with nitrilotriacetate (Fe-NTA; 1, 4, 6, 10, and 20 μg Fe/mL). Data are means ± SD for 3 to 6 determinations (flasks), as microgram of protein per milligram of cell protein. Asterisk (*) indicates increases that are statistically significant (P < .01) by t-test, in comparison with the control. (C) LDH activity in the medium from cells untreated (control) or treated with 4, 10, 15, or 20 μg/mL doses of iron (Fe-NTA), ± brefeldin A (4 μg/mL), for 48 hours. After the control, under successive bars (from left to right) are the results at a given iron dose (for example, 4 μg Fe/mL) followed by the same, but also treated with BFA. The only significant change in LDH was with the highest dose of Fe-NTA (asterisk; P < .01).
Effects of Iron and Brefeldin A on Release of Ferritin from Hepatoma Cells
| Treatment . | Dose . | Total Ferritin in Medium (μg) . | Inhibition by BFA (%) . | Total Albumin in Medium (μg) . | Inhibition by BFA (%) . |
|---|---|---|---|---|---|
| . | (μg Fe/mL) . | . | . | . | . |
| None (6) | 0 | 4.3 ± 0.6 | 192 ± 12 | ||
| Fe-NTA (3) | 10 | 8.5 ± 2.43-150 | 144 ± 43-150 | ||
| +BFA (3) | 10 | 3.6 ± 0.13-151 | 58 | 45 ± 53-151 | 69 |
| Fe-NTA (3) | 20 | 6.6 ± 0.73-150 | 135 ± 53-150 | ||
| +BFA (3) | 20 | 3.2 ± 0.13-151 | 52 | 45 ± 13-151 | 67 |
| Imferon (3) | 15 | 5.6 ± 0.63-150 | 208 ± 5 | ||
| +BFA (3) | 15 | 3.9 ± 0.13-151 | 31 | 87 ± 33-151 | 59 |
| Treatment . | Dose . | Total Ferritin in Medium (μg) . | Inhibition by BFA (%) . | Total Albumin in Medium (μg) . | Inhibition by BFA (%) . |
|---|---|---|---|---|---|
| . | (μg Fe/mL) . | . | . | . | . |
| None (6) | 0 | 4.3 ± 0.6 | 192 ± 12 | ||
| Fe-NTA (3) | 10 | 8.5 ± 2.43-150 | 144 ± 43-150 | ||
| +BFA (3) | 10 | 3.6 ± 0.13-151 | 58 | 45 ± 53-151 | 69 |
| Fe-NTA (3) | 20 | 6.6 ± 0.73-150 | 135 ± 53-150 | ||
| +BFA (3) | 20 | 3.2 ± 0.13-151 | 52 | 45 ± 13-151 | 67 |
| Imferon (3) | 15 | 5.6 ± 0.63-150 | 208 ± 5 | ||
| +BFA (3) | 15 | 3.9 ± 0.13-151 | 31 | 87 ± 33-151 | 59 |
Conditions were the same as described in Table 2, except that the data are given as micrograms of protein per flask and that treatments were with iron, in the form of a 1:1 molar Fe(III)-nitrilotriacetate complex (Fe-NTA) or iron dextran (Imferon), ± brefeldin A (BFA) (4 μg/mL). Values are means ± SD (for the number of flasks indicated in parentheses). There was no significant effect of any of the treatments on cell growth or viability, as determined by the amounts of cell protein per flask.
P < .05 to P < .001 for difference from controls.
Significant inhibition by BFA (P < .001).
Effects of transcriptional inhibition, and regulatory interactions between iron and cytokines. Iron stimulates synthesis of intracellular ferritin primarily by altering the rate of translation of existing mRNA,6,8-10 and iron stimulation of intracellular ferritin biosynthesis is not altered by inhibitors of transcription, such as actinomycin D.5 Similarly, it has been reported that IL-1 stimulates synthesis of intracellular ferritin in hepatic cells by enhancing translation28,29 and without changing levels of ferritin mRNA.23,24,28 We wondered whether regulation of serum ferritin secretion might be different and require enhanced transcription. To test this possibility, we used dichlororibofuranosylbenzimidazole (DRB), which inhibits transcription by binding to RNA polymerase II.34 It is not as toxic as actinomycin D and can be used in more long-term experiments. In this case, we added it to 4-day cultures in protein-free medium, at the same time as the cytokines or iron, and cells and media were harvested 24 hours later. The data in Table 4 show that without DRB, there was the usual enhancement of ferritin secretion in response to IL-1, TNF or Fe-NTA, iron again being the most effective at the doses applied. Simultaneous treatment with DRB almost completely blocked the stimulatory effects of each factor. The same was true for albumin secretion. There was no increase in LDH release or a reduction in the number of cells per flask with DRB treatment (data not shown), indicating the treatment was not toxic to the cells over the 24-hour time period examined. Thus, the stimulatory effects of the two cytokines and iron appear to involve increased transcription of the mRNA for secreted (serum) ferritin and/or of factors that control this process.
Effects of DRB on Secretion of Ferritin and Albumin by Hepatoma Cells
| Treatment . | Ferritin . | Albumin . | ||
|---|---|---|---|---|
| . | Secretion . | Inhibition by DRB (%) . | Secretion . | Inhibition by DRB (%) . |
| . | (μg/mg cell protein) . | . | (μg/mg cell protein) . | . |
| Controls | ||||
| −DRB | 0.8 ± 0.0 | 53 ± 1 | ||
| +DRB | 0.02 ± 0.004-150 | 98 | 0.4 ± 0.04-150 | 99 |
| IL-1 | ||||
| −DRB | 1.9 ± 0.14-151 | 48 ± 1 | ||
| +DRB | 0.3 ± 0.04-150 | 86 | 0.4 ± 0.0 | 99 |
| TNF | ||||
| −DRB | 1.5 ± 0.04-151 | 45 ± 24-151 | ||
| +DRB | 0.1 ± 0.04-150 | 98 | 0.4 ± 0.0 | 99 |
| Fe-NTA | ||||
| −DRB | 4.5 ± 0.64-151 | 49 ± 3 | ||
| +DRB | 0.4 ± 0.04-150 | 91 | 0.5 ± 0.0 | 98 |
| Treatment . | Ferritin . | Albumin . | ||
|---|---|---|---|---|
| . | Secretion . | Inhibition by DRB (%) . | Secretion . | Inhibition by DRB (%) . |
| . | (μg/mg cell protein) . | . | (μg/mg cell protein) . | . |
| Controls | ||||
| −DRB | 0.8 ± 0.0 | 53 ± 1 | ||
| +DRB | 0.02 ± 0.004-150 | 98 | 0.4 ± 0.04-150 | 99 |
| IL-1 | ||||
| −DRB | 1.9 ± 0.14-151 | 48 ± 1 | ||
| +DRB | 0.3 ± 0.04-150 | 86 | 0.4 ± 0.0 | 99 |
| TNF | ||||
| −DRB | 1.5 ± 0.04-151 | 45 ± 24-151 | ||
| +DRB | 0.1 ± 0.04-150 | 98 | 0.4 ± 0.0 | 99 |
| Fe-NTA | ||||
| −DRB | 4.5 ± 0.64-151 | 49 ± 3 | ||
| +DRB | 0.4 ± 0.04-150 | 91 | 0.5 ± 0.0 | 98 |
Cells were grown almost to confluence in serum-containing medium, then switched to protein-free medium for 24 hours before harvest of cells and medium. Treatments with cytokines, iron, and DRB were for the final 24 hours. Doses of IL-1, TNF, and Fe-NTA were 5.0 ng/mL, 2.5 ng/mL, and 10 μg/mL, respectively, and the dose of DRB was 4 ng/mL. Data are means ± SD, for groups of three flasks that were treated identically. It is noteworthy that the treatments with DRB caused little or no change in cell number (as judged by recovery of cell protein from each flask) and no enhanced leakage of LDH.
Difference from −DRB is significant (P < .001).
Difference from control is significant (P < .001).
Interactive effects of cytokines and iron on the secretion of ferritin (A) and albumin (B) by hepatoma cells. Bars indicate mean amounts of ferritin and albumin in the medium, per milligram of hepatoma cell protein, as a percent of that for control (untreated) cells, after 48 hours incubation. Doses were 5.0 ng/mL IL-1, 2.5 ng/mL TNF, and 10 μg/mL Fe-NTA. Mean values ± SD are for three flasks in the case of treated cells, and six flasks in the case of controls. All differences from the values for controls were significant (P < .01) except in the case of albumin from cells treated only with TNF.
Interactive effects of cytokines and iron on the secretion of ferritin (A) and albumin (B) by hepatoma cells. Bars indicate mean amounts of ferritin and albumin in the medium, per milligram of hepatoma cell protein, as a percent of that for control (untreated) cells, after 48 hours incubation. Doses were 5.0 ng/mL IL-1, 2.5 ng/mL TNF, and 10 μg/mL Fe-NTA. Mean values ± SD are for three flasks in the case of treated cells, and six flasks in the case of controls. All differences from the values for controls were significant (P < .01) except in the case of albumin from cells treated only with TNF.
Finally, potential interactions between the cytokines and iron were examined. Figure 4 shows that at the concentration of 10 μg/mL, iron (as the NTA complex) had a stimulatory effect greater than that produced by IL-1 and TNF, together. Iron increased ferritin secretion more than sevenfold, while TNF plus IL-1 caused a sixfold enhancement. Unexpectedly, secretion was significantly diminished when TNF was given with the iron. However, IL-1 given along with the Fe and TNF appeared to prevent this inhibition, and all three factors together had the same effect as IL-1 plus TNF, without the iron. Clearly, some complex interactions of stimulatory cascades induced by these factors are occurring that will need to be dissected out.
DISCUSSION
We have shown for the first time that cells of hepatic origin secrete ferritin; that secretion is stimulated by inflammatory hormones; that two cytokines together have much more of a stimulatory effect than one alone; and that transcription is required for this hepatic cell response. We have also shown for the first time that iron enhances ferritin secretion, again by a mechanism involving transcription, and that there is a complex interaction between the inflammatory cytokines and iron in regulating secretion. Although indirect evidence strongly suggested that serum ferritin is at least in part a secreted protein, actual secretion of ferritin has never before been demonstrated for cells from a major organ of the body, such as liver.
Ferritin concentrations in human serum have been measured in many different physiologic states and are routinely used to assess body iron stores. As indicated earlier, concentrations of ferritin in serum increase in a variety of conditions that include cancer, liver disease, inflammation, iron overload, or iron treatment. In the present studies, we have attempted to model two of these conditions using cultured, differentiated hepatic cells, with apparently positive results.
Our cells acted like hepatocytes. They synthesized and secreted albumin, and the secretion of albumin was slowed by treatment with IL-1. This reflects the in vivo situation, in which there is decreased albumin secretion during inflammation.35 To monitor ferritin release, we used immunoassays based on antibodies to intracellular ferritin, as is the case for clinical assays of ferritin. The assay showed that hepatic cells release ferritin, and that the release was greatly stimulated by a combination of cytokines that circulate during inflammation, and also by iron. BFA inhibited ferritin release, indicating that the ferritin was being secreted rather than leaked from the hepatic cells by the usual Golgi-mediated mechanism (BFA being a specific inhibitor of Golgi function).33 BFA inhibited not just ferritin, but also albumin and ceruloplasmin secretion. Proteins using this secretory mechanism are synthesized on ER-bound polyribosomes and have coding for a signal peptide sequence in their mRNAs (which is not the case for intracellular H and L ferritins).
The response to IL-1 + TNF can explain why serum ferritin concentrations increase in vivo during inflammation and indicates that serum ferritin can legitimately be added to the list of acute phase reactant proteins. The response to iron can explain why serum ferritin concentrations increase in vivo, when iron is given to treat iron deficiency,36 or in iron overload, when the flux of iron in and out of cells is high. Our results also indicate that liver cells are a significant source of serum ferritin.
Our results for secreted ferritin should not be confused with those for intracellular ferritin, which appears to be a different protein.21 Synthesis of intracellular ferritin by liver,26-29 or expression of ferritin H mRNA in adipocytes, muscle cells, and myoblasts,37-39 as well as in fibroblasts and monocytes40,41 is stimulated by the same cytokines we have been studying. In most cells it is also stimulated by iron. However, our studies show that the mechanisms underlying synthesis and secretion of serum ferritin (at least by hepatic cells) differ from those for intracellular ferritin. In hepatic cells, stimulation of intracellular ferritin biosynthesis by iron5,6 and IL-128 occurs primarily at the translational level via a change in the use of existing mRNA, and with little or no change in rate of transcription or concentration of ferritin mRNA.23,24,28,29 In the case of iron, this involves release of iron response proteins (IRP1 and IRP2) from a stem loop structure in the 5′-untranslated region (5′UTR) of the ferritin H and L mRNA.4-13 In the case of IL-1, this may also involve binding of regulatory proteins to a specific nucleotide sequence in the 5′UTR.29 We have now shown that for secreted (serum) ferritin the situation is quite different. We found that stimulation of serum ferritin secretion by hepatic cells, induced by iron or IL-1, was markedly inhibited by DRB and is thus primarily or exclusively under transcriptional control. This is supported by our observations of a 10-fold to 13-fold increase in mRNA for serum ferritin in these hepatic cells and liver,21 as determined with our partial cDNA clones. All these findings are consistent with the concept that serum ferritin is the product of separate mRNAs and separate genes. The details of the regulatory mechanisms involved and the intriguing interactions between the mechanisms for iron and the cytokines will be worked out when full-sized clones of the serum ferritin subunit cDNAs (and the genes for these messages) become available.21
Supported in part by Public Health Service Grant No. SO6 GM 08258, by REU Grant No. 5369 from the National Science Foundation, and by two minigrants from California State University, Fullerton.
Address reprint requests to Maria C. Linder, PhD, Department of Chemistry and Biochemistry, California State University, Fullerton, CA 92834-6866; email: mlinder@fullerton.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal