Abstract
Follicular dendritic cells (FDCs) in the lymphoid follicle (LF) are essential to the sequential processes of B-cell proliferation, selection, and differentiation. Although the importance of some cytokines in these processes has been pointed out, there is little information about the follicular localization of their receptors. We investigated, with special reference to FDCs, the localization of cytokine receptors as well as cytokines themselves in human tonsils by several means, including immunochemistry, immunoelectron microscopy, reverse transcriptase polymerase chain reaction, and in situ hybridization. FDCs in the follicular apical light zone expressed transforming growth factor-β receptor II (TGF-βR II), granulocyte-macrophage colony-stimulating factor receptor α (GM-CSFRα; CDw116), tumor necrosis factor receptor I (TNFR I; CD120a), interleukin-1 receptor II (IL-1R II; CDw121b), IL-2 receptor β (IL-2Rβ; CD122), IL-4 receptor (IL-4R; CDw124), and IL-6 receptor (IL-6R; CD126), among the 10 receptors examined. Those in the basal light zone expressed strongly TNFR I and weakly GM-CSFR α, IL-1R II, IL-2Rβ, IL-4R, and IL-6R, and often those in the outer and mantle zones expressed GM-CSFR α, IL-4R, and IL-6R. FDCs in the apical light zone expressed only TGF-β among the 7 cytokines examined. On the other hand, follicular lymphocytes mainly in the light zone expressed 9 kinds of receptors, with the exception being TGF-βR II; expression was rather frequent for TNF-α, IL-1α, and IL-2 and less frequent for TGF-β, GM-CSF, IL-4, and IL-6. These data indicate that only FDCs mainly in the light zone express many cytokine receptors, although FDCs may produce the cytokine, TGF-β. Cytokines may act not only on some follicular lymphocytes but also on most FDCs in the light zone expressing cytokine receptors.
LYMPHOID TISSUES ARE composed of B-cell–dependent and T-cell–dependent areas. Follicular B cells and follicular dendritic cells (FDCs) play a central role in events related to humoral immunity in the lymphoid follicle (LF). Mantle zone (MZ) B cells activated in the T-cell zone may migrate to the dark zone (DZ) to proliferate with the somatic mutation of the V region of the Ig gene, resulting in class switches from IgM to IgA, IgE, or IgG.1-4 Whereas B cells with low affinity to antigen die by means of apoptosis in the basal light zone (BLZ), those with high affinity for the antigen survive and mature into plasmablasts and memory B cells in the apical light zone (ALZ).5 FDCs are essential to these sequential events of B cells by (1) supporting the framework of the LF by extending their long cytoplasmic processes,6-8 (2) trapping and retaining immune complexes on their surfaces with well-developed labyrinth-like structures, and (3) presenting antigen to B cells.9 10
It has been pointed out that some cytokines play an important role in the sequential processes of B cells from proliferation in the DZ to maturation in the ALZ.8,11-13 However, the results of some studies were controversial, partly because of technical problems involved in detecting cytokines.14-16 On the other hand, there is little information about cytokine receptors, although the follicular localization of some receptors, such as tumor necrosis factor receptor type I and II (TNFR I and II), interleukin-2 receptor α (IL-2Rα), and IL-4R, has been reported.17-19 Evaluation from the viewpoint of cytokine receptors is crucial to understanding the cytokine network in the LF. The aim of the present study is to investigate the localization of cytokine receptors, as well as cytokines themselves, with special reference to FDCs in human LFs by means of immunochemistry, reverse transcriptase-polymerase chain reaction (RT-PCR), and in situ hybridization. We showed here that FDCs mainly in the follicular light zone (LZ) express seven cytokine receptors such as transforming growth factor-β receptor type II (TGF-βR II), granulocyte-macrophage colony-stimulating factor receptor α (GM-CSFRα; CDw116), TNF receptor I (TNFR I, CD120a), IL-1 receptor II (IL-1R II; CDw121b), IL-2 receptor β (IL-2Rβ; CD122), IL-4 receptor (IL-4R; CDw124), and IL-6 receptor (IL-6R; CD126). These data indicate that FDCs mainly in the LZ express a variety of cytokine receptors and that cytokines may act not only on some follicular lymphocytes but also on most FDCs in the LZ-expressing cytokine receptors.
MATERIALS AND METHODS
Tissue Samples
Human tonsillar tissues were obtained from 15 patients with chronic tonsillitis. The tissue samples were cut into sections 5-mm thick and then immediately fixed in periodate-lysine-paraformaldehyde (PLP),20 4% paraformaldehyde (PFA), or buffered 10% formalin for 6 hours at 4°C. Samples fixed in PLP or 4% PFA were washed with 0.01 mol/L phosphate-buffered saline (PBS; pH 7.4) containing a graded series of sucrose (10%, 15%, and 20%), embedded and frozen in Tissue-Tek II OCT compound (Miles Inc, Elkhart, IN) in acetone cooled with dry ice, and stored at −80°C until cryostat sectioning for immunohistochemistry and in situ hybridization. Buffered 10% formalin and 4% PFA-fixed samples were embedded in paraffin and cut into 5-μm–thick sections for immunohistochemistry.
Immunohistochemistry
The antibodies used for immunohistochemistry are listed in Table 1. Representative cryostat and paraffin sections were immunostained using the avidin-biotin-peroxidase (ABC) complex.21 Paraffin sections were digested with 0.1% trypsin (DIFCO Laboratories, Detroit, MI) for 30 minutes at 37°C and immunostained for TGF-β R II. Biotinylated F(ab′ )2 fragment of affinity-isolated rabbit antimouse Igs (DAKO, Glostrup, Denmark), antirabbit IgG (Protos Immuno Research, San Francisco, CA), and biotinylated affinity-isolated rabbit antirat Igs (DAKO) were used as secondary antibodies. The sections were incubated with the ABC reagent (Vectastain, ABC Elite; Vector Laboratories, Burlingame, CA), followed by 3,3′-diaminobenzidine (DAB; Dojin Chemicals, Kumamoto, Japan) as the chromogen. Finally, the slides were counterstained with methyl green. Negative controls were run in parallel with replacement of the specific antibody with 0.01 mol/L PBS (pH 7.4) or mouse IgG1 or IgG2 (DAKO). Secondary LF were divided into the MZ, OZ, DZ, BLZ, and ALZ, as previously described.22 Each zone was recognized by findings of hematoxylin and eosin stain and CD23 immunostain using serial cryostat sections. The reactivity in each zone was estimated as follows: −, negative; +/−, often positive; +, weakly positive; and ++, strongly positive.
List of Antibodies
| Antibody . | CD . | Subclass . | . | Source . | Used for . |
|---|---|---|---|---|---|
| Cytokines | |||||
| TGF-β | Mouse | IgG2 | GZM | IH, IC | |
| GM-CSF | Mouse | IgG1 | GZM | IH, IC | |
| TNF-α | Rabbit | H | GZM | IH, IC | |
| IL-1α | Rabbit | H | GZM | IH, IC | |
| IL-2 | Mouse | IgG1 | GZM | IH, IC | |
| IL-4 | Mouse | IgG1 | GZM | IH, IC | |
| IL-6 | Mouse | IgG1 | GZM | IH, IC | |
| Cytokine receptors | |||||
| IL-2 R α | 25 | Mouse | IgG1 | Dako | IH, IC |
| GM-CSF R α | w116 | Mouse | IgG2a | UBI | IH, IC, IEM |
| TNF R I | 120a | Mouse | IgG2a | Bender | IH, IC, IEM |
| TNF R II | 102b | Rat | IgG2b | GZM | IH |
| IL-1 R I | 121a | Mouse | IgG1 | GZM | IH |
| IL-1 R II | 121b | Rat | IgG2b | GZM | IH, IC |
| IL-2 R | 122 | Mouse | IgG1 | IT | IH, IC |
| IL-4 R | 124 | Mouse | IgG1 | IT | IH, IC, IEM |
| IL-6 R | 126 | Mouse | IgG1 | IT | IH, IC |
| TFG-β R II | Rabbit | H | UBI | IH, IC | |
| Others | |||||
| L26 | 20 | Mouse | IgG2a | DAKO | IC |
| UCHL-1 | 45 | Mouse | IgG2a | DAKO | IC |
| SK11 | 62L | Mouse | IgG2a | BD | IC |
| Fc ɛ R II | 23 | Mouse | IgG2a | BD | IC |
| EMA | Mouse | IgG2a | DAKO | IC | |
| Ki-M4 | Mouse | IgG2a | BMA | IH | |
| DRC-1(R4/23) | Mouse | IgM | DAKO | IC |
| Antibody . | CD . | Subclass . | . | Source . | Used for . |
|---|---|---|---|---|---|
| Cytokines | |||||
| TGF-β | Mouse | IgG2 | GZM | IH, IC | |
| GM-CSF | Mouse | IgG1 | GZM | IH, IC | |
| TNF-α | Rabbit | H | GZM | IH, IC | |
| IL-1α | Rabbit | H | GZM | IH, IC | |
| IL-2 | Mouse | IgG1 | GZM | IH, IC | |
| IL-4 | Mouse | IgG1 | GZM | IH, IC | |
| IL-6 | Mouse | IgG1 | GZM | IH, IC | |
| Cytokine receptors | |||||
| IL-2 R α | 25 | Mouse | IgG1 | Dako | IH, IC |
| GM-CSF R α | w116 | Mouse | IgG2a | UBI | IH, IC, IEM |
| TNF R I | 120a | Mouse | IgG2a | Bender | IH, IC, IEM |
| TNF R II | 102b | Rat | IgG2b | GZM | IH |
| IL-1 R I | 121a | Mouse | IgG1 | GZM | IH |
| IL-1 R II | 121b | Rat | IgG2b | GZM | IH, IC |
| IL-2 R | 122 | Mouse | IgG1 | IT | IH, IC |
| IL-4 R | 124 | Mouse | IgG1 | IT | IH, IC, IEM |
| IL-6 R | 126 | Mouse | IgG1 | IT | IH, IC |
| TFG-β R II | Rabbit | H | UBI | IH, IC | |
| Others | |||||
| L26 | 20 | Mouse | IgG2a | DAKO | IC |
| UCHL-1 | 45 | Mouse | IgG2a | DAKO | IC |
| SK11 | 62L | Mouse | IgG2a | BD | IC |
| Fc ɛ R II | 23 | Mouse | IgG2a | BD | IC |
| EMA | Mouse | IgG2a | DAKO | IC | |
| Ki-M4 | Mouse | IgG2a | BMA | IH | |
| DRC-1(R4/23) | Mouse | IgM | DAKO | IC |
Abbreviations: H, heterologous; GZM, Genzyme; BD, Becton Dickinson; UBI, Upstate Biotechnology Inc; IT, Immunotech; BMA, Biomedicals Ltd; IH, immunohistochemical; IC, immunocytochemical; IEM, immunoelectron microscopy.
Immunoelectron Microscopy
Immunoelectron microscopy was used for the localization of GM-CSFRα, TNFR I, and IL-4R in tonsillar LFs. PLP-fixed tonsillar tissues were sliced to a 60-μm thickness using a Microslicer (Dosaka EM, Kyoto, Japan) and immunostained by means of the streptavidin-biotin complex (SAB).23 24 Biotinylated antimouse and rabbit IgG (Vector Laboratories) were used as secondary antibodies. Sections were incubated with streptavidin-DAB. Immunostained tissue sections were postfixed in 1.25% glutaraldehyde and 2% osmium, dehydrated in graded ethanols and propylene, and embedded in Epon 812. Representative areas of LFs were selected for electron microscopy. The ultrathin sections were observed using a Zeiss EM109 electron microscope (Carl Zeiss, Oberkochen, Germany). For the negative control, the primary antibodies were replaced with 0.01 mol/L PBS.
Isolation of FDCs and Germinal Center (GC) Lymphocytes
FDCs were isolated by a magnetic cell sorter (MACS; Miltenyi Biotec, Bergisch-Gladbach, Germany).25 Tonsillar tissues were cut in 300-μm–thick sections by Microslicer (Dosaka EM). GCs were enucleated under a stereoscope as described26 and digested with an enzyme cocktail of RPMI 1640 (Life Technologies, Gaithersburg, MD) containing collagenase IV (450 U/mL; Sigma Chemical Co, St Louis, MO) and deoxyribonuclease I (28k U/mL; Sigma) for 1 hour at 37°C. The cell suspension was washed with RPMI 1640 and passed through a 133-μm nylon mesh to remove nondigested tissue debris. A discontinuous density gradient was prepared by adjusting BSA (Patho-o-cyte; Miles Inc) with RPMI 1640 to a density of 1.052 g/mL and 1.030 g/mL. Diluted Patho-o-cyte (6 mL) at a density of 1.052 g/mL was placed in a centrifugation tube and overlaid with 4 mL of 1.030 g/mL solution. The cell suspension (4 mL) was layered on top of the gradient and centrifuged at 8,500g for 1 hour. The interphase was collected, washed with RPMI 1640, incubated with the biotinylated monoclonal antibody R4/23 (DAKO; mouse IgM)27-29 for 30 minutes at 4°C, and then incubated with streptavidin conjugated microbeads (Miltenyi Biotec) for 30 minutes at 4°C. The cell suspension was divided into the biotinylated FDC-rich fraction and nonbiotinylated cell (lymphocytes) fraction using MACS.
Cytospin slides were prepared from the two collected fractions. Immunocytochemistry using antibodies listed in Table 1, of which subclasses were all mouse IgG or heterologous rabbit sera, was performed using alkaline phosphatase-antialkaline phosphatase (APAAP).30 AP-conjugated affinity-isolated rabbit antimouse and swine antirabbit Igs (DAKO) were used as secondary antibodies. New fuchsin (DAKO) was used as the substrate. After immunostaining, the slides were double-stained by R4/23 using ABC method followed by DAB as the chromogen. Finally, the slides were counterstained with hematoxylin.
RT-PCR
Total cellular RNA was extracted from four tonsillar samples by means of acid guanidinium thiocyanate-phenol-chloroform (AGPC) using ISOGEN (Nippon Gene Co, Ltd, Tokyo, Japan) according to the manufacturer's instructions, as described by Chomczynski et al.31 In brief, tonsillar samples were homogenized with ISOGEN (1 mL/100 mg). Samples were centrifuged at 12,000g for 10 minutes at 4°C; and the supernatant was collected with a Pasteur pipette. Chloroform (0.2 mL) was added and centrifuged at 12,000g for 15 minutes at 4°C. RNA in the aqueous phase was transferred to a fresh tube, mixed with 0.5 mL of isopropanol, and centrifuged at 12,000g for 10 minutes at 4°C. The precipitate was dissolved in 1 mL of 70% ethanol, vortexed, and centrifuged at 7,500g for 5 minutes at 4°C. The precipitate was dried and dissolved with diethylpyrocarbonate (DEPC; Boehringer Ingelheim Bioproducts Partnership, Heidelberg, Germany)-treated water at a concentration of 1 to 2 mg/mL. RNA was separated from the GC and the extra-GC (EGC) in the tonsillar samples. Initially, whole tonsillar samples were digested as described above.25,26 Thereafter, the GC was enucleated under a stereoscope. RNA obtained from GC and EGC was extracted using AGPC and ISOGEN. Oligonucleotide primers for the RT-PCR amplification of TGF-β (amplified PCR fragment, 161 bp; Table 2A) and GM-CSF R α (amplified PCR fragment, 682 bp; Table 2A) mRNA were obtained from Clontech (Palo Alto, CA). The cDNA was synthesized from mRNA using a RNA LA PCR Kit (Takara, Ohtsu, Japan) including Avian Myeloblastosis Virus reverse transcriptase and random 9 mers.32 The final volume of 20 μL consisted of 1× RNA PCR buffer, 5 mmol/L MgCl2 , 1 mmol/L dNTP mixture, 1 U/μL RNase inhibitor, 0.25 U/μL reverse transcriptase, 2.5 mmol/L random 9 mers, and 1 μg total RNA sample. The reaction was incubated for 10 minutes at 30°C and for 1 hour at 42°C and terminated by heating the mixture for 5 minutes at 99°C. The PCR mixture consisted of 2.5 mmol/L MgCl2 , 1× LA PCR buffer II, 2.5 U/100 μL Takara LA Taq, 20 pmol of the upstream and downstream primers, as well as the synthesized cDNA. Finally, 100 μL of mineral oil (Sigma) was added to prevent evaporation. The samples were amplified in an ASTEC (PC-800, Tokyo, Japan) thermal cycler for 30 cycles (45 seconds at 94°C, 45 seconds at 60°C, and 2 minutes at 72°C). The PCR products were stained with ethidium bromide and resolved by 2% agarose gel electrophoresis.
The Primer of RT-PCR and the Oligonucleotide of In Situ Hybridization
| Table 2A. Upstream and downstream primer for RT-PCR |
| TGF-β (PCR product: 161 bp) |
| Upstream: 5′ GCCCTGGACACCAACTATTGCT 3′ |
| Downstream: 5′ AGGCTCCAAATGTAGGGGCAGG 3′ |
| GM-CSF R α (PCR product: 682 bp) |
| Upstream: 5′ AGAAATCGGATCTGCGAACAGTGGCACC 3′ |
| Downstream: 5′ TCCAGGTACGACAGCTTCTGATAGGTCC 3′ |
| G3PDH (PCR product: 983 bp) |
| Upstream: 5′ TGAAGGTCGGAGTCAACGGATTTGGT 3′ |
| Downstream: 5′ CATGTGGGCCATGAGGTCCACCAC 3′ |
| Table 2B. Oligonucleotide probe for in situ hybridization |
| TGF-β R II |
| 5′ CCCACGTTGCGCAGGTCCTCCCAGCTGATGACATGCCGCG 3′ |
| GM-CSF R α |
| 5′ TGATAGGTCCTGGGCTGTTTCCACCGTACGAGGCAGTGCG 3′ |
| Table 2A. Upstream and downstream primer for RT-PCR |
| TGF-β (PCR product: 161 bp) |
| Upstream: 5′ GCCCTGGACACCAACTATTGCT 3′ |
| Downstream: 5′ AGGCTCCAAATGTAGGGGCAGG 3′ |
| GM-CSF R α (PCR product: 682 bp) |
| Upstream: 5′ AGAAATCGGATCTGCGAACAGTGGCACC 3′ |
| Downstream: 5′ TCCAGGTACGACAGCTTCTGATAGGTCC 3′ |
| G3PDH (PCR product: 983 bp) |
| Upstream: 5′ TGAAGGTCGGAGTCAACGGATTTGGT 3′ |
| Downstream: 5′ CATGTGGGCCATGAGGTCCACCAC 3′ |
| Table 2B. Oligonucleotide probe for in situ hybridization |
| TGF-β R II |
| 5′ CCCACGTTGCGCAGGTCCTCCCAGCTGATGACATGCCGCG 3′ |
| GM-CSF R α |
| 5′ TGATAGGTCCTGGGCTGTTTCCACCGTACGAGGCAGTGCG 3′ |
Immunochemical Expression on FDC in Tonsillar Follicles
| Antibody . | Immunohistochemistry . | Immunocytochemistry of Isolated FDC . | ||||
|---|---|---|---|---|---|---|
| . | MZ . | OZ . | DZ . | BLZ . | ALZ . | . |
| TFG-β R II | − | − | − | − | ++ | ND |
| GM-CSF R α | ± | ± | − | + | ++ | + |
| TNF R I | − | − | − | ++ | + | + |
| TNF R II | − | − | − | − | − | − |
| IL-1 R I | − | − | − | − | − | − |
| IL-1 R II | − | − | − | + | + | ND |
| IL-2 R α | − | − | − | − | − | − |
| IL-2 R β | − | − | − | + | + | + |
| IL-4 R | ± | ± | − | + | + | + |
| IL-6 R | ± | ± | − | + | + | + |
| TGF-β | − | − | − | − | + | + |
| GM-CSF | − | − | − | − | − | ND |
| TNF-α | − | − | − | − | − | ND |
| IL-1 α | − | − | − | − | − | ND |
| IL-2 | − | − | − | − | − | ND |
| IL-4 | − | − | − | − | − | ND |
| IL-6 | − | − | − | − | − | ND |
| Antibody . | Immunohistochemistry . | Immunocytochemistry of Isolated FDC . | ||||
|---|---|---|---|---|---|---|
| . | MZ . | OZ . | DZ . | BLZ . | ALZ . | . |
| TFG-β R II | − | − | − | − | ++ | ND |
| GM-CSF R α | ± | ± | − | + | ++ | + |
| TNF R I | − | − | − | ++ | + | + |
| TNF R II | − | − | − | − | − | − |
| IL-1 R I | − | − | − | − | − | − |
| IL-1 R II | − | − | − | + | + | ND |
| IL-2 R α | − | − | − | − | − | − |
| IL-2 R β | − | − | − | + | + | + |
| IL-4 R | ± | ± | − | + | + | + |
| IL-6 R | ± | ± | − | + | + | + |
| TGF-β | − | − | − | − | + | + |
| GM-CSF | − | − | − | − | − | ND |
| TNF-α | − | − | − | − | − | ND |
| IL-1 α | − | − | − | − | − | ND |
| IL-2 | − | − | − | − | − | ND |
| IL-4 | − | − | − | − | − | ND |
| IL-6 | − | − | − | − | − | ND |
Abbreviations: ND, not done; MZ, mantle zone; OZ, outer zone; DZ, dark zone; BLZ, basal light zone; ALZ, apical light zone; −, negative; ±, often positive; +, weakly positive; ++, strongly positive.
Immunochemical Expression on Lymphocytes in Tonsillar Follicles
| Antibody . | Immunohistochemistry . | Immunocytochemistry of Isolated Lymphocytes . | ||||
|---|---|---|---|---|---|---|
| . | MZ . | OZ . | DZ . | BLZ . | ALZ . | . |
| TGF-β R II | − | − | − | − | − | ND |
| GM-CSF R α | − | − | − | ± | ± | ND |
| TNF R I | − | − | − | ± | ± | ND |
| TNF R II | − | − | − | + | + | ND |
| IL-1 R I | − | − | − | + | + | Nd |
| IL-1 R II | − | − | − | ± | ± | ND |
| IL-2 R α | − | + | + | + | + | ND |
| IL-2 R β | − | − | − | + | + | ND |
| IL-4 R | − | + | − | + | ++ | ND |
| IL-6 R | − | + | − | + | + | ND |
| TFG-β | − | − | − | + | + | <1% |
| GM-CSF | − | − | + | + | + | <1% |
| TNF-α | + | + | + | + | + | 8.0% ± 2.3% |
| IL-1α | + | + | + | + | + | 3.8% ± 1.1% |
| IL-2 | + | + | + | ++ | ++ | 11.5% ± 4.8% |
| IL-4 | + | + | + | + | + | <1% |
| IL-6 | − | + | ± | + | + | <1% |
| Antibody . | Immunohistochemistry . | Immunocytochemistry of Isolated Lymphocytes . | ||||
|---|---|---|---|---|---|---|
| . | MZ . | OZ . | DZ . | BLZ . | ALZ . | . |
| TGF-β R II | − | − | − | − | − | ND |
| GM-CSF R α | − | − | − | ± | ± | ND |
| TNF R I | − | − | − | ± | ± | ND |
| TNF R II | − | − | − | + | + | ND |
| IL-1 R I | − | − | − | + | + | Nd |
| IL-1 R II | − | − | − | ± | ± | ND |
| IL-2 R α | − | + | + | + | + | ND |
| IL-2 R β | − | − | − | + | + | ND |
| IL-4 R | − | + | − | + | ++ | ND |
| IL-6 R | − | + | − | + | + | ND |
| TFG-β | − | − | − | + | + | <1% |
| GM-CSF | − | − | + | + | + | <1% |
| TNF-α | + | + | + | + | + | 8.0% ± 2.3% |
| IL-1α | + | + | + | + | + | 3.8% ± 1.1% |
| IL-2 | + | + | + | ++ | ++ | 11.5% ± 4.8% |
| IL-4 | + | + | + | + | + | <1% |
| IL-6 | − | + | ± | + | + | <1% |
Abbreviations: ND, not done; MZ, mantle zone; OZ, outer zone; DZ, dark zone; BLZ, basal light zone; ALZ, apical light zone; −, negative; ±, often positive; +, weakly positive; ++, strongly positive.
The controls for TGF-β and GM-CSFRα were as follows: (1) positive controls using a glyceraldehyde 3-phosphate dehydrogenase (G3PDH) primer for housekeeping mRNA (Table 2A; amplified PCR fragment, 983 bp; Clontech), (2) cDNA fragment of each previously confirmed primer (Clontech), and negative controls using (3) DEPC-treated water instead of reverse transcriptase, (4) upstream and downstream primer, and (5) Taq polymerase. One hundred basepair ladders (Pharmacia Biotech, Uppsala, Sweden) were molecular weight markers.
In Situ Hybridization
The antisense oligonucleotide probes TGF-β R II (1012-51, 40 mer; Table 2B)33 and GM-CSF R α (841-880, 40 mer; Table 2B)34 were constructed by means of a DNA synthesizer (Nihon Gene Research Laboratory, Inc, Sendai, Japan) and labeled with digoxigenin using a Digoxigenin Oligotailing Kit (Boehringer Mannheim, GmBH, Mannheim, Germany). The cDNA probe TGF-β (1.05 kb) purchased from American Type Culture Collection (Rockville, MD) was labeled with digoxigenin using a Nick Translation Kit (Boehringer Mannheim GmBH). Four percent PFA-fixed paraffin-embedded and cryostat tonsillar sections were digested with 0.1 to 10 mg/mL proteinase-K (DAKO) for 10 minutes at 37°C and postfixed with 4% PFA/PBS for 5 minutes at 4°C. The sections were acetylated with 0.1 mol/L triethanolamine (pH 8.0) containing 0.25% acetic anhydride for 10 minutes at room temperature and dehydrated. The hybridization temperature was 40°C, and the probe concentration was 2 ng/mL. The sections were washed with 2× SSC/50% formamide (1× SSC: 0.15 mol/L sodium chloride, 0.03 mol/L sodium citrate) for 30 minutes at 37°C, 2× SSC for 10 minutes at 37°C, and 1× SSC for 10 minutes at room temperature. Digoxigenin-labeled probes were detected using a Digoxigen Detection Kit (Boehringer Mannheim GmBH) and AP-conjugated rabbit antidigoxigenin antibody, with 5-bromo-4-chloro-3-indoxyl phosphate/nitro blue tetrazolium chloride solution (BCIP/NBT; Boehringer Mannheim GmBH) as the substrate. The slides were counterstained with methyl green.
Expression of TGF-β and TGF-β receptor II in tonsillar lymphoid follicle. (A) Immunostain of TGF-β. There is the reticular and dotted positive stain in the apical and basal light zones but not the other follicular zones. MZ, mantle zone; LZ, light zone; DZ, dark zone. Counterstained with methyl green. Original magnification × 132. (B) Immunostain of TGF-βR II. There is the reticular expression in the apical light zone. OZ, outer zone; ALZ, apical light zone; BLZ, basal light zone; DZ, dark zone. Counterstained with methyl green. Original magnification × 100. (C) Immunocytochemical single staining (red color) of TGF-β on an isolated FDC. Original magnification × 1,280. (D) Double staining of TGF-β and R4/23 on the same FDC. There is a positive signal in the cytoplasm of FDC (brown). Counterstained with hematoxylin. Original magnification × 1,280. (E) In situ hybridization of TGF-β cDNA in the ALZ. Dendritic-shaped cells heavily labeled (arrows). Scattered lymphocytes are also stained (arrowheads). Uncounterstained. Original magnification × 860. (F) In situ hybridization of a TGF-βR II oligonucleotide in the ALZ. Many dendritic-shaped cells are strongly positive. Uncounterstained. Original magnification × 400.
Expression of TGF-β and TGF-β receptor II in tonsillar lymphoid follicle. (A) Immunostain of TGF-β. There is the reticular and dotted positive stain in the apical and basal light zones but not the other follicular zones. MZ, mantle zone; LZ, light zone; DZ, dark zone. Counterstained with methyl green. Original magnification × 132. (B) Immunostain of TGF-βR II. There is the reticular expression in the apical light zone. OZ, outer zone; ALZ, apical light zone; BLZ, basal light zone; DZ, dark zone. Counterstained with methyl green. Original magnification × 100. (C) Immunocytochemical single staining (red color) of TGF-β on an isolated FDC. Original magnification × 1,280. (D) Double staining of TGF-β and R4/23 on the same FDC. There is a positive signal in the cytoplasm of FDC (brown). Counterstained with hematoxylin. Original magnification × 1,280. (E) In situ hybridization of TGF-β cDNA in the ALZ. Dendritic-shaped cells heavily labeled (arrows). Scattered lymphocytes are also stained (arrowheads). Uncounterstained. Original magnification × 860. (F) In situ hybridization of a TGF-βR II oligonucleotide in the ALZ. Many dendritic-shaped cells are strongly positive. Uncounterstained. Original magnification × 400.
Four control studies were performed to test the specificities of the oligonucleotide probes and the stainability as follows: (1) a positive control using a fluorescein isothiocyanate (FITC)-labeled β-actin probe (DAKO), followed by AP-conjugated rabbit anti-FITC antibody and BCIP/NBT as a substrate, and negative controls using (2) PBS instead of the probe for the hybridization, (3) a sense probe instead of the antisense probe, and (4) RNase-digested sections (200 mg/mL for 1 hour at 37°C).
Control Studies
Isolation of FDCs and GC lymphocytes.More than 80% of FDCs isolated from GC were positive for the antibodies R4/23 and Ki-M4, and none were positive for epithelial membrane antigen. More than 90% of lymphocytes isolated from GC were positive for the B-cell marker, CD20; about 5% for the T-cell marker, CD45RO; and less than 1% for the marker of MZ lymphocytes, CD62L, showing that they were isolated from GC.
RT-PCR.The agarose-gel resolution of RNA isolated from whole tonsillar tissue and GC and EGC areas showed 18S and 28S bands. No bands were seen in the absence of reverse transcriptase, 5′ and 3′ primer, and Taq polymerase. Positive bands were observed on confirmed PCR products that were positive for G3PDH (983 bp; see Table 6 and Fig 2C).
The Results of RT-PCR
| Probe . | Total Tissue . | GC . | EGC . |
|---|---|---|---|
| TGF-β | + | + | + |
| GM-CSF R α | + | + | + |
| G3PDH (control) | + | + | + |
| Probe . | Total Tissue . | GC . | EGC . |
|---|---|---|---|
| TGF-β | + | + | + |
| GM-CSF R α | + | + | + |
| G3PDH (control) | + | + | + |
Abbreviations: +, positive; −, negative; GC, germinal center; EGC, extragerminal center.
The RT-PCR expression of TGF-β, GM-CSFRα, and G3PDH. (A) RT-PCR of TGF-β in samples of complete tonsil, germinal center (GC), and extra-germinal center (EGC), showing a single-positive band (161 bp). Lane 1, 100-bp ladder; lane 2, total tonsil (case 1); lane 3, total tonsil except reverse transcriptase (patient 1); lanes 4 and 5, total tonsil (patients 2 and 3); lane 6, GC (patient 4); lane 7, EGC (patient 4); lane 8, primer confirmed as a positive control. (B) RT-PCR of GM-CSFRα in samples of total tonsil and GC, showing a single-positive band (682 bp). Lane 1, 100-bp ladder; lane 2, total tonsil (patient 1); lane 3, total tonsil except reverse transcriptase (patient 1); lanes 4 and 5, total tonsil (patients 2 and 3); lane 6, GC (patient 4); lane 7, EGC (patient 4); lane 8, primer confirmed as a positive control. (C) RT-PCR of G3PDH in samples of total tonsil, GC, and EGC, showing a single-positive band (983 bp). Lane 1, 100-bp ladder; lane 2, total tonsil (patient 1); lanes 3 and 4, total tonsil (patients 2 and 3); lane 5, GC (patient 4); lane 6, EGC (patient 4); lane 7, primer confirmed as a positive control.
The RT-PCR expression of TGF-β, GM-CSFRα, and G3PDH. (A) RT-PCR of TGF-β in samples of complete tonsil, germinal center (GC), and extra-germinal center (EGC), showing a single-positive band (161 bp). Lane 1, 100-bp ladder; lane 2, total tonsil (case 1); lane 3, total tonsil except reverse transcriptase (patient 1); lanes 4 and 5, total tonsil (patients 2 and 3); lane 6, GC (patient 4); lane 7, EGC (patient 4); lane 8, primer confirmed as a positive control. (B) RT-PCR of GM-CSFRα in samples of total tonsil and GC, showing a single-positive band (682 bp). Lane 1, 100-bp ladder; lane 2, total tonsil (patient 1); lane 3, total tonsil except reverse transcriptase (patient 1); lanes 4 and 5, total tonsil (patients 2 and 3); lane 6, GC (patient 4); lane 7, EGC (patient 4); lane 8, primer confirmed as a positive control. (C) RT-PCR of G3PDH in samples of total tonsil, GC, and EGC, showing a single-positive band (983 bp). Lane 1, 100-bp ladder; lane 2, total tonsil (patient 1); lanes 3 and 4, total tonsil (patients 2 and 3); lane 5, GC (patient 4); lane 6, EGC (patient 4); lane 7, primer confirmed as a positive control.
In situ hybridization.The FITC-labeled β-actin probe as the positive control was detected in LF. Negative controls were not detected on any sections.
RESULTS
TGF-β and TGF-β Receptor II
Immunohistochemically, the ALZ but not the other follicular zones was positive in the reticular meshwork and dotted patterns for TGF-β and TGF-βR II (Tables 3 and 4 and Fig 1A and B). Immunocytochemically, FDCs isolated with MACS were single-positive for TGF-β in their cytoplasm (Fig 1C) and double-positive for TGF-β and R4/23 (Fig 1D). Less frequently, lymphocytes isolated with MACS were positive for TGF-β (<1%). In situ hybridization using a cDNA probe for TGF-β showed the reticular and dotted patterns only in the ALZ labeling cells such as dendritic-shaped cells and lymphocytes and the scattered pattern labeling vascular walls within the whole LF (Table 5 and Fig 1E). In situ hybridization using an oligonucleotide probe for TGF-βRII showed only the reticular pattern in the ALZ labeling dendritic-shaped cells (Fig 1F). Vascular walls within the whole LF were also reacted. Follicular lymphocytes were negative. RT-PCR of samples obtained from the whole tonsillar sample as well as the GC and EGC showed a single-positive band of TGF-β (161 bp; Table 6 and Fig 2A).
The Results of In Situ Hybridization
| Probe . | FDC . | Lymphocyte . | Vascular Endothelium . |
|---|---|---|---|
| TGF-β | + (within the ALZ) | + (within the ALZ) | + (within the whole LF) |
| TGF-βRII | + (within the ALZ) | — | + (within the whole LF) |
| GM-CSFRα | + (except the DZ) | + (within the LZ) | — |
| Probe . | FDC . | Lymphocyte . | Vascular Endothelium . |
|---|---|---|---|
| TGF-β | + (within the ALZ) | + (within the ALZ) | + (within the whole LF) |
| TGF-βRII | + (within the ALZ) | — | + (within the whole LF) |
| GM-CSFRα | + (except the DZ) | + (within the LZ) | — |
Abbreviations: ALZ, apical light zone; DZ, dark zone; LZ, light zone; LF, lymphoid follicle.
GM-CSF and GM-CSFRα
Some GC lymphocytes were positive for GM-CSF (Fig 3A). No distinct positive reticular pattern was found in GC. On the other hand, the ALZ was heavily labeled in the reticular pattern and rarely in the dotted pattern for GM-CSFRα (Fig 3B). The BLZ was also weakly positive, and the OZ and MZ were often reacted. Immunoelectron microscopy confirmed that FDCs were positive for GM-CSFRα on their surface (Fig 3C). In situ hybridization also showed the expression of mRNA of GM-CSFRα in the cytoplasm of dendritic-shaped cells in the LZ and often in the OZ and MZ (Table 5 and Fig 3D). A few of the lymphocytes were positive for GM-CSFRα in the LZ. RT-PCR of samples from complete tonsillar tissue as well as GC and EGC showed a single-positive band (682 bp; Table 6 and Fig 2B).
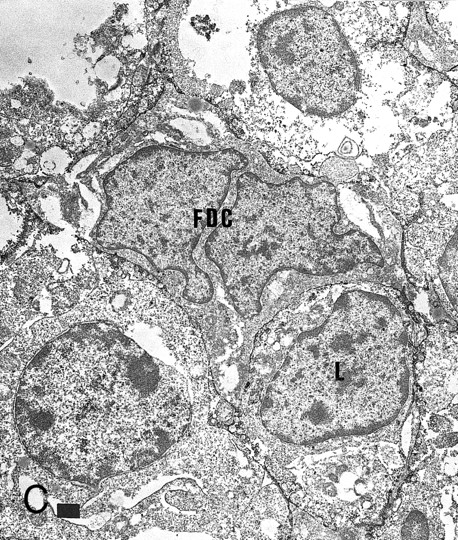
Expression of GM-CSF and GM-CSFRα in tonsillar lymphoid follicle. (A) Immunostain of GM-CSF. Some positive lymphocytes are scattered in the germinal center, but the distinct reticular pattern was not found. Counterstained with methyl green. Original magnification × 90. (B) Immunostain of GM-CSFRα. The distinct lacy pattern is found in the light zone and often in the outer and mantle zones. MZ, mantle zone; ALZ, apical light zone. Counterstained with methyl green. Original magnification × 240. (C) Immunoelectron micrograph of GM-CSFRα on FDC. Note a positive reaction on the surface of binucleated FDC embracing lymphocyte (L) of FDC. Bar = 1 nm. Original magnification × 5,700. (D) In situ hybridization of GM-CSFRα oligonucleotide. Dendritic-shaped cells are heavily labeled. Uncounterstained. Original magnification × 386.
Expression of GM-CSF and GM-CSFRα in tonsillar lymphoid follicle. (A) Immunostain of GM-CSF. Some positive lymphocytes are scattered in the germinal center, but the distinct reticular pattern was not found. Counterstained with methyl green. Original magnification × 90. (B) Immunostain of GM-CSFRα. The distinct lacy pattern is found in the light zone and often in the outer and mantle zones. MZ, mantle zone; ALZ, apical light zone. Counterstained with methyl green. Original magnification × 240. (C) Immunoelectron micrograph of GM-CSFRα on FDC. Note a positive reaction on the surface of binucleated FDC embracing lymphocyte (L) of FDC. Bar = 1 nm. Original magnification × 5,700. (D) In situ hybridization of GM-CSFRα oligonucleotide. Dendritic-shaped cells are heavily labeled. Uncounterstained. Original magnification × 386.
TNF-α, TNFR I, and TNFR II
Immunohistochemically, the LZ was positive in the reticular pattern for TNFR I (Fig 4A). Particularly, the BLZ was more heavily labeled than the ALZ (Fig 4B). Isolated FDCs were positive for TNFR I. Immunoelectron microscopy also confirmed that FDCs were positive for TNFR I (Fig 4C and D). A small group of GC lymphocytes were positive for TNF-α (about 8% of lymphocytes isolated with MACS) and a few in the LZ for TNFR I and II. However, positive dendritic pattern for TNF-α and TNFR II was not found.
Expression of TNFR I in tonsillar lymphoid follicle. (A and B) Immunostains of TNFR I. A lacy positive pattern (A) is evident in the apical and basal light zones. High-power view of the basal light zone, showing a heavily labeled lacy pattern (B). MZ, mantle zone; LZ, light zone; DZ, dark zone. (A) Original magnification × 98, (B) Original magnification × 300. (C and D) Immunoelectron micrographs of TNFR I in the tonsillar light zone. The cell surface of a binucleated FDC and the surface of the labyrinth-like structure of FDC embracing lymphocyte (L) are positively stained. Bar = 1 nm. (C) Original magnification × 5,700, (D) Original magnification × 10,080.
Expression of TNFR I in tonsillar lymphoid follicle. (A and B) Immunostains of TNFR I. A lacy positive pattern (A) is evident in the apical and basal light zones. High-power view of the basal light zone, showing a heavily labeled lacy pattern (B). MZ, mantle zone; LZ, light zone; DZ, dark zone. (A) Original magnification × 98, (B) Original magnification × 300. (C and D) Immunoelectron micrographs of TNFR I in the tonsillar light zone. The cell surface of a binucleated FDC and the surface of the labyrinth-like structure of FDC embracing lymphocyte (L) are positively stained. Bar = 1 nm. (C) Original magnification × 5,700, (D) Original magnification × 10,080.
IL-1α, IL-1R I, and IL-1R II
Immunohistochemically, the LZ was positive in the lacy pattern for IL-1R II but not for IL-1α and IL-1R I. IL-1R II was also detected on tonsillar epithelium. GC lymphocytes were positive for IL-1α, and those in the LZ were positive for IL-1R I and often for IL-1R II. Immunocytochemically, a few lymphocytes isolated with MACS were positive for IL-1α (3.8%).
IL-2, IL-2R α (CD25), and IL-2Rβ (CD122)
Immunohistochemically, the positive reticular reaction for IL-2Rβ but not for IL-2 and IL-2Rα was found in the LZ. Isolated FDCs were positive for IL-2Rβ but negative for IL-2Rα. GC lymphocytes, particularly in the LZ, were positive for IL-2, IL-2Rα, and IL-2Rβ. A relatively large percentage of isolated lymphocytes were positive for IL-2 (11.5%).
IL-4 and IL-4R (CDw124)
Immunohistochemically, the reticular reaction for IL-4R, but not for IL-4 in the LZ as well as often the OZ and MZ, was found (Fig 5A). Isolated FDC with MACS was single-positive for IL-4R (Fig 5B) and double-positive for IL-4R and R4/23 on their cell surface (Fig 5C). Immunoelectron microscopically, IL-4R was found on the surface of FDCs. Lymphocytes in the LF were positive for IL-4, and IL-4R was found especially in the ALZ, but not in the DZ. Isolated lymphocytes were positive for IL-4 (<1%).
Expression of IL-4 R and IL-6R in tonsillar lymphoid follicle. (A and D) Immunostains of IL-4R (A) and IL-6R (D). There is a positive lacy pattern of dendritic-shaped cells as well as a few lymphocytes mainly in the light zone. Counterstained with methyl green. (A and D) Original magnification × 328. (B, C, and E) Immunocytochemical stainings of IL-4R (B, single staining of IL-4R; C, double staining of IL-4R and R4/23) and IL-6R (E, single staining of IL-6R) on isolated FDCs. The positive reaction on FDCs is found. Counterstained with hematoxylin. (B), (C), and (E) Original magnification × 1,280.
Expression of IL-4 R and IL-6R in tonsillar lymphoid follicle. (A and D) Immunostains of IL-4R (A) and IL-6R (D). There is a positive lacy pattern of dendritic-shaped cells as well as a few lymphocytes mainly in the light zone. Counterstained with methyl green. (A and D) Original magnification × 328. (B, C, and E) Immunocytochemical stainings of IL-4R (B, single staining of IL-4R; C, double staining of IL-4R and R4/23) and IL-6R (E, single staining of IL-6R) on isolated FDCs. The positive reaction on FDCs is found. Counterstained with hematoxylin. (B), (C), and (E) Original magnification × 1,280.
IL-6 and IL-6R (CD126)
Immunohistochemically, the LZ, as well as often the OZ and MZ, was positive in the reticular pattern for IL-6R (Fig 5D), but not for IL-6. GC lymphocytes were positive for IL-6. Isolated FDC was positive for IL-6R (Fig 5E). Less frequently, isolated lymphocytes were positive for IL-6 (<1%). Lymphocytes in the LZ, but not in the DZ, were positive for IL-6R.
DISCUSSION
With special reference to FDCs, we investigated the localization of cytokine receptors as well as cytokines themselves in humans by several means. Dendritic-shaped cells were positive for various types of cytokine receptors, including TGF-βR II in the ALZ, TNFR I relatively markedly in the BLZ, and GM-CSFRα, IL-1R II, IL-2Rβ, IL-4R, and IL-6R, and the cytokine TGF-β in the BLZ and ALZ. GM-CSFRα, IL-4R, and IL-6R were often found in the OZ and MZ. Immunocytochemistry, using isolated FDCs, showed that TGF-β is localized diffusely in the cytoplasm of FDCs, whereas GM-CSFRα, TNFR I, and IL-4R were found on the surface of FDCs. In situ hybridization showed that dendritic-shaped cells in the LZ were positive for mRNA of TGF-β, TGF-βRII, and GM-CSFRα, and RT-PCR also showed that the GC contained single bands of TGF-β and GM-CSFRα. These data showed that FDCs exhibiting mainly in the LZ expressed a variety of cytokine receptors and the cytokine TGF-β.
TGF-β plays a potent, modulating, and immunosuppressive role.35,36 It promotes murine B cells stimulated with lipopolysaccharide to undergo isotype switching to IgA, whereas the persistent presence of TGF-β inhibits IgA secretion. Among the three types of TGF-βR, types I and II are essential for signal transduction.33,37 The expression of TGF-β in the LF has been reported,15 but the precise follicular localization of it and its receptor is not fully understood. The present immunochemical and in situ hybridization studies showed that most FDCs and very few lymphocytes in the ALZ are positive for TGF-β. Our RT-PCR study also indicated TGF-β in the GC as well as the extra-GC area. These data showed that FDCs as well as lymphocytes are the main source of TGF-β in the LF. TGF-βR II was positive in FDCs in the ALZ but not on follicular lymphocytes. To date, the effect of TGF-β is not completely clear, but these data indicate that FDCs may themselves amplify the autocrine or paracrine response to TGF-β that may be associated with the function of FDCs, in the ALZ, where B cells mature into memory B cells and plasmablasts.
Cultured FDCs can be maintained for a long time in the presence of GM-CSF.38 This study showed that a few lymphocytes but none of the FDCs in the GC expressed GM-CSF, and most FDCs in the LZ as well as often the OZ and MZ and a few LZ lymphocytes expressed its receptor under immuno-light and electron microscopy. RT-PCR and in situ hybridization showed the localization of GM-CSFRα and its mRNA on/in the FDCs. GM-CSF released from lymphocytes in the LZ may be important for FDC survival.
Ruco et al39 have reported that FDCs produce TNF-α. However, as indicated by others,40 we found that TNF-α was expressed on follicular lymphocytes but not on FDCs. Whereas TNFR I was expressed on FDCs and weakly on lymphocytes in the LZ according to light and electron microscopic findings, TNFR II was found only on lymphocytes in the LZ. Ryffel et al19 have reported that FDCs express TNFR I and TNF-α acting through autocrine and paracrine pathways. It has been recently shown that TNFα is essential to form primary LF, FDC network, and GC.4,41,42 TNFR also associates with apoptosis and a high level of intercellular adhesion molecule-1 expression.43
In the signal transduction of IL-1, IL-1RI positively transduces signals, but IL-1RII is an antagonist of IL-1.44 Ruco et al39 have described the positive staining of FDCs for IL-1β. This study suggested that IL-1 released from follicular lymphocytes acts on lymphocytes in the LZ via both types of receptors, but on FDCs in the LZ only via IL-1RII. It has been pointed out that the synergetic effect of IL-1α and CD23 induces B cells to differentiate into IgG-positive plasma cells in the ALZ.45
Zola et al46 have described the p55 and p75 expression of tonsillar B cells at low levels. In the present immunohistochemical study, FDCs in the LZ expressed IL-2Rβ but not IL-2Rα. A relatively large amount of IL-2–expressing lymphocytes were found extensively in the GC as well as in the extrafollicular area. IL-2Rα was the only 1 among the 10 receptors examined that was expressed on lymphocytes in the DZ, showing that it may involve in B-cell proliferation. B cells as well as FDCs in the LZ may mediate IL-2 signals.
CD57+ T cells in the LF produce IL-412 and express biphasic IL-4 mRNA14,47 and tonsillar B cells express IL-4R.18 FDCs express CD23 after stimulation by IL-4 and secrete soluble CD23.48 Here, FDCs in the LZ as well as often the OZ and MZ and lymphocytes in the GC expressed IL-4R, indicating that IL-4 produced by GC T cells bind to FDCs as well as to lymphocytes to secrete soluble CD23, which is important in promoting B-cell differentiation and proliferation.
IL-6 induces Ig production in the late phase of B-cell differentiation.49 IL-6 localizes outside the GC,50 and isolated and cultured FDCs express IL-6 mRNA51 and produce IL-6.38 Here, FDCs in the LZ as well as often the OZ and MZ expressed IL-6R but not IL-6, and FDCs may relate to B-cell differentiation into plasmablasts.
We showed here that FDCs in the follicular LZ express 7 of 10 cytokine receptors examined, but only TGF-β among the 7 cytokines examined. On the other hand, follicular lymphocytes expressed 9 receptors (except for TGF-βR II) and frequently expressed TNF-α, IL-1α, and IL-2 and less frequently TGF-β, GM-CSF, IL-4, and IL-6. These data indicate that cytokines affect only FDCs in the LZ as well as lymphocytes expressing receptors for some cytokines and may be important in regulating the function of FDCs. The simultaneous evaluation of both cytokines and their receptors may provide a powerful strategy for clarifying the cytokine network in the LF. This work, therefore, significantly increases our knowledge on the network in the LF, which is important for the understanding of the differentiation of B cells in lymphoid tissues.
ACKNOWLEDGMENT
We thank the doctors of the Department of Otorhinolaryngology of Yamagata Saiseikan, Yamagata Kahoku, Yonezawa City Hospitals, and Yamagata University School of Medicine; the Department of Pathology of Yamagata Saiseikan Hospital; and the Department of Surgery, Yamagata Saisei Hospital for providing fresh materials. We also thank Dr Kanichi Nakagawara (Nihon Gene Research Laboratories, Inc, Sendai, Japan) for the synthesis of cDNA probes and Prof Takeshi Kasajima (Second Department of Pathology, Tokyo Women's Medical College) and Junko Higuchi (Second Department of Pathology, Yamagata University School of Medicine) for technical support.
Address reprint requests to Kazuhiko Yamada, MD, First Department of Surgery, Yamagata University School of Medicine, 2-2-2 Iida-Nishi, Yamagata 990-23, Japan.










This feature is available to Subscribers Only
Sign In or Create an Account Close Modal