Abstract
Structural analysis of naturally processed peptides bound to the HLA class I and class II molecules of chronic myeloid leukemia (CML) blast cells was performed to characterize the antigen processing and autoantigen repertoire in this hematopoietic malignancy. Self-peptides derived from the carboxy-terminal end of the breakpoint cluster region (bcr) protein, as well as several differentiation stage- and tissue-specific self-antigens characteristic of early stages of myeloid differentiation, such as c-fes, c-pim, granulocyte-macrophage colony-stimulating factor receptor α chain, proteinase 3, and cathepsin G, were identified. A common characteristic of several of the high copy-number self-peptides identified in this study is the participation of their parent proteins in signal transduction or myeloid effector function. Because bcr-abl junctional peptides bind to a limited number of major histocompatibility complex (MHC) class I alleles, an effective peptide-based immunotherapy strategy for CML requires identification of further tumor-associated or tissue-specific peptide antigens binding to common MHC alleles such as HLA-A2. The differentiation stage- and tissue-specific MHC-bound peptides found in this study, as well as the naturally processed proteins from which they are derived, may represent autoantigens towards which T-cell responses may potentially be developed for immunotherapy of hematopoietic malignancies such as CML.
CHRONIC MYELOID LEUKEMIA (CML), a clonal proliferative disorder of the primitive hematopoietic stem cell, is characterized by the (9; 22)(q34; q11) chromosomal translocation.1 This reciprocal translocation between the breakpoint cluster region gene (bcr) and the c-abl gene forms a novel bcr-abl oncogene usually producing one of two 210-kD fusion proteins (p210b2a2 or p210b3a2) with tyrosine kinase activity.2
Antigen-specific immunotherapy of CML using tumorspecific bcr-abl fusion region peptides has been proposed.3-5 The bcr-abl fusion protein is located in the cytoplasm and is speculated to be available to the antigen processing machinery that generates peptides destined to associate primarily with major histocompatibility complex (MHC) class I molecules.6 Typically, cytoplasmic proteins are presented by MHC class I molecules, and exogenous or membrane bound proteins are presented by MHC class II molecules for immune surveillance. Recent studies showing recognition of p210b3a2 expressing CML blast cells by a p210b3a2 peptide-specific CD4+ T-cell line in an HLA-restricted manner7 suggests that processing and presentation of the p210bcr-abl protein occurs, albeit by an alternate processing mechanism.8 However, there is as yet no clear evidence that peptides derived from the p210bcr-abl protein can be naturally processed and presented to MHC class I-restricted cytotoxic T cells. Peptides derived from the fusion region of bcr-abl have been shown in in vitro binding studies to be antigenic in the context of some (eg, HLA-A3, A11, B8, DR2, DR4, and DR11) but not all MHC restriction elements.4,5,9-11 An alternative strategy for CML immunotherapy is to seek tissue-specific proteins, possibly overexpressed as a result of malignant transformation, that contain antigenic determinants and are presented by common MHC class I alleles such as HLA-A2 and can serve as targets for therapeutically directed immune responses.12 The premise that all autoreactive T cells are deleted or rendered tolerant conflicts with increasing evidence of the immunogenicity of normal self-peptides in a variety of tumors and autoimmune diseases. The utility of tissue-specific self-peptides as targets for immunotherapy has found experimental validation in melanoma for MART-1 and tyrosinase,13 ovarian and breast cancer for HER2/neu,14 and most recently in hematopoietic malignancies.15
It has been previously shown that the MHC-bound self-peptide repertoire reflects the differentiation-stage of the presenting cell.16 The present study is the first to characterize the high copy-number self-peptide repertoire naturally processed and bound to the HLA-A2, HLA-B, and HLA-DR molecules purified from CML blast cells. Our data show that the bcr protein enters into the endogenous antigen processing pathway and that several cytoplasmic proteins are presented by MHC class II molecules. In addition to a peptide derived from the carboxy-terminal of the bcr protein, we have identified several differentiation stage- and tissue-specific self-antigens characteristic of early stages of myeloid differentiation. These differentiation stage- and tissue-specific MHC bound peptides or their parent proteins, expressed by CML blast cells, may represent structures towards which specific T-cell responses can be developed for immunotherapy of hematopoietic malignancies.
MATERIALS AND METHODS
Reagents. The murine monoclonal antibodies (MoAbs) used in the affinity purification of HLA molecules were obtained from culture supernatants and purified by protein A affinity chromatography with reagents supplied by Bio-Rad (Richmond, CA). Cyanogen bromide-activated Sepharose 4B, purified normal mouse IgG, aprotinin, tosyllysylchloroketone (TLCK), tosylprolylchloroketone (TPCK), and iodoacetamide were obtained from Sigma Co. (St Louis, MO). Pepstatin A, leupeptin, and phenylmethylsulphonyl fluoride (PMSF ) were obtained from Boehringer Mannheim (San Diego, CA). All reverse-phase liquid chromatography solvents, trifluoroacetic acid (TFA), Nonidet-40, and the bicinchonnic acid (BCA) protein assay kit were obtained from Pierce (Rockford, IL). All other reagents were of the highest commercially available quality.
Cell lines and culture conditions. The murine hybridomas producing the anti–HLA-DR MoAb L243, recognizing the nonpolymorphic α1 and the β1, β3, and β5 chains of mature HLA class II molecules, and the anti–HLA-A,-B,-C MoAb W6/32 were obtained from the ATCC (Rockville, MD). The anti–HLA-B,-C MoAb producing hybridoma Bl.23.2 was a kind gift of Dr Alessandro Sette (Cytel, San Diego, CA). All hybridomas were cultured in RPMI 1640 supplemented with IgG-free fetal bovine serum.
Patient material. Approximately 1 × 1011 fresh CML blast cells were obtained with informed consent after a therapeutic leukapheresis from a patient with bilineage myeloid/extramedullary T-lymphoid CML in myeloid blast crisis. The peripheral blood leukocyte count was 503 × 109/L (myeloblasts 86%). Immunophenotypic analysis of peripheral blood mononuclear cells showed CD2+ (4%), CD3+ (3%), CD5+ (2%), CD13+ (90%), CD14+ (15%), CD19+ (<1%), CD33+ (>50%), and CD34+ (76%). The CML cells collected by leukapheresis were 94% CD34+, 95% HLA class I positive, 82% HLA class II positive, CD3−, and CD14−, with side and forward scatter measurements consistent with a blast cell population. Bone marrow was morphologically diagnostic of blastic transformation of a myeloproliferative disorder, showing cells staining myeloperoxidase positive and terminal deoxynucleotide transferase negative. An axillary lymph node biopsy showed diffuse infiltration of T lymphoblasts that were cytoplasmic CD3 and Leu22 positive, intranuclear TdT positive, and L26 and myeloperoxidase negative. Karyotyping showed 48XY, t(9,22)(q34; q11), del 16(q22), del 19(q12), +12(M). Reverse transcriptase-polymerase chain reaction (RT-PCR) using bcr-abl–specific primers confirmed a b3a2 translocation. The patient was typed for HLA class I and class II antigens by genomic typing of in vitro-amplified DNA with sequence specific-oligonucleotide primers and probes. The patient's HLA genotype was HLA-A0201, B5101, B63, DRB1*0101, DRB1*0301.
Immunofluorescent flow cytometry. The cell surface expression of HLA class I, HLA class II, and CD34 was determined on the collected CML blasts by direct or indirect immunofluorescence using a Becton Dickinson FACScan instrument (Mountain View, CA). The phycoerythrin-labeled anti-CD34 MoAb, isotype control, and fluorescein isothiocyanate-labeled antimouse Ig were obtained from Becton Dickinson.
Immunoaffinity purification of class I and class II molecules and elution of bound peptides. The immunoaffinity purification of HLA-A,-B,-C, and -DR molecules and the elution of bound peptides were performed as previously described.16 17 Briefly, a detergent lysate containing protease inhibitors was prepared from approximately 1011 cells. The lysate was cleared by centrifugation and filtration. The clarified lysate was then passed over a series of fast-flow sepharose 4B columns conjugated to (1) normal mIg, (2) anti–HLA-DR MoAb (L243), (3) anti–HLA-B,-C (B1.23.2), and (4) anti–HLA-A,-B,-C MoAb (W6/32). The columns were then washed with buffered detergent solutions and the bound HLA molecules were eluted from the affinity columns with dilute TFA. Aliquots of the purified HLA-A2, HLA-B,-C, and HLA-DR protein obtained were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining and judged to be greater than 89% pure. The final yield of HLA-A2 molecules was 1,500 μg, of HLA-B,-C molecules was 1,340 μg, and of HLA-DR1, DR3 molecules was 620 μg, as determined using the BCA protein assay. Peptides were eluted from the HLA molecules by acid/heat denaturation and separated from the high molecular weight material by ultrafiltation.
High-performance liquid chromatography (HPLC) purification and sequencing of eluted peptides. The separation of MHC-bound peptides, eluted from the HLA-A2, HLA-B or the HLA-DR molecules of the CML cells, was performed as previously described.16 17 Briefly, the acid-eluted peptides from the HLA molecules were separated by C18 reverse-phase HPLC using a 2-hour gradient. From the absorbance trace at 215 nm of the eluate, the single fractions containing absorbance peaks were selected for microsequencing by N-terminal Edman degradation using a Hewlett-Packard G 1000A protein sequencer (Palo Alto, CA). Single fraction sequencing regularly yields cycles containing multiple phenylthiohydantoin (PTH)-amino acids and mixed sequences. For the assignment of major and minor sequences from these data, we used previously published criteria. Although the material in the fractions was not homogeneous, in many instances we were able to identify the amino acid sequence of major and minor peptide sequences in each fraction. Major and minor sequences were assignable on the basis of the picomolar yield of each PTH-amino acid derivative in the cycle. The assignment was corroborated by the repetitive yield of each sequence. The repetitive yields for all reported sequences ranged from 85% to 95%. Sequences were assigned only when the yield of two or more PTH amino acid derivatives in a cycle differed by more than 35%. In some fractions it was not possible to assign major or minor sequences. The sequences of the MHC bound peptides were compared with existing protein sequences contained within the EMBL and Swissprot Databases.
RT-PCR. Normal CD34+ cells were obtained with informed consent from a patient without hematopoietic disease undergoing bone marrow harvest. Bone marrow was processed through a CellPro CELPRATE stem cell concentrator column (CellPro, Bothell, WA) for CD34+ selection. Cells eluted from the column (>80% CD34+) were then further purified with magnetic beads coated with anti-CD34 MoAb (Dynal, New Hyde Park, NY) according to the manufacturer's instructions. The purity of CD34+ cells from the second round of selection was estimated to be greater than 99%. Leukemic blasts obtained by leukapheresis were 94% CD 34+ and were used without further purification. Total RNA was prepared from blast cells and directly from normal CD34+ cells bound to the magnetic beads by the guanidine isothiocyante extraction method.18 cDNA was prepared using random hexamers and reverse transcriptase from Clonetech (Palo Alto, CA) according to the manufacturer's instructions. Equal amounts of RNA from normal and leukemic CD34+ cells (as estimated by the optical density [OD] 260 nm) were used for RT-PCR with cathepsin G primers (5′-TGA GAG TGC AGA GGG ATA GG-3′, 5′-CAG GAA ACT TGA GAC CCT GG-3′) and specific β-actin primers (Clonetech) as quality controls. PCR amplification was performed in a total volume of 50 μL using Amplitaq Gold (Perkin-Elmer, San Jose, CA) in a Perkin-Elmer thermocycler for 30 cycles of 45 seconds at 94°C, 45 seconds at 60°C, 1 minute at 72°C, and 7 minutes of extension at 72°C.
RESULTS
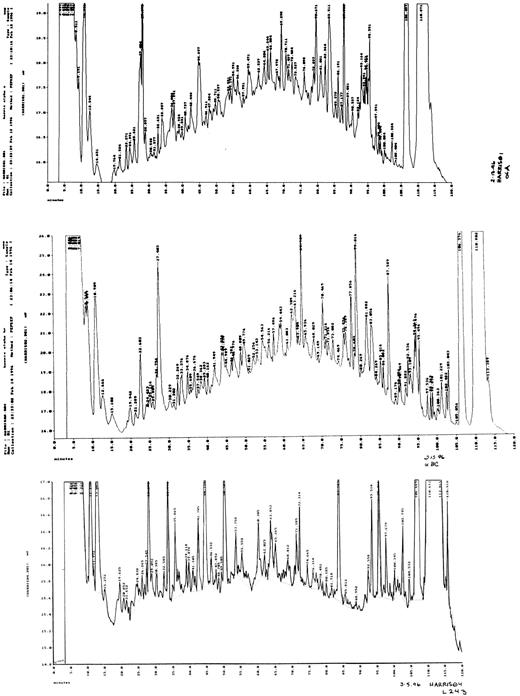
The cumulative yield of major and minor sequence peptides was estimated to be approximately 3.8 × 103 pmol from HLA-A2, 1.8 × 103 pmol from HLA-B, -C, and 2.2 × 103 pmol from HLA-DR (or approximately one-sixth of the total HLA-bound peptides). The peptides eluted from the HLA-A2, HLA-B,-C, and HLA-DR heterodimers expressed by the leukemic blast cells were fractionated by reverse-phase HPLC (Fig 1).
Reverse-phase HPLC separation of peptide pools eluted from HLA-A, B/C, and DR molecules. The chromatograms represent the peptide repertoire as detected by UV absorbance at 210 nm (20 mAU full scale, Y axis). X axis, time (0 to 120 minutes). The gradient was run for 0 to 5 minutes at 2% acetonitrile/TFA solution B and then for 5 to 120 minutes at 2% to 80% acetonitrile/TFA solution B, with a flow rate of 0.150 mL/min. (Top panel) HLA-A2 eluted peptides. (Middle panel) HLA-B63 and HLA-B5101 eluted peptides. (Bottom panel) HLA-DR1 and HLA-DR3 eluted peptides.
Reverse-phase HPLC separation of peptide pools eluted from HLA-A, B/C, and DR molecules. The chromatograms represent the peptide repertoire as detected by UV absorbance at 210 nm (20 mAU full scale, Y axis). X axis, time (0 to 120 minutes). The gradient was run for 0 to 5 minutes at 2% acetonitrile/TFA solution B and then for 5 to 120 minutes at 2% to 80% acetonitrile/TFA solution B, with a flow rate of 0.150 mL/min. (Top panel) HLA-A2 eluted peptides. (Middle panel) HLA-B63 and HLA-B5101 eluted peptides. (Bottom panel) HLA-DR1 and HLA-DR3 eluted peptides.
The sequences of the HLA-A2–bound peptides corresponded with the allele-specific motif for HLA-A0201. Similarly, the peptides bound to the HLA-B, -C, and HLA-DR molecules showed anchor residues characteristic of either HLA-B63 or HLA-B5101, and either HLA-DR1 or HLA-DR3 bound peptides, respectively. Searches of the databases for peptide sequence homology to known protein precursors showed 12 of 85 sequences bound to HLA-A2 (Table 1), 13 of 48 sequences bound to HLA-B (Table 2), and 9 of 53 sequences bound to HLA-DR (Table 3) with 100% similarity to known precursor proteins.
HLA-A2–Bound Peptides From CD34+ Blast Cells of a Patient With CML
| Precursor Protein . | Sequence* . | Yield† (pmol) . | Location‡ . | Predominant Distribution . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | . | . | . |
| Breakpoint Cluster Region Protein | F | L | L | D | H | L | K | R | V | 43 | C | Widespread | ||
| Ribosomal S6 kinase | Y | M | A | P | E | I | L | M | R | S | 3 | C | Widespread | |
| Interferon α/β receptor α chain | X | I | A | L | F | A | L | P | F | 18 | M | Widespread | ||
| Cathepsin G | F | L | L | P | T | G | A | E | A | 41 | L | Myeloid | ||
| c-pim | L | L | Y | D | M | V | X | G | D | I | P | 23 | C | Myeloid |
| c-fes | I | P | R | A | E | V | A | E | L | L | 9 | C | Myeloid (endothelial) | |
| GM CSF receptor α chain | F | I | Y | N | A | D | L | M | N | X | 21 | M | Myeloid | |
| Globin α chain | V | L | S | P | A | D | K | T | N | V | K | 23 | C | Erythroid |
| Globin β chain | K | V | N | V | D | E | V | G | G | E | 6 | C | Erythroid | |
| Dematin | S | L | P | H | F | H | H | P | E | T | 14 | M | Erythroid | |
| IL-7 | K | Q | Y | E | S | V | L | M | V | S | I | 67 | C | Stromal cell (lymphoid) |
| CD3 ζ chain | L | L | D | P | K | L | X | Y | L | L | 52 | C | T-lymphoid cell | |
| Precursor Protein . | Sequence* . | Yield† (pmol) . | Location‡ . | Predominant Distribution . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | . | . | . |
| Breakpoint Cluster Region Protein | F | L | L | D | H | L | K | R | V | 43 | C | Widespread | ||
| Ribosomal S6 kinase | Y | M | A | P | E | I | L | M | R | S | 3 | C | Widespread | |
| Interferon α/β receptor α chain | X | I | A | L | F | A | L | P | F | 18 | M | Widespread | ||
| Cathepsin G | F | L | L | P | T | G | A | E | A | 41 | L | Myeloid | ||
| c-pim | L | L | Y | D | M | V | X | G | D | I | P | 23 | C | Myeloid |
| c-fes | I | P | R | A | E | V | A | E | L | L | 9 | C | Myeloid (endothelial) | |
| GM CSF receptor α chain | F | I | Y | N | A | D | L | M | N | X | 21 | M | Myeloid | |
| Globin α chain | V | L | S | P | A | D | K | T | N | V | K | 23 | C | Erythroid |
| Globin β chain | K | V | N | V | D | E | V | G | G | E | 6 | C | Erythroid | |
| Dematin | S | L | P | H | F | H | H | P | E | T | 14 | M | Erythroid | |
| IL-7 | K | Q | Y | E | S | V | L | M | V | S | I | 67 | C | Stromal cell (lymphoid) |
| CD3 ζ chain | L | L | D | P | K | L | X | Y | L | L | 52 | C | T-lymphoid cell | |
Standard IUPAC amino acid abbreviations are used. X denotes an unidentifiable PTH-amino acid.
Initial yield of peptides calculated from the repetitive yields of PTH amino acid derivatives of the sequence. The total yield of peptides in Table 1 accounted for approximately 9% of the mass of material found in all the HLA-A2 fractions combined.
C, cytoplasmic; L, lumenal; M, membrane associated.
HLA-B63, B5101–Bound Peptides From CD34+ Blast Cells of a Patient With CML
| Precursor Protein . | Sequence* . | Yield† (pmol) . | Location‡ . | Predominant Distribution . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | . | . | . |
| c-cbl | I | L | R | E | F | V | S | I | S | 5 | C | Widespread | |
| G 3-PDH | M | A | H | M | A | S | K | E | V | 81 | C | Widespread | |
| Transketolase | I | D | R | D | A | I | A | Q | A | V | 17 | C | Widespread |
| AF6 | S | L | N | D | A | P | F | S | P | 9 | C | Widespread (?) | |
| a-Myb | I | M | T | E | Q | A | R | R | Y | 18 | C | Widespread | |
| Rhombotin 1 | M | V | L | D | K | E | D | G | V | P | 21 | C | Widespread (?) |
| P19-INK4D | V | E | H | G | A | D | V | N | V | 14 | C | Widespread | |
| UBR1 (yeast) | X | P | A | Y | H | D | H | S | P | 15 | C | Widespread | |
| Flavin monooxygenase | F | P | F | P | E | D | Y | P | N | 15 | M | Widespread | |
| CD44 | V | T | V | G | D | S | N | S | N | V | 32 | M | Widespread |
| CD5 | A | E | N | P | T | A | S | H | V | D | 8 | M | Lymphoid (myeloid) |
| Proteinase 3 | R | L | V | N | V | V | L | G | A | H | 33 | L | Myeloid |
| Tryptase | L | A | S | R | A | Y | A | A | P | 25 | L | Myeloid | |
| Precursor Protein . | Sequence* . | Yield† (pmol) . | Location‡ . | Predominant Distribution . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | . | . | . |
| c-cbl | I | L | R | E | F | V | S | I | S | 5 | C | Widespread | |
| G 3-PDH | M | A | H | M | A | S | K | E | V | 81 | C | Widespread | |
| Transketolase | I | D | R | D | A | I | A | Q | A | V | 17 | C | Widespread |
| AF6 | S | L | N | D | A | P | F | S | P | 9 | C | Widespread (?) | |
| a-Myb | I | M | T | E | Q | A | R | R | Y | 18 | C | Widespread | |
| Rhombotin 1 | M | V | L | D | K | E | D | G | V | P | 21 | C | Widespread (?) |
| P19-INK4D | V | E | H | G | A | D | V | N | V | 14 | C | Widespread | |
| UBR1 (yeast) | X | P | A | Y | H | D | H | S | P | 15 | C | Widespread | |
| Flavin monooxygenase | F | P | F | P | E | D | Y | P | N | 15 | M | Widespread | |
| CD44 | V | T | V | G | D | S | N | S | N | V | 32 | M | Widespread |
| CD5 | A | E | N | P | T | A | S | H | V | D | 8 | M | Lymphoid (myeloid) |
| Proteinase 3 | R | L | V | N | V | V | L | G | A | H | 33 | L | Myeloid |
| Tryptase | L | A | S | R | A | Y | A | A | P | 25 | L | Myeloid | |
Standard IUPAC amino acid abbreviations are used. X denotes an unidentifiable PTH-amino acid.
Initial yield of peptides calculated from the repetitive yields of PTH amino acid derivatives of the sequence. The total yield of peptides in Table 2 accounted for approximately 1.2% of the mass of material found in all the HLA-B fractions combined.
C, cytoplasmic; L, lumenal; M, membrane associated.
HLA-DR 1- and HLA-DR 3-Bound Peptides From CD34+ Blast Cells of a Patient With CML
| Precursor Protein . | Sequence/Anchors3-150 . | Predominant Distribution . | ||||
|---|---|---|---|---|---|---|
| . | i+3+8 . | Yield3-151 (pmol) . | Location3-152 . | . | ||
| . | . | . | . | . | . | . |
| Tubulin β chain | TSRGSQQYRALTVPELT | 180 | C | Widespread | ||
| HLA-DQ(5) β chain | NSQKEVLEGTRAELDTV | 67 | M | Widespread | ||
| Integrin β 5 subunit | DAPEGGFDAVLQAAVXK | 27 | M | Widespread | ||
| Erythrocyte membrane protein p55 | VPYTTRPPRKSEEDGKEY | 32 | M | Widespread (erythroid) | ||
| Prolidase | AVDDVQYVDEIASVLTSQ | 21 | L | Widespread (secreted) | ||
| Thrombospondin 1 | DNXPFHYNPAQYDYD | 45 | L | Widespread (secreted) | ||
| MRP14 | EGPGHHHKPGLGEG | 45 | M/C | Myeloid | ||
| Leukotriene-B4 Omega-Hydroxylase | WPDPEVYDPFRFDPKNIK | 89 | M | Myeloid | ||
| Granzyme H | QPFLLLLAFLLTP | 93 | L | T lymphoid, NK cells | ||
| Precursor Protein . | Sequence/Anchors3-150 . | Predominant Distribution . | ||||
|---|---|---|---|---|---|---|
| . | i+3+8 . | Yield3-151 (pmol) . | Location3-152 . | . | ||
| . | . | . | . | . | . | . |
| Tubulin β chain | TSRGSQQYRALTVPELT | 180 | C | Widespread | ||
| HLA-DQ(5) β chain | NSQKEVLEGTRAELDTV | 67 | M | Widespread | ||
| Integrin β 5 subunit | DAPEGGFDAVLQAAVXK | 27 | M | Widespread | ||
| Erythrocyte membrane protein p55 | VPYTTRPPRKSEEDGKEY | 32 | M | Widespread (erythroid) | ||
| Prolidase | AVDDVQYVDEIASVLTSQ | 21 | L | Widespread (secreted) | ||
| Thrombospondin 1 | DNXPFHYNPAQYDYD | 45 | L | Widespread (secreted) | ||
| MRP14 | EGPGHHHKPGLGEG | 45 | M/C | Myeloid | ||
| Leukotriene-B4 Omega-Hydroxylase | WPDPEVYDPFRFDPKNIK | 89 | M | Myeloid | ||
| Granzyme H | QPFLLLLAFLLTP | 93 | L | T lymphoid, NK cells | ||
Alignment of self-peptide sequences along the motifs of HLA-DR1 and DR3 according to the notation introduced by Hill et al.20 The italicized letter i indicates the first or N terminal anchor residue, followed by the number of intervening amino acid residues to the last or C terminal anchor position (DR 1 = i + 8, DR 3 = i + 3).19
The total yield of peptides in Table 3 accounted for approximately 27% of the mass of material found in all the HLA-DR fractions combined.
C, cytoplasmic; L, lumenal; M, membrane associated.
Autologous peptides bound to the HLA-class I molecules of CML blasts. The precursor proteins of the HLA class I-bound peptides could be classified into two groups based on their expression in adult tissue: (1) widespread tissue expression and (2) restricted lineage- and differentiation-specific expression (Tables 1 and 2). The former group includes peptide FLLDHLKRV (residues 1181-1189) derived from the carboxy-terminal of the p160bcr cytoplasmic breakpoint cluster region protein,21 c-cbl–derived peptide ILREFVSIS,22 and YMAPEILMRS (residues 236-245) derived from cytoplasmic ribosomal S6 kinase.23 The binding affinity to HLA-A2 of the p160bcr-derived peptide FLLDHLKRV, relative to other abl, bcr, and bcr-abl junctional derived peptides, was calculated using the previously published schema developed by Parker et al24 (Table 4).
Predicted HLA-A0201 Binding Half-Lives of Peptides Derived From BCR-ABL, ABL, and BCR
| Precursor Protein . | Sequence4-150 . | T1/2 (min) . |
|---|---|---|
| BCR-ABL junctional region only | TGFKQSSKA4-151 | 0.120 |
| BCR-ABL junctional region only | KALQRPVAS | 0.013 |
| ABL and BCR-ABL fusion protein | NLFVALYDFV | 1040 |
| ABL and BCR-ABL fusion protein | NLYTFCVSYV | 2815 |
| BCR and BCR-ABL fusion protein | QQYDCKWYI | 572 |
| BCR and BCR-ABL fusion protein | QLLKDSFMV | 1492 |
| BCR (carboxyl terminal) | NLLTFLFLL | 4006 |
| BCR (carboxy terminal) | FLLDHLKRV‡ | 11,163 |
| Precursor Protein . | Sequence4-150 . | T1/2 (min) . |
|---|---|---|
| BCR-ABL junctional region only | TGFKQSSKA4-151 | 0.120 |
| BCR-ABL junctional region only | KALQRPVAS | 0.013 |
| ABL and BCR-ABL fusion protein | NLFVALYDFV | 1040 |
| ABL and BCR-ABL fusion protein | NLYTFCVSYV | 2815 |
| BCR and BCR-ABL fusion protein | QQYDCKWYI | 572 |
| BCR and BCR-ABL fusion protein | QLLKDSFMV | 1492 |
| BCR (carboxyl terminal) | NLLTFLFLL | 4006 |
| BCR (carboxy terminal) | FLLDHLKRV‡ | 11,163 |
Pairs of sequences, derived from the parent protein, with the highest binding affinity for HLA-A2 as determined from the algorithm of Parker et al.24
K is the unique junctional amino acid encoded by the b3a2 translocation.
The naturally processed self-peptide bound to HLA-A0201 in this study.
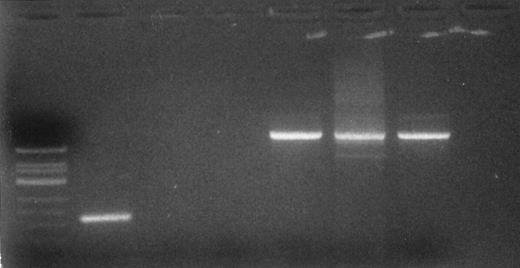
Several of the high copy number peptides bound to the HLA class I molecules of the CML blast cells corresponded to self-antigens with a narrower tissue distribution. The peptides FLLPTGAEA (residues 10-18), RLVNVVLGAH (residues 79-88), and LASRAYAAP (residues 12-20) are derived from cathepsin G,25 proteinase 3,26 and tryptase,27 respectively. These three proteins are lineage- and differentiaton stage-specific members of the neutral serine proteinase family, found in the primary granules of myeloid cells.28 RT-PCR analysis of cathepsin G showed differential expression of mRNA for this protein in the CD34+ CML blast cells as compared with normal CD34+ cells (Fig 2). The peptide corresponding to residues 231-241 of the protooncogene c-pim was identified bound to HLA-A2. p33pim is a 33-kD cytoplasmic kinase whose normal expression is largely restricted to primitive hematopoietic tissue and adult myeloid cells.29 The proto-oncogene c-fes also served as a source of HLA-A2–bound peptide. p92fes is a cytoplasmic tyrosine-specific kinase expressed exclusively in hematopoeitic progenitor and mature myeloid cells30 and in vascular endothelial cells in adults.31 The transmembrane protein granulocyte-macrophage colony-stimulating factor (GM-CSF ) receptor α chain (residues 127-136), normally expressed predominantly by hematopoeitic cells,32 was found to contribute to the HLA class I self-peptide repertoire.
Detection of cathepsin G mRNA transcripts in CD34+ CML blast cells. Total RNA was extracted from CD34+ CML blast cells. Ethidium bromide-stained gel of RT-PCR products. Lane 1 (left to right), molecular weight standard; lanes 2 and 5, CD34+ CML blast cells; lanes 3 and 6, normal CD34+ cells; lanes 4 and 7, breast cancer cell line as a negative control. Lanes 2 through 4 are RT-PCR products after amplification with cathepsin G primer set. Lanes 5 through 7 are RT-PCR products after amplification with β-actin primers and serve as a control.
Detection of cathepsin G mRNA transcripts in CD34+ CML blast cells. Total RNA was extracted from CD34+ CML blast cells. Ethidium bromide-stained gel of RT-PCR products. Lane 1 (left to right), molecular weight standard; lanes 2 and 5, CD34+ CML blast cells; lanes 3 and 6, normal CD34+ cells; lanes 4 and 7, breast cancer cell line as a negative control. Lanes 2 through 4 are RT-PCR products after amplification with cathepsin G primer set. Lanes 5 through 7 are RT-PCR products after amplification with β-actin primers and serve as a control.
Two peptides were derived from α globin (residue 1-11) and β globin (residue 17-26), respectively. The expression of β globin mRNA by the CML blast cells in this study was confirmed by RNA primer extension assay (data not shown).
Autologous peptides bound to the HLA-class II molecules of CML blasts. The tissue specificity and subcellular origin of self-peptides bound to the HLA class II molecules of the CML cells are shown in Table 3.
The widely expressed intracellular protein tubulin β-2 supplied the major HLA-DR bound peptide of the CML cells (Table 3). Other broadly expressed proteins that supplied peptides to the class II molecules included the MHC class II histocompatibility antigen HLA-DQB*0501 chain molecule, the transmembrane integrin β5 subunit, and the erythrocyte membrane protein p55. Secreted proteins, associated with the exocytic pathway, are frequently a source of MHC class II-bound peptides. Consistent with this, peptides derived from the ubiquitous enzyme prolidase and the glycoprotein thrombospondin 1 also were found bound to the HLA-DR1, DR3 molecules of the CML cells.
Three HLA-DR–bound peptides with more limited tissue expression were identified. One was derived from MRP14, a protein produced predominantly by myeloid cells and keratinocytes,33 and another from a myeloid-restricted microsomal leukotriene-B4 ω-hydroxylase.34 Finally, a peptide derived from granzyme H protein, expressed in T cells and NK cells, was found on the HLA class II molecules.
DISCUSSION
The structural characterization of self-peptides bound to the MHC class I and class II molecules of CML blast cells was performed to gain a better understanding of the antigen processing pathways operative in CML blast cells and of the repertoire of self-peptides presented by myeloid leukemia blast cells. Additionally, we identified HLA-bound self-peptides within the repertoire that were derived from proteins with tissue-restricted expression. Such proteins have the potential to carry autoimmunogenic epitopes that could serve as targets of therapeutic T-cell autoreactivity.12
We have previously shown that, as in professional antigen presenting cells, the distinction between endogenous and exogenous antigen processing pathways is not rigorous in myeloid-lineage cells.35 Although the mechanisms responsible for the cross-talk between these antigen processing pathways have not been analyzed in detail, this phenomenon results in the presentation by MHC class I molecules of self-peptides usually associated with the endocytic compartments of the exogenous antigen processing pathway36 and, conversely, the presentation of endogenous proteins by MHC class II molecules.35 In this study, we found that a significant percentage of the total MHC class II-bound peptides was contributed by cytosolic tubulin β chain. Tubulin has a long half-life, which is consistent with the model proposed by Gueguen and Long37 for selection of cytosolic proteins for MHC class II presentation. These investigators have provided experimental evidence that class I molecules present both short- and long-lived forms of a cytosolic protein, whereas MHC class II molecules present only the long-lived form. Hence, the half-life of cytosolic peptides suggests a mechanism for selective expression of cytosolic proteins by MHC class II molecules. A possible pathway for the presentation of endogenous cytosolic protein-derived peptides by MHC class II molecules may be via autophagy, a poorly understood process whereby cytosolic proteins are engulfed into lysosomes. The intracellular abundance of a cytosolic protein may also favor MHC class II presentation. We identified a MHC class II-bound peptide derived from the cytosolic Ca2+ binding phosphoprotein MRP14, which represents a major portion of cellular protein in myeloid cells. Bosch et al7 have reported that CD4+ T cells primed in vitro with synthetic junctional region peptides responded to naturally processed bcr-abl peptides presented by MHC class II molecules of the CML cell. The aforementioned mechanisms of MHC class II presentation of peptides derived from endogenous proteins that appear to be operative in CML blasts may help to elucidate this finding.
As expected in the CML blast cells examined, many of the peptides bound to MHC molecules derived from widely expressed cellular proteins. We also identified MHC bound peptides derived from interleukin-7, CD3 ζ chain protein, and granzyme H, together with α-globin, β-globin, and dematin. This likely represents aberrant expression of lineage- and differentiation stage-specific proteins in a pluripotential malignant stem cell. The T-cell phenotype of the lymphoblastic cells in the patient's axillary lymph nodes provides clinical corroborative support for the bilineal potential of these CML blast cells.38
The HLA-A2 allele expressed by CML blast cells in this study occurs with high frequency (40% to 50%) in the general population. Tumor-specific peptides binding to this allele would be of obvious relevance when considering a broad-based immunotherapy strategy. The junctional sequences of p210bcr-abl fusion proteins have been proposed to represent tumor-specific immunogens. Recognition and lysis of CML cells by bcr-abl–specific cytotoxic T lymphocytes will require that the fusion protein enter into the endogenous antigen processing pathway and that peptides spanning the junctional region successfully compete with other peptides for binding to the MHC class I molecules. However, peptides derived from the junctional region of bcr-abl show little or no binding affinity for HLA-A2, based on predicted binding affinity and on HLA-A2 refolding assays.4,9,10 As anticipated, we were unable to show the presence of bcr-abl junctional region-derived peptides bound to the HLA-A2 molecules of the CML cells studied. However, we did identify an HLA-A2–bound peptide derived from the carboxy-terminal end of the bcr protein. There is evidence in CML for the simultaneous expression of bcr-abl, abl, abl-bcr, and bcr mRNA,39 with proteins derived from the latter two transcripts being possible sources for the peptide isolated. The relative amounts of bcr and bcr-abl transcripts in CML cells are similar, with relatively prolonged half-lives compared with abl.21 In the context of protein trafficking and antigen presentation, it has been shown that the amino-terminal of a protein influences its processing and presentation by MHC class I molecules.40 Because p160bcr and p210bcr-abl share the amino-terminal sequence, it is reasonable to predict that they share certain characteristics in processing. Peptides derived from the native p160bcr, such as that characterized in this study, may thus conceivably compete and bind to other MHC class I alleles with greater affinity than peptides derived from the junctional region of p210bcr-abl. Indeed, for most HLA class I alleles reported to bind peptides derived from the junctional region of bcr-abl, there exist nonjunctional p210bcr-abl peptides of equal or greater binding affinity that can elicit cytotoxic T-cell responses.41 For HLA-A2, the naturally processed bcr-derived peptide characterized in this study shows the highest predicted binding affinity of possible nonameric peptide sequences contained within the bcr, abl, and bcr-abl proteins (Table 4). Junctional peptides have been shown to be antigenic, but it remains to be shown that these peptide antigens are naturally processed and presented by MHC class I molecules of CML. Our findings suggest that this may be due, in part, to the fact that junctional peptides compete (unsuccessfully) with peptides of higher affinity for MHC class I molecule binding. These observations underscore the difficulties associated with whole protein vaccination and may prove crucial in immunotherapy using bcr-abl–derived peptides. Other autoantigens, perhaps in the form of self-peptides derived from tissue-specific proteins, may thus have greater utility in the development of HLA-restricted T-cell responses towards CML.
We have identified eight peptides bound to MHC molecules from protein precursors with restricted tissue expression that are relevant to CML. Among these are HLA-A2–bound peptides derived from the protooncogenes p33pim and p92fes, the GM-CSF receptor α chain, and HLA-DR–bound MRP14. Both p33pim and p92fes may contribute to malignant transformation and are overexpressed in CML and various other myeloid leukemias.29,42 Because there is no evidence for gene amplification, it is speculated that transcriptional or posttranscriptional events may be responsible for the overexpression of these proto-oncogenes. The overexpression of p33pim and p92fes in CML cells, analogous to HER2/neu, and presentation of their peptides by MHC class I molecules fulfill the requirements of potential antigenic targets. It is of interest that both p33pim and p92fes are involved in signal transduction induced by GM-CSF and interleukin-3 via the GM-CSF receptor. p210bcr-abl appears to induce an increase in phosphorylation of p92fes. p92fes has also been shown to form a stable complex with p160bcr. This complex associates with the adaptor protein GRB-2 that binds SOS and activates the RAS pathway.43 Ribosomal S6 kinase is a further downstream component of the RAS pathway. p210bcr-abl also induces the formation of a multimeric complex containing the adaptor protein CRKL, p120cbl, p210bcr-abl, and phosphotidylinositol-3′ kinase, which is involved in signal transduction pathways mediating proliferation.44 p120cbl is one of the major proteins phosphorylated by p210bcr-abl and results in p120cbl ubiquitination.45 Finally, MRP14 also appears to function in signal transduction and modulation of protein kinase activity. HLA class I bound peptides derived from many of the component members of these signaling pathways, namely GM-CSFR α chain, p92fes, p160bcr, and p120cbl, have been identified in this study. These observations suggest that processing and presentation of peptides derived from proteins involved in signaling pathways may be a characteristic of myeloid transformation and a reflection of increased trafficking and turnover of these proteins in CML cells.
The ubiquitin-dependent proteasome pathway is the main nonlysosomal proteolytic system in the cytoplasm and a prime candidate for MHC class I peptide production.46 The MHC class I presentation of globin α and CD3 ζ chain-derived peptides reported in this study provides in vivo confirmation of the previously demonstrated in vitro ubiquitin-dependent proteasomal degradation and processing of these proteins.47,48 The rapidity and irreversibility of selective protein degradation by proteasomes makes this an ideal mechanism for controlling regulatory proteins involved in signal transduction pathways and cell lineage specificity.49 Ubiquitination/deubiquitination may regulate the function of signaling and cell cycle proteins and receptors. Furthermore, phosphorylation of proteins may influence the rate of ubiquitination and thus the rate of degradation of these ubiquitinated proteins. The formation of multimeric complexes such as those involving bcr-abl, cbl, and CRKL may facilitate ubiquitination of these phosphoproteins by trans-acting mechanisms. The constitutive tyrosine kinase activity of bcr-abl may thus play a role in phosphorylation of signaling proteins that alter not only their function, but also their fate with regard to ultimate proteasomal degradation and MHC class I presentation. The determinants of recognition for protein degradation and the role of ubiquitination and phosphorylation of proteins, particularly proto-oncogenes and those involved in signal transduction and cell cycle control in CML, remain to be elucidated. Understanding of these mechanisms may not only clarify the pathways of antigen presentation by these malignant hematopoietic cells, but also define a possible role for alterations in ubiquitin-dependent protein degradation in malignant transformation.
The remaining four peptides relevant to CML derive from proteins involved in myeloid effector functions, namely cathepsin G, proteinase 3, tryptase, and leukotriene-B4 omega-hydroxylase. Cathepsin G expression is normally limited to the promyelomonocyte stage of myeloid cell development and mast cells.50 Autoantibodies to cathepsin G have been implicated in ulcerative colitis and primary sclerosing cholangitis, suggesting that this protein contains immunogenic epitopes.51 Currently, it is not technically possible to characterize the MHC self-peptide repertoire of normal CD34+ progenitors due to the limited numbers of available cells. To determine whether a specific self-peptide might have CML blast cell-restricted presentation, relative to normal CD34+ progenitor cells, we have relied on detection of mRNA coding for the precursor protein. In this context, the detection of cathepsin G mRNA in CD34+ CML blasts but not normal CD34+ cells makes the cathepsin G protein another potential candidate antigen for immunotherapy in CML. Functional data regarding the immunogenic potential of the tissue-specific parent proteins identified in these studies are provided, in part, by a recent report of Molldrem et al.15 Cytotoxic T cells raised against an HLA-A2 binding peptide derived from proteinase 3, also a member of the neutral serine proteinase family and the likely target of autoantibodies in Wegener's granulomatosis, have been shown to lyse myeloid leukemic cells preferentially. We have identified a peptide derived from proteinase 3 bound to the HLA-B allele, providing confirmatory evidence that this protein is naturally processed and presented on MHC molecules in CML.
In preliminary experiments we were unable to show immunogenicity of c-fes–derived peptide (IPRAEVAELL) using peripheral blood mononuclear cells from HLA-A2–positive responders. It is not unreasonable to conclude that T cells able to recognize this high copy-number MHC class I-bound peptide have been deleted from the peripheral T-cell repertoire of these responders. However, within the primary structure of c-fes, there exists alternative peptide nonamers, with lower predicted HLA-A2 binding affinities, that may be naturally processed and presented. Because c-fes is tissue-specific and overexpressed in CML, these latter peptides may not have participated in T-cell selection-thymic education and could be antigenic. We are currently determining whether subdominant or normally cryptic peptide antigens can be found within the tissue-specific parent proteins identified in this study. Demonstration of cytotoxic T-cell–mediated specific lysis of malignant CD34+ cells with sparing of normal CD34+ cells may define a role for these peptides in adoptive or vaccine immunotherapy in CML and other myeloid malignancies.
ACKNOWLEDGMENT
The authors thank Dr Haralambos Raftopoulos for performing the β globin RNA primer extension assay. A.M. is on leave from the International Institute of Genetics and Biophysics, CNR (Naples, Italy).
Supported by grants from the Public Health Services (R01 AI25210-10; to N.S.-F.) and from the Office of Clinical Trials of the College of Physicians and Surgeons of Columbia University (to P.E.H.).
Address reprint requests to Paul E. Harris, PhD, P&S 14-401, 630 W 168th St, New York, NY 10032.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal