Abstract
Chronic graft-versus-host disease (GVHD) is likely caused by donor T lymphocytes. Because unmodified blood stem cell grafts contain one log more T lymphocytes than unmodified marrow grafts, we evaluated the incidence of chronic GVHD in previously reported 37 blood stem cell recipients and 37 computer-matched historical control marrow recipients (Bensinger et al, Blood 88:2794, 1996). All patients have been followed until death, relapse, or occurrence of chronic GVHD or for a minimum of 2 years. In a univariable proportional hazards regression model, the relative risk of developing clinical chronic GVHD (includes clinical limited and clinical extensive disease) by 2 years posttransplant among the peripheral blood stem cell recipients compared with the marrow recipients was 2.22 (95% confidence interval, 1.04 to 4.74; P = .039). For clinical extensive chronic GVHD, the relative risk was 2.37 (95% confidence interval, 1.07 to 5.29; P = .035). In multivariable analyses, considering also the covariables of patient age, patient cytomegalovirus serostatus, and donor cytomegalovirus serostatus, the relative risks of clinical chronic GVHD and clinical extensive chronic GVHD were also greater than 2 (P < .05). We conclude that the transplantation of unmanipulated filgrastim-mobilized blood stem cells may result in a relatively high incidence of chronic GVHD.
ENTHUSIASM FOR transplantation of allogeneic cytokine-mobilized peripheral blood stem cells (PBSCs) has recently developed because several phase I/II studies suggest that transplanting PBSCs instead of marrow results in faster engraftment and possibly improved immune reconstitution.1-12 Donor T lymphocytes likely cause both acute and chronic graft-versus-host disease (GVHD).13 Despite the fact that PBSC grafts contain one log more T lymphocytes than marrow grafts,8,10,11 a higher incidence of acute GVHD after PBSC compared with marrow transplantation has not been observed in the phase I/II studies.1-9 However, it is possible that PBSC recipients have a higher incidence of chronic GVHD than marrow recipients, given the historical example of donor blood buffy coat infusions to patients with aplastic anemia: the recipients of buffy coat plus marrow had a significantly higher incidence of chronic GVHD than did the recipients of marrow, whereas the two groups did not differ markedly in the incidence of acute GVHD.14 15 Therefore, we evaluated whether the transplantion of unmodified PBSCs might be associated with a higher incidence of chronic GVHD than the transplantation of unmodified marrow.
PATIENTS AND METHODS
Patient characteristics have been described.8 Briefly, 37 consecutive adult patients who received granulocyte colony-stimulating factor (G-CSF )–mobilized PBSCs from an HLA-identical sibling as their first allotransplant for an advanced hematologic malignancy were included. The historical control group was composed of 37 HLA-identical sibling marrow transplant recipients who were computer-matched for the following variables: diagnosis, disease phase at transplant, patient age, and GVHD prophylaxis.
Diagnosis of clinical chronic GVHD and its classification into clinical limited or clinical extensive disease was performed by two chart reviewers (J.S. and M.S.) using published criteria.16 Concordant diagnosis and classification was made in 44 of the 46 patients who survived relapse-free beyond day 80. Discordant diagnosis or classification was made in the following 2 cases: (1) 1 marrow recipient treated with corticosteroids for acute skin, liver, and gut GVHD from the first month posttransplant until his death on day 81, who died of liver failure and disseminated aspergillosis; and (2) 1 PBSC recipient who at 6 months posttransplant developed rash (skin biopsy positive for GVHD) and primarily cholestatic liver test abnormalities (alkaline phosphatase 9 times the upper normal limit, biopsy not performed) that resolved with corticosteroids. In this report, both patients are considered to have had clinical extensive chronic GVHD.
Cox regression models were fit to compare the hazard of clinical chronic GVHD among patients who received PBSCs with the hazard among those who received marrow. A univariable model was fit in addition to a multivariable model. Each model contained the source of stem cells as an explanatory variable, and the multivariable model also considered pretransplant variables known to be associated with the development of chronic GVHD. Estimates of the incidence of GVHD were obtained using cumulative incidence estimates,17 in which death or relapse without chronic GVHD was considered a competing risk. Patients alive without relapse and without chronic GVHD at last follow-up were censored at this time. Prevalence estimates were also used to describe the incidence of active clinical extensive chronic GVHD among patients alive without relapse.18 All P values arising from regression models were obtained from the Wald test and are two-sided. No adjustments were made for multiple comparisons.
RESULTS
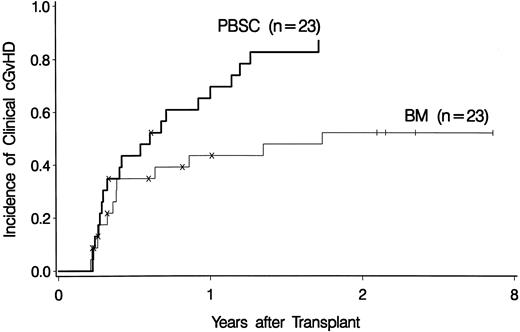
Among all 74 patients, 12 of 37 (32%) marrow recipients versus 20 of 37 (54%) PBSC recipients developed clinical chronic GVHD (Fig 1). Among 46 patients alive and relapse-free at day 80, 12 of 23 (52%) marrow recipients versus 20 of 23 (87%) PBSC recipients developed clinical chronic GVHD (Fig 2). Clinical extensive chronic GVHD occurred in 11 marrow and 18 PBSC recipients. All patients have been observed until death, relapse, or occurrence of chronic GVHD or for a minimum of 2 years.
The cumulative incidence of clinical chronic GVHD among all 37 bone marrow (BM) and all 37 PBSC recipients. The “×” tick marks denote patients who died or relapsed. The “‖” tick marks denote patients who have not developed clinical chronic GVHD by the time of the last follow-up. Note that the horizontal axis scale between 0 and 2 years differs from that between 2 and 8 years posttransplant.
The cumulative incidence of clinical chronic GVHD among all 37 bone marrow (BM) and all 37 PBSC recipients. The “×” tick marks denote patients who died or relapsed. The “‖” tick marks denote patients who have not developed clinical chronic GVHD by the time of the last follow-up. Note that the horizontal axis scale between 0 and 2 years differs from that between 2 and 8 years posttransplant.
The cumulative incidence of clinical chronic GVHD among the 23 bone marrow (BM) and the 23 PBSC recipients surviving without relapse beyond day 80. The tick marks and the horizontal axis scale are as in Fig 1.
The cumulative incidence of clinical chronic GVHD among the 23 bone marrow (BM) and the 23 PBSC recipients surviving without relapse beyond day 80. The tick marks and the horizontal axis scale are as in Fig 1.
In the univariable proportional hazards regression model, PBSC recipients had 2.22 times higher hazard of developing clinical chronic GVHD by 2 years than did marrow recipients (95% confidence interval, 1.04 to 4.74; P = .039). In the multivariable models, the following potential predictors of chronic GVHD were considered as covariables: patient age, patient cytomegalovirus (CMV) serostatus pretransplant and donor CMV serostatus pretransplant.19-22 None of these covariables significantly altered the apparent association between the source of stem cells and the hazard of chronic GVHD that was seen in the univariable model. For clinical extensive chronic GVHD, the hazard of developing it by 2 years was 2.37 times higher among PBSC than marrow recipients in the univariable model (95% confidence interval, 1.07 to 5.29; P = .035). Again, none of the above potential predictors of chronic GVHD significantly altered the apparent association between the source of stem cells and the hazard of clinical extensive chronic GVHD in the multivariable models. Splenectomy, another potential predictor of chronic GVHD,22 was not included as a covariable because none of the patients surviving beyond day 80 had a history of splenectomy. Female donor parity, identified in previous work as possibly being associated with an increased hazard of chronic GVHD (Hansen, unpublished data),20 was not included as a covariable because of a small number of patients with parous female donors that would make a multivariable model unstable. Nevertheless, it is unlikely that the observed high incidence of chronic GVHD in PBSC recipients was due to a high proportion of PBSC recipients with parous female donors, because 3 of 23 PBSC versus 9 of 23 marrow recipients surviving without relapse beyond day 80 had parous female donors.
Organs Involved With Chronic GVHD Among Patients Developing Clinical Chronic GVHD
| Organ . | Marrow Recipients . | PBSC Recipients . | P Value† . |
|---|---|---|---|
| . | (n = 12) . | (n = 20) . | . |
| Skin | 3 (25%)* | 13 (65%) | .07 |
| Liver | 9 (75%) | 12 (60%) | .47 |
| Eyes | 5 (42%) | 8 (40%) | 1.00 |
| Mouth | 7 (58%) | 15 (75%) | .44 |
| Gastrointestinal tract | 3 (25%) | 7 (35%) | .70 |
| Contractures | 0 (0%) | 2 (10%) | .52 |
| Organ . | Marrow Recipients . | PBSC Recipients . | P Value† . |
|---|---|---|---|
| . | (n = 12) . | (n = 20) . | . |
| Skin | 3 (25%)* | 13 (65%) | .07 |
| Liver | 9 (75%) | 12 (60%) | .47 |
| Eyes | 5 (42%) | 8 (40%) | 1.00 |
| Mouth | 7 (58%) | 15 (75%) | .44 |
| Gastrointestinal tract | 3 (25%) | 7 (35%) | .70 |
| Contractures | 0 (0%) | 2 (10%) | .52 |
Values are the number of marrow recipients who had clinical skin involvement, with the percentage of patients with clinical skin involvement of all marrow recipients with clinical chronic GVHD in parentheses.
P value for the difference between the percentage of marrow recipients with the involved organ and the percentage of PBSC recipients with the involved organ (Fisher exact test).
The prevalence of clinical extensive chronic GVHD among bone marrow (BM) and PBSC recipients surviving without relapse. Patients were considered to have ongoing clinical extensive chronic GVHD if they had been diagnosed with clinical extensive chronic GVHD and continued to receive immunosuppressive drugs. Note that the horizontal axis scale between 0 and 2 years differs from that between 2 and 8 years posttransplant.
The prevalence of clinical extensive chronic GVHD among bone marrow (BM) and PBSC recipients surviving without relapse. Patients were considered to have ongoing clinical extensive chronic GVHD if they had been diagnosed with clinical extensive chronic GVHD and continued to receive immunosuppressive drugs. Note that the horizontal axis scale between 0 and 2 years differs from that between 2 and 8 years posttransplant.
De novo chronic GVHD (without preceding acute GVHD) appeared relatively common after PBSC grafting: Among the 12 marrow and the 20 PBSC recipients developing clinical chronic GVHD, 2 of 12 (17%) versus 9 of 20 (45%) patients had de novo disease.
The involvement of various organs with chronic GVHD is displayed in Table 1. There appears to be no major difference in the organs affected by post-PBSC and post-marrow transplant chronic GVHD.
The median Karnofsky performance score at 1 year after grafting was 90% (range, 80% to 100%) among the 10 marrow recipients and 85% (range, 60% to 100%) among the 14 PBSC recipients surviving without relapse by day 365. The difference was not statistically significant (P = .23, Mann-Whitney test).
Among the 37 PBSC recipients, 14 were alive at last contact. Their median length of follow up is 682 days (range, 506 to 1,128 days). Among the 37 marrow recipients, 11 were alive at last contact, and the median length of follow-up is 1,260 days (range, 819 to 2,605 days).
Of the 37 marrow recipients, 4 patients that have not developed relapse or clinical extensive chronic GVHD (“‖” tick marks in Figs 1 and 2) were surviving at the time of the last follow-up (931, 1057, 1,488, and 2,705 days posttransplant). None of the 37 PBSC recipients survived without developing relapse or clinical extensive chronic GVHD (Fig 3). All marrow as well as PBSC recipients who had developed clinical extensive chronic GVHD were still on immunosuppressive drugs at the time of the last follow-up.
DISCUSSION
These data suggest that the incidence of chronic GVHD may be higher after PBSC than after marrow grafting. This was not detected at the time of our previous report8 most likely due to the short follow-up of the PBSC recipients (median, 285 days; range, 110 to 720 days) compared with that of the marrow recipients (median, 817 days; range, 295 to 2,224 days). Other groups have also reported a high incidence of chronic GVHD after PBSC transplantation.23-26 However, none of the previous reports presents a formal comparison of the PBSC recipients with similar marrow recipients.
It is not known why the incidence of acute GVHD does not whereas the incidence of chronic GVHD does appear higher after PBSC than marrrow transplantation.1-9,23,24 The relatively low incidence of acute GVHD in PBSC recipients despite the abundance of T cells in the PBSC grafts may be attributed to (1) G-CSF–induced preferential differentiation of T-helper cells into TH2 rather than TH1 cells, resulting in PBSC grafts with relatively few TH1 cells, that have been implicated in the pathogenesis of acute GVHD27-29; or (2) the large numbers of monocytes in PBSC grafts (up to 2-logs more than in marrow grafts) that suppress T cells.30-33 We speculate that the TH1 → TH2 polarization may wane or that the immunosuppressive monocytes may die within months after grafting. The latter hypothesis would be consistent with our unpublished data and the published data of Ottinger et al10 showing that, whereas the counts of both monocytes and T cells are higher in PBSC than in marrow recipients during the first 2 months, thereafter the monocyte counts are similar, whereas the T-cell counts remain higher in the PBSC recipients compared with marrow recipients for at least 6 months after grafting.
Chronic GVHD and/or its treatment is associated with immune deficiency. Infections occur more frequently in long-term marrow transplant survivors with chronic GVHD than in those without chronic GVHD.34-36 Because PBSC grafts contain more than 1 log more CD4 T cells than marrow grafts,8,10,11 because this appears to result in higher CD4 T-cell counts in the PBSC compared with marrow recipients,10 and because CD4 T-cell counts appear to inversely correlate with infectious morbidity in long-term transplant survivors,37 infections could theoretically occur less frequently in PBSC recipients than in marrow recipients with chronic GVHD. This is in accord with the following observation. Among our 10 of 37 PBSC and 3 of 37 marrow recipients who survived until 1 year without relapse and who developed clinical chronic GVHD by 1 year posttransplant, the average number of infections (defined as in Sullivan et al38 ) between days 180 and 365 per patient was 0.4 in the PBSC and 1.3 in the marrow recipients.
Chronic GVHD has been associated with a low incidence of leukemic relapse.39,40 Therefore, PBSC transplantation could be associated with a low incidence of relapse.41 Indeed, Korbling et al25 reported a relapse rate of 18% among 100 PBSC recipients versus 40% among an unspecified number of marrow recipients by 2 years posttransplant. However, information on the comparability of the PBSC and the marrow recipients in, for example, disease type or disease status pretransplant was not reported. In our series, we have not observed a lower incidence of relapse among the PBSC recipients: 15 of 37 PBSC and 14 of 37 marrow recipients relapsed by 1 year posttransplant.
Currently, it is unclear whether the advantage of fast engraftment1-9 and the possible advantages of superior graft-versus-leukemia reaction25 and superior immune reconstitution10 11 outweigh the disadvantage of frequent chronic GVHD. Therefore, at present, allogeneic PBSC transplantation should not be routinely performed instead of marrow transplantation, except in the context of controlled trials. Whether the incidence of chronic GVHD is truly higher in PBSC than marrow recipients and whether this disadvantage is outweighed by the advantages of PBSCs will hopefully be resolved by ongoing controlled randomized trials.
Supported by National Institutes of Health Grants No. CA68496, CA18221, and CA18029.
Address reprint requests to Jan Storek, MD, FHCRC, M318, 1124 Columbia St, Seattle, WA 98104-2092.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal