Abstract
CGP 57148 is a compound of the 2-phenylaminopyrimidine class that selectively inhibits the tyrosine kinase activity of the ABL and the platelet-derived growth factor receptor (PDGFR) protein tyrosine kinases. We previously showed that CGP 57148 selectively kills p210BCR-ABL–expressing cells. To extend these observations, we evaluated the ability of CGP 57148 to inhibit other activated ABL tyrosine kinases, including p185BCR-ABL and TEL-ABL. In cell-based assays of ABL tyrosine phosphorylation, inhibition of ABL kinase activity was observed at concentrations similar to that reported for p210BCR-ABL. Consistent with the in vitro profile of this compound, the growth of cells expressing activated ABL protein tyrosine kinases was inhibited in the absence of exogenous growth factor. Growth inhibition was also observed with a p185BCR-ABL–positive acute lymphocytic leukemia (ALL) cell line generated from a Philadelphia chromosome–positive ALL patient. As CGP 57148 inhibits the PDGFR kinase, we also showed that cells expressing an activated PDGFR tyrosine kinase, TEL-PDGFR, are sensitive to this compound. Thus, this compound may be useful for the treatment of a variety of BCR-ABL–positive leukemias and for treatment of the subset of chronic myelomonocytic leukemia patients with a TEL-PDGFR fusion protein.
ACTIVATION OF THE ABL oncogene has been associated with a variety of human leukemias. Chronic myelogenous leukemia (CML) is the most common of these with 95% of patients expressing a 210-kD ABL fusion protein in which the first exon of c-ABL has been replaced by BCR sequences encoding 927 or 902 amino acids.1,2 p210BCR-ABL is also present in approximately 5% to 10% of adults with acute leukemia for whom there is no evidence of antecedent CML. Another BCR-ABL fusion protein, p185BCR-ABL, contains only sequences from BCR exon 1 (426 amino acids) fused to exons 2 to 11 of c-ABL and is seen in 5% to 10% of de novo acute lymphocytic leukemias (ALL).3 4
Approximately one third of CML patients are eligible for allogeneic bone marrow transplantation and the cure rate is 70% to 90% for patients who survive the transplant.5 6 For CML patients not eligible for allogeneic bone marrow transplantation, no curative therapy is available, but the average survival is close to 6 years. For patients with BCR-ABL–positive acute leukemias, the prognosis is quite poor. Thus, new therapies are needed for these disorders.
ABL tyrosine kinase activity is required for the transforming function of BCR-ABL proteins.7,8 Sequences from the first exon of BCR are required for activation of the ABL tyrosine kinase and these sequences promote oligomerization of BCR-ABL.9-11 However, sequences other than BCR may be capable of substituting for BCR in activating the transforming ability and tyrosine kinase activity of ABL. An example of this is the TEL gene that encodes a putative transcription factor with homology to the ETS family of DNA binding proteins. An ABL fusion to the TEL gene has been reported in one case of acute myelogenous leukemia (AML), one case of ALL, and a single case of atypical CML.12-14 Fusion of TEL sequences to ABL results in activation of ABL kinase activity and a pattern of cellular tyrosine phosphorylation similar to that induced by BCR-ABL fusion proteins.15
TEL was cloned as a result of its fusion to the intracellular tyrosine kinase domain of the platelet-derived growth factor receptor (PDGFR) in chronic myelomonocytic leukemia (CMML) patients with a 5; 12 chromosome translocation.16 This fusion event results in homodimerization and activation of the PDGFR tyrosine kinase activity.17
We previously reported that the tyrosine kinase inhibitor, CGP 57148, inhibits the ABL and PDGFR tyrosine kinases.18 19 Our data showed that this compound is capable of inhibiting the growth of p210BCR-ABL–or v-ABL–positive cells in vitro and in vivo, but does not inhibit the growth of normal cells or cells transformed by other tyrosine kinases such as v-SRC. To extend these observations, we evaluated the ability of CGP 57148 to inhibit the growth of cells transformed by activated PDGFR or activated ABL tyrosine kinases other than p210BCR-ABL or v-ABL. In this report we show that CGP 57148 has the ability to specifically inhibit the growth of p185BCR-ABL–positive cells, TEL-ABL, and TEL-PDGFR–expressing cells.
MATERIALS AND METHODS
Antibodies and Reagents
A stock solution of CGP 57148B at a concentration of 10 mmol/L was prepared by dissolving the compound in sterile phosphate buffered saline (PBS) and was diluted in tissue culture media before use. The antiphosphotyrosine antibody 4G10 was generated using phosphotyramine as the immunogen and was used as described.20 The anti-ABL monoclonal antibody 24-21 was a gift from Naomi Rosenberg, New England Medical Center, Boston, MA and the PDGFR antibody was from UBI (Lake Placid, NY). Other reagents were obtained from Sigma Chemical Co (St Louis, MO) unless otherwise indicated.
Cell Lines
The 32Dcl3 cell line is a murine myeloid cell line that was obtained from Joel Greenberger, University of Massachusetts Medical Center, Worcester, MA.21 BaF3 is a murine progenitor cell line that has characteristics of immature B cells and was obtained from James D. Griffin, Dana Farber Cancer Institute, Boston, MA. Both 32Dcl3 and BaF3 cells are dependent on interleukin-3 (IL-3) for proliferation. Cells were cultured in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; UBI) and 15% WEHI-3B conditioned media as a source of IL-3. MO7e is a human megakaryocytic leukemia cell line that requires either granulocyte-macrophage colony-stimulating factor, IL-3, or stem cell factor (SCF) for proliferation.22
Derivatives of these cell lines expressing p210BCR-ABL, p185BCR-ABL, TEL-ABL, or TEL-PDGFR were created by electroporation with a retrovirus, pGD, containing the respective cDNAs, as described.15,17,23,24 32Dp210, 32Dp185, BaF3 TEL-ABL, and BaF3 TEL-PDGFR cells are factor-independent for proliferation and were grown in RPMI 1640 media supplemented with 10% FBS. K562 cells are a p210BCR-ABL–positive cell line derived from a CML patient in blast crisis.25 A p185BCR-ABL–positive ALL cell line was provided by Fatih Uckin, University of Minnesota.
Proliferation and Differentiation Assays
Cell count assays. Cells were plated at a density of 2 × 105 cells/mL in RPMI 1640 media with 10% FBS with or without growth factors. Inhibitor was added at various concentrations. Controls using an identical dilution of buffer in which the inhibitor had been dissolved were also performed. Viable cells were counted, as assessed by exclusion of trypan blue, at 24-hour intervals.
MTT assays. Between 5 × 103 and 2 × 104 cells were plated per well in quadruplicate in RPMI 1640 media with 10% FBS with or without growth factors and various concentrations of inhibitors. Controls were performed using an identical dilution of buffer in which the inhibitor had been dissolved. Wells were assayed for uptake of MTT at daily intervals as described.26 One plate per day contained serial dilutions of cells to ensure that the assay remained linear with respect to the cell concentration used. Results are expressed as the mean optical density (OD).
Immunoblotting Experiments
Inhibition of BCR-ABL, TEL-ABL, and TEL-PDGFR tyrosine kinases. 32Dcl3 or BaF3 cells expressing activated tyrosine kinases were incubated in the presence of various concentrations of inhibitor. After a 1.5-hour incubation, cells were washed twice with PBS and lysed in 1% NP40, 150 mmol/L NaCl, 20 mmol/L Tris pH 7.4, 10% glycerol containing 1 mmol/L phenylmethylsulfonylfluoride, 20 μg/mL aprotinin, and 1 mmol/L sodium orthovanadate at 5 × 107 cells/mL. Equal amounts of lysates (approximately 250 μg) were analyzed by immunoblotting with antiphosphotyrosine or anti-ABL antibodies as described.27 Gels were scanned using a laser densitometer to quantitate the signal intensity.
Inhibition of IL-3–induced tyrosine kinase activity. 32Dcl3 cells were washed twice with RPMI 1640 and resuspended at 2 × 106 cells/mL of RPMI 1640 containing 1% bovine serum albumin. Cells were incubated in this media for 6 to 8 hours. Various concentrations of inhibitor were added for the final 1.5 hours of incubations. At the end of this period, cells were stimulated with 10 ng/mL of murine recombinant IL-3 (UBI). Cells were lysed as described previously and analyzed by antiphosphotyrosine immunoblotting.
RESULTS
CGP 57148
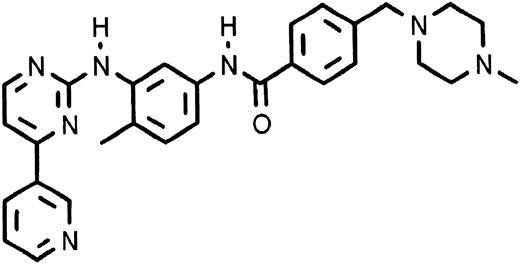
CGP 57148 is a compound of the 2-phenylaminopyrimidine class. This class of compounds was designed based on the known structure of the adenosine triphosphate (ATP) binding site of protein kinases. A series of structurally modified congeners of a base compound of this class were produced and analyzed for their ability to inhibit a series of protein kinases. CGP 57148 (Fig 1) was found to be a potent inhibitor of the ABL tyrosine kinase.
A number of other protein kinases were initially examined for inhibition by this compound, including the epidermal growth factor receptor, HER-2/neu, insulin receptor; insulin-like growth factor-1 receptor; PDGFR; c-FGR; c-LCK; c-LYN; c- and v-SRC; and a variety of protein serine/threonine kinases including protein kinase A, phosphorylase kinase, protein kinase C types α, β1, β2, γ, σ, ε, η, and ζ, cdc2/cyclin, and casein kinases 1 and 2.18 With the exception of the PDGFR, no inhibitory activity of CGP 57148 was observed at concentrations less than 100 μmol/L.18 19 The PDGFR belongs to a family of receptor tyrosine kinases characterized by five extracellular immunoglobulin-like domains and an interrupted intracellular tyrosine kinase domain. Other members include FMS (CSF-1R), c-KIT, and FLT3. Although CGP 57148 does not inhibit the FLT3 or FMS tyrosine kinases, inhibition of tyrosine phosphorylation induced by stimulation of cells with SCF, the ligand for c-KIT, has been observed (E. Buchdunger, manuscript in preparation).
Inhibition of Protein Tyrosine Kinase Activity In Vitro
Initial studies showed that CGP 57148 was capable of inhibiting substrate phosphorylation by a bacterially expressed v-ABL tyrosine kinase with an IC50 of 0.038 μmol/L,19 where IC50 represents the concentration of compound required to achieve a 50% reduction in the phosphorylation of an exogenous substrate. Additional studies using cell-based assays for inhibition of autophosphorylation by p210BCR-ABL kinase showed an IC50 of 0.25 μmol/L.18 Similar results have been obtained in MO7e cells that express p210BCR-ABL, K562 cells, a p185BCR-ABL ALL cell line, and 32Dcl3 cells expressing p185BCR-ABL. A representative experiment using 32Dcl3 cells expressing p185BCR-ABL is shown in Fig 2. Antiphosphotyrosine and anti-ABL immunoblots were performed on cellular lysates of p185-expressing 32Dcl3 cells incubated for 1.5 hours with various concentrations of inhibitor. No significant differences in the amount of ABL protein were noted on the ABL immunoblot (Fig 2B). However, there was a significant decrease in the amount of phosphotyrosine detected in the presence of CGP 57148 (Fig 2A). Densitometric scans of the BCR-ABL phosphotyrosine band shows that a 50% reduction in the intensity of the phosphotyrosine staining (IC50) of p185BCR-ABL using CGP 57148 occurred at a concentration of 0.25 μmol/L.
IC50 of inhibition of p185BCR-ABL kinase. 32Dp185 cells were incubated for 1.5 hours in the presence of the indicated concentration of inhibitor. Cells were lysed and equal amounts of lysate were analyzed by immunoblotting with antiphosphotyrosine (A) or anti-ABL antibodies (B). The migration of BCR-ABL is marked with an arrow on the left of the panel. These gels were scanned using a laser densitometer. No significant differences in the amount of ABL protein were noted on the ABL immunoblot. The scanned data from the antiphosphotyrosine immunoblot were used to calculate the IC50, defined as a 50% reduction of the intensity of the BCR-ABL phosphotyrosine band. The data shown are representative of two separate experiments and similar values were obtained for the IC50s.
IC50 of inhibition of p185BCR-ABL kinase. 32Dp185 cells were incubated for 1.5 hours in the presence of the indicated concentration of inhibitor. Cells were lysed and equal amounts of lysate were analyzed by immunoblotting with antiphosphotyrosine (A) or anti-ABL antibodies (B). The migration of BCR-ABL is marked with an arrow on the left of the panel. These gels were scanned using a laser densitometer. No significant differences in the amount of ABL protein were noted on the ABL immunoblot. The scanned data from the antiphosphotyrosine immunoblot were used to calculate the IC50, defined as a 50% reduction of the intensity of the BCR-ABL phosphotyrosine band. The data shown are representative of two separate experiments and similar values were obtained for the IC50s.
CGP 57148 also inhibits ligand-stimulated PDGFR tyrosine phosphorylation with an IC50 of 0.3 μmol/L,19 and c-ABL with an IC50 of 0.25 μmol/L suggesting that CGP 57148 is equipotent for inhibition of ABL and PDGFR kinases. Experiments were performed on cell lines expressing TEL-ABL and TEL-PDGFR to determine whether similar effects would be seen in cells expressing ABL or PDGFR activated by other means. These experiments showed an IC50 of 0.35 μmol/L for TEL-ABL and 0.15 for TEL-PDGFR (Fig 3), suggesting that the mechanism of activation of these kinases did not alter the ability of CGP 57148 to inhibit their activity.
IC50 of inhibition of TEL-ABL and TEL-PDGFR kinases. BaF3 TEL-ABL (A) or BAF3 TEL-PDGFR (B) cells were incubated for 1.5 hours in the presence of the indicated concentration of inhibitor. Cells were lysed and equal amounts of lysate were analyzed by immunoblotting with antiphosphotyrosine (top panel of A and B) or anti-ABL or anti-PDGFR antibodies (bottom panel of A and B). Data were analyzed as for Fig 2. The data shown are representative of two separate experiments and similar values were obtained for the IC50s.
IC50 of inhibition of TEL-ABL and TEL-PDGFR kinases. BaF3 TEL-ABL (A) or BAF3 TEL-PDGFR (B) cells were incubated for 1.5 hours in the presence of the indicated concentration of inhibitor. Cells were lysed and equal amounts of lysate were analyzed by immunoblotting with antiphosphotyrosine (top panel of A and B) or anti-ABL or anti-PDGFR antibodies (bottom panel of A and B). Data were analyzed as for Fig 2. The data shown are representative of two separate experiments and similar values were obtained for the IC50s.
Cell Proliferation Assays
To determine whether inhibition of the ABL or PDGFR kinases would have any effects on the cells expressing these kinases, cell proliferation assays were performed. Preliminary studies with CGP 57148 were performed with concentrations of CGP 57148 ranging from 0.01 μmol/L to 100 μmol/L. No toxicity against parental BaF3, 32Dcl3, or MO7e cells was seen at doses up to 10 μmol/L (Fig 4). However, at 100 μmol/L CGP 57148, these cells were killed. 32Dcl3 and BaF3 cells are absolutely dependent on the presence of exogenous IL-3 for survival and proliferation. Because IL-3 signal transduction is known to activate a number of tyrosine kinases, including JAK-2,28 we examined antiphosphotyrosine immunoblots of BaF3 or 32Dcl3 cells stimulated with IL-3 in the presence or absence of CGP 57148. No inhibition of tyrosine phosphorylation was seen even at concentrations of CGP 57148 of 100 μmol/L, at which cell death was observed (data not shown).
Dose response of inhibition of growth of 32Dcl3 cells. 32Dcl3 cells were incubated in quadruplicate in the presence of regular growth media with the indicated amount of inhibitor. Wells were assayed for uptake of MTT at daily intervals. The data shown are representative of three separate experiments. Similar results have been obtained using MO7e and BaF3 cells.
Dose response of inhibition of growth of 32Dcl3 cells. 32Dcl3 cells were incubated in quadruplicate in the presence of regular growth media with the indicated amount of inhibitor. Wells were assayed for uptake of MTT at daily intervals. The data shown are representative of three separate experiments. Similar results have been obtained using MO7e and BaF3 cells.
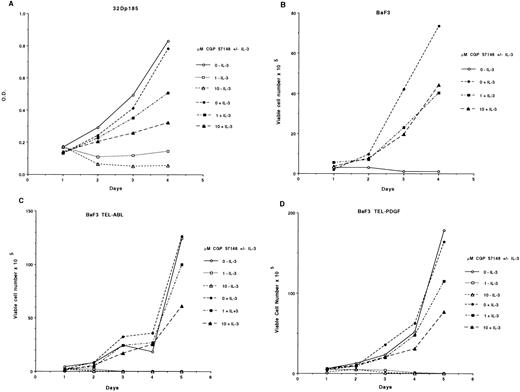
We previously showed that CGP 57148 inhibited the growth of 32Dcl3 and MO7e cells expressing p210BCR-ABL at concentrations of 1 or 10 μmol/L.18 To extend these observations, we tested cell lines that expressed p185BCR-ABL, TEL-ABL, and TEL-PDGF. CGP 57148 at 1 or 10 μmol/L killed cells expressing these constructs in the absence of exogenous growth factor (Fig 5). This included a p185BCR-ABL–positive ALL cell line generated from a Philadelphia chromosome–positive ALL patient (data not shown). However, 32Dcl3 cells expressing p185BCR-ABL and BaF3 cells expressing TEL-ABL or TEL-PDGFR were rescued by the addition of IL-3 (Fig 5). This is consistent with our data for another murine cell 32Dp210. However, a human cell line, MO7p210, cannot be rescued by the addition of growth factors normally required for cellular proliferation.18
Cell proliferation assays. (A) 32Dp185 cells were plated in quadruplicate in the presence of regular growth media with or without IL-3 and the indicated amount of inhibitor. Wells were assayed for uptake of MTT at daily intervals. Results are expressed as the mean OD. These assays have been performed four times, all with similar results. (B through D) Cells were plated in the presence of regular growth media with or without IL-3 and the indicated amount of inhibitor. Each day, viable cells were counted as assessed by exclusion of trypan blue. (B) BaF3 cells, (C) BaF3 TEL-ABL cells, (D) BaF3 TEL-PDGFR cells. The data shown are representative of three separate experiments.
Cell proliferation assays. (A) 32Dp185 cells were plated in quadruplicate in the presence of regular growth media with or without IL-3 and the indicated amount of inhibitor. Wells were assayed for uptake of MTT at daily intervals. Results are expressed as the mean OD. These assays have been performed four times, all with similar results. (B through D) Cells were plated in the presence of regular growth media with or without IL-3 and the indicated amount of inhibitor. Each day, viable cells were counted as assessed by exclusion of trypan blue. (B) BaF3 cells, (C) BaF3 TEL-ABL cells, (D) BaF3 TEL-PDGFR cells. The data shown are representative of three separate experiments.
DISCUSSION
CGP 57148 is a tyrosine kinase inhibitor that shows specificity at the submicromolar level for the ABL and PDGFR kinases. This compound was designed based on the structure of the ATP binding site of protein kinases. Given the high degree of conservation of protein kinases in this region, it is a bit surprising to see selective inhibition of the ABL and PDGFR tyrosine kinases by this compound. Therefore, this specificity may reflect subtle differences in the structure of the protein kinases, even in this highly conserved region. It is also of interest that this compound inhibits the two tyrosine kinases that have been associated with proliferative hematologic malignancies.
We previously showed that CGP 57148 selectively kills p210BCR-ABL-expressing cells.18 This includes K562 cells and MO7e and 32Dcl3 cells engineered to express p210BCR-ABL. No effects of this compound, CGP 57148, were observed using 32Dv-SRC cells or parental cells, 32Dcl3, and MO7e. These observations have been extended to include other activated versions of ABL, such as p185BCR-ABL and TEL-ABL. The IC50 for inhibition of these activated versions of ABL is similar to that observed for c-ABL and BCR-ABL. In assays of substrate phosphorylation, the IC50 for ABL using CGP 57148 was 0.038 μmol/L and in cell-based assays of autophosphorylation the IC50 was 0.25 to 0.35 μmol/L. For cell killing, the concentration of compound required was 1 μmol/L with little or no effect observed at 0.1 μmol/L. This range of concentrations likely reflects the difference in the endpoint used for the assay. Thus, phosphorylation of an exogenous substrate is more readily inhibited than autophosphorylation. These data also suggest that greater than 50% of the tyrosine kinase activity of BCR-ABL must be suppressed for inhibition of cellular proliferation.
Consistent with the in vitro profile of this compound, the growth of cells expressing these activated ABL protein tyrosine kinases was inhibited in the absence of exogenous growth factor. This suggests that these cells are dependent on the activated ABL protein tyrosine kinase for proliferation and/or survival. This also suggests that inhibition of the ABL kinase occurs regardless of the mechanism of activation or that TEL and BCR activate ABL in a similar fashion. As these cells can be rescued by the addition of exogenous growth factor, our data are consistent with BCR-ABL activation of a signaling pathway that overlaps growth factor-activated pathways. These data also suggest that cells expressing an activated ABL or PDGFR could be resistant to the effects of CGP 57148 if other pathways for survival or proliferation are activated by genetic changes in addition to BCR-ABL or a TEL fusion.
Nonspecific toxicity of CGP 57148 was observed at concentrations of 100 μmol/L. The term nonspecific toxicity is used because we have not observed inhibition of cellular tyrosine phosphorylation at these concentrations and because BCR-ABL–expressing cells are killed at concentrations of 1 μmol/L of CGP 57148. Of course, it is possible that inhibition of tyrosine kinases at these higher concentrations does occur but is not detected by immunoblotting. It is also possible that, at higher concentrations, other ATP-dependent reactions could be inhibited or that other unknown processes are affected.
As CGP 57148 inhibits the PDGFR kinase, we also showed that cells expressing an activated PDGFR tyrosine kinase, TEL-PDGFR, are sensitive to this compound. Thus, this compound may be useful for the treatment of a variety of BCR-ABL–positive leukemias and for treatment of the subset of CMML patients with a TEL-PDGFR fusion protein.
Supported in part by Grant No. CA01422 (B.J.D.) and Novartis Pharma. B.J.D. and D.G.G. are recipients of Translational Research Awards from the Leukemia Society of America.
Address reprint requests to Brian J. Druker, MD, Division of Hematology and Medical Oncology, L592, Oregon Health Sciences University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97201-3098.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal